Abstract
Background
Observational studies have demonstrated an association between anemia and adverse outcomes in patients with chronic kidney disease (CKD). However, randomized trials failed to identify a benefit of higher hemoglobin concentrations, suggesting that the anemia‐outcome association may be confounded by unknown factors.
Methods and Results
We evaluated the influence of fluid status on hemoglobin concentrations and the cardiovascular and renal outcomes in a prospective cohort of 326 patients with stage 3 to 5 CKD. Fluid status, as defined by overhydration (OH) level measured with bioimpedance, was negatively correlated with hemoglobin concentrations at baseline (r=−0.438, P<0.001). In multivariate regression analysis, OH remained an independent predictor of hemoglobin, second only to estimated glomerular filtration rate. Patients were stratified into 3 groups: no anemia (n=105), true anemia (n=82), and anemia with excess OH (n=139) (relative OH level ≥7%, the 90th percentile for the healthy population). During a median follow‐up of 2.2 years, there was no difference in cardiovascular and renal risks between patients with true anemia and those with no anemia in the adjusted Cox proportional hazards models. However, patients with anemia with excess OH had a significantly increased risk of cardiovascular morbidity and mortality and CKD progression relative to those with true anemia and those with no anemia, respectively.
Conclusions
Fluid retention is associated with the severity of anemia and adverse cardiovascular and renal outcomes in patients with CKD. Further research is warranted to clarify whether the correction of fluid retention, instead of increasing erythropoiesis, would improve outcomes of CKD‐associated anemia.
Keywords: anemia, bioimpedance, cardiovascular, kidney, overhydration
Introduction
Observational studies have shown a strong detrimental relationship among anemia, chronic kidney disease (CKD), and mortality1; it is therefore logical to consider whether correcting anemia can improve patient outcomes. However, the failure of randomized trials to find a benefit of higher hemoglobin concentrations suggests that the anemia‐outcome association may be confounded by unknown factors.2–5 The cause of anemia in CKD is multifactorial. A low hemoglobin concentration may result from reduced red blood cell volume (true anemia) and/or from an increased extracellular water (ECW) volume (hemodilution) (Figure 1).6–8 Our recent study has shown that ECW volume overload is a common issue in non‐dialysis‐dependent CKD (ND‐CKD) patients.9 However, the prevalence of hemodilution in CKD is unclear, and its clinical impact has not been elucidated.
Figure 1.
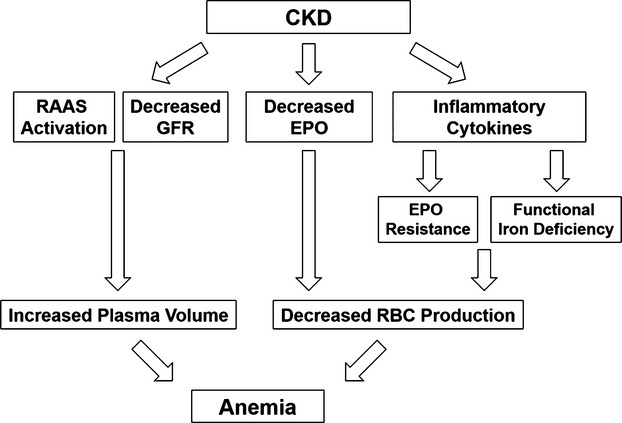
A low hemoglobin concentration may result from reduced red blood cell volume (true anemia) and/or from an increased extracellular water volume (hemodilution). CKD indicates chronic kidney disease; EPO, erythropoietin; GFR, glomerular filtration rate; RAAS, renin–angiotensin–aldosterone system; RBC, red blood cell.
In a secondary analysis of the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT), patients with a poor response to darbepoetin alfa had an increased risk of cardiovascular outcomes.10 However, it is difficult to ascertain whether this increased risk was due to the preexisting characteristics of the patients, an increased dose of erythropoiesis‐stimulating agents (ESAs), or both. We recently found that volume overload is strongly associated with both traditional and nontraditional risk factors for CKD progression and cardiovascular disease (CVD) in ND‐CKD patients.9 Because ESA treatment further increases the blood volume, the benefits of anemia correction by ESA may have been masked by the negative effects of increased fluid retention. Therefore, testing for an interaction between fluid status, hemoglobin concentrations, and outcomes in CKD patients seems warranted.
In the present study, we hypothesized that fluid status would be closely associated with hemoglobin concentrations and outcomes in patients with CKD. To test this hypothesis, we evaluated the influence of fluid retention on hemoglobin concentrations and the cardiovascular and renal outcomes in a prospective cohort of 326 patients with stage 3 to 5 ND‐CKD, using a novel bioimpedance spectroscopy device to measure the fluid status.
Patients and Methods
Study Population
The study design and patients were previously described.9 Briefly, 338 clinically stable stage 3 to 5 ND‐CKD patients were recruited from the outpatient clinics between September 1, 2011 and December 31, 2012. All patients received the integrated multidisciplinary CKD care program in Taiwan,11 focusing on dietary salt and protein restriction, nephrotoxin avoidance, and strict blood pressure and glycemic control. For each patient, a thorough medical history was taken and the medical chart was reviewed at the time of study enrollment. The definition of CVD included coronary artery disease, as documented by coronary angiography or a history of myocardial infarction, class III to IV congestive heart failure, or cerebrovascular accident. All patients were followed up every 3 months until June 30, 2014. The study protocol complies with the Declaration of Helsinki and was approved by the institutional review board of Taipei Tzu Chi Hospital. All participants gave their written informed consent before inclusion.
Measurements
Fluid status was assessed by using the Body Composition Monitor (BCM, Fresenius Medical Care, Bad Homburg, Germany), a novel bioimpedance spectroscopy device, and was represented by the level of overhydration (OH). The BCM measures the electrical responses at 50 different frequencies between 5 and 1000 kHz. OH is derived from the impedance data based on a 3‐compartment model developed by Chamney et al12 The 3 compartments are lean tissue mass, adipose tissue mass, and OH. OH is the difference between the amount of ECW in tissue that is detected by the BCM and the amount of water present in tissue predicted using physiological models under normal (euvolemic) conditions. Therefore, the OH value obtained from the BCM can be compared directly with that of the normal population. Volume overload was defined as a relative OH value (OH normalized to ECW or OH/ECW) ≥7%, corresponding to the value of the 90th percentile for the reference cohort.13 The BCM device has been validated in a study involving 350 healthy people with the same ethnic background in Taiwan. Both investigators and patients were blinded to the OH results. We performed a repeat body composition measurement 6 months after enrollment and found no significant changes in the OH levels over time (paired t test, P=0.563). Anemia was defined in accordance with the World Health Organization as hemoglobin concentrations <13.0 g/dL in men and <12.0 g/dL in women.
Outcomes
The primary outcome, morbidity and mortality from cardiovascular causes, was a composite of myocardial infarction, hospitalization for congestive heart failure or unstable angina, or death from cardiovascular causes. The secondary outcome, the renal outcome, was the first occurrence of a decline in the estimated glomerular filtration rate (eGFR) ≥50% or end‐stage renal disease needing chronic dialysis. Changes in the eGFR were confirmed at least 4 weeks after treatment of potentially reversible factors. The timing of initiation of chronic dialysis was determined in accordance with the regulations of the National Health Insurance Administration of Taiwan, which suggest beginning dialysis at an eGFR level <5 mL/min per 1.73 m2. For the primary cardiovascular outcome, patients were censored at the time of their last outpatient visit, noncardiovascular death, or end of follow‐up period, whereas for the secondary renal outcome, patients were censored at the time of their last outpatient visit, death, or end of follow‐up period.
Statistical Analyses
All variables were expressed as frequencies and percentages for categorical data and as the means±SDs or medians and interquartile ranges for continuous data with or without a normal distribution, respectively. The baseline characteristics were compared using a χ2 test for categorical variables and Student t test or Mann–Whitney U test for continuous variables. Univariate correlations between hemoglobin and potential explanatory variables were assessed by Pearson correlation analyses. Multivariate regression analysis was used to identify variables that were independently associated with hemoglobin. Age, sex, and clinically relevant variables with a P value ≤0.10 in the univariate analysis were fitted. CKD patients were stratified by the presence or absence of anemia and volume overload into 3 groups: no anemia, true anemia (relative OH <7%), or anemia with excess OH (relative OH ≥7%). Cox proportional hazards modeling was used to estimate hazard ratios (HRs) and 95% CIs with unadjusted and multivariate adjusted models for the cardiovascular and renal outcomes separately. The proportional hazard assumption, the constant HR over time, was evaluated by comparing estimated log–log survival curves for all time‐independent covariates. All assessed log–log survival plots graphically showed 2 parallel lines, indicating no violation of the assumption. A 2‐tailed P‐value <0.05 was considered statistically significant. Analyses were performed using SPSS (Statistical Package for the Social Sciences) version 20.0 software (SPSS Inc, Chicago, IL).
Results
Baseline Characteristics
Out of a total of 338 stage 3 to 5 ND‐CKD patients, 12 patients were excluded from the analysis due to starting chronic dialysis within the first month after enrollment (n=4) or were lost to follow‐up after the initial visit (n=8). Thus, the final sample consisted of 326 CKD patients (Table 1). Overall, 68% of the study population had anemia (n=221). Patients with anemia were further divided based on the presence of volume overload (anemia with excess OH) or not (true anemia). The patients in the 2 groups were similar with regard to age, sex, smoking history, eGFR, and ferritin levels, but diabetes and CVD were more prevalent in the volume overload group. Additionally, patients with anemia with excess OH were found to have significantly lower serum albumin, as well as higher systolic blood pressure, urine protein‐to‐creatinine ratio, N‐terminal pro‐brain natriuretic peptide, and interleukin‐6 at baseline, compared with patients with true anemia.
Table 1.
Baseline Characteristics of the Study Cohort According to the Presence or Absence of Anemia and Volume Overload
| Characteristics | No Anemia | Anemia | P Value* | |
|---|---|---|---|---|
| OH <7% | OH ≥7% | |||
| (N=105) | (N=82) | (N=139) | ||
| Hemoglobin, g/dL | 14.1±1.0 | 11.0±1.2 | 10.4±1.5 | 0.001 |
| Age, y | 61.8±14.1 | 68.7±13.0 | 67.1±12.2 | 0.35 |
| Male sex, n (%) | 85 (81.0) | 46 (56.1) | 93 (66.9) | 0.108 |
| Smoking history, n (%) | 23 (21.9) | 18 (22.0) | 26 (18.7) | 0.559 |
| Diabetes mellitus, n (%) | 36 (34.3) | 25 (30.5) | 87 (62.6) | <0.001 |
| CVD, n (%) | 15 (14.3) | 13 (15.9) | 48 (34.5) | 0.003 |
| Systolic BP, mm Hg | 135±17 | 133±15 | 142±17 | <0.001 |
| Diuretics, n (%) | 22 (21.0) | 27 (32.9) | 61 (43.9) | 0.108 |
| RAAS blockers, n (%) | 72 (68.6) | 44 (53.7) | 80 (57.6) | 0.573 |
| Statins, n (%) | 33 (31.4) | 17 (20.7) | 36 (25.9) | 0.385 |
| Body mass index, kg/m2 | 26.8±3.7 | 24.9±4.2 | 25.7±4.3 | 0.192 |
| Relative OH, % | 3.6±5.9 | 1.7±4.1 | 15.6±6.5 | <0.001 |
| Lean tissue index, kg/m2 | 16.7±2.9 | 14.9±3.1 | 14.4±3.1 | 0.304 |
| Fat tissue index, kg/m2 | 9.7±4.2 | 9.9±4.2 | 9.9±4.4 | 0.995 |
| eGFR,* mL/min per 1.73 m2 | 39.8±12.0 | 24.6±12.9 | 23.1±13.0 | 0.43 |
| UPCR | 0.49 (0.16 to 1.35) | 0.58 (0.30 to 1.34) | 1.90 (0.64 to 4.34) | <0.001 |
| Albumin, g/dL | 3.8±0.3 | 3.7±0.4 | 3.4±0.4 | <0.001 |
| Ferritin, ng/mL | 210 (137 to 391) | 222 (124 to 383) | 250 (119 to 390) | 0.62 |
| NT‐proBNP, ng/L | 58 (31 to 150) | 184 (99 to 481) | 613 (277 to 1387) | <0.001 |
| IL‐6, pg/mL | 2.8 (1.6 to 4.2) | 3.1 (1.8 to 4.8) | 4.7 (2.8 to 8.8) | <0.001 |
BP indicates blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; IL‐6, interleukin‐6; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; OH, overhydration; RAAS, renin–angiotensin–aldosterone system; UPCR, urine protein‐to‐creatinine ratio.
Comparison between anemic patients with relative OH <7% and ≥7%.
Calculated by using the Modification of Diet in Renal Disease (MDRD) formula.
Impact of OH on Hemoglobin and Outcomes
Correlations between hemoglobin and other variables in all patients (n=326) are shown in Table 2. Fluid status, as defined by OH, was negatively correlated with hemoglobin concentrations at baseline (r=−0.438, P<0.001) (Figure 2). In multivariate regression analysis, OH remained an independent predictor of hemoglobin concentrations, second only to eGFR.
Table 2.
Determinants of Hemoglobin Concentrations in 326 Patients With CKD
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| Coefficient β* | P Value | Coefficient β* | P Value | |
| Age, y | −0.169 | 0.002 | −0.089 | 0.031 |
| Male sex | 0.406 | <0.001 | 0.258 | <0.001 |
| Smoking history | 0.101 | 0.070 | — | — |
| Diabetes mellitus | −0.200 | <0.001 | — | — |
| CVD | −0.136 | 0.014 | — | — |
| RAAS blockers | 0.149 | 0.007 | −0.090 | 0.023 |
| Relative OH, % | −0.438 | <0.001 | −0.366 | <0.001 |
| ln NT‐proBNP,* ng/L | −0.624 | <0.001 | — | — |
| eGFR, mL/min per 1.73 m2 | 0.598 | <0.001 | 0.440 | <0.001 |
| ln Ferritin, ng/mL | −0.009 | 0.870 | — | — |
| ln IL‐6, pg/mL | −0.217 | <0.001 | — | — |
Adjusted R2 = 0.537. CKD indicates chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; IL‐6, interleukin‐6; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; OH, overhydration; RAAS, renin–angiotensin–aldosterone system.
Coefficient β refers to how many SDs a dependent variable will change, per SD increase in the predictor variable. The higher the coefficient β value, the greater the impact of a predictor variable on hemoglobin concentrations.
NT‐proBNP is highly correlated with overhydration and thus not included in the multivariate models to avoid collinearity.
Figure 2.
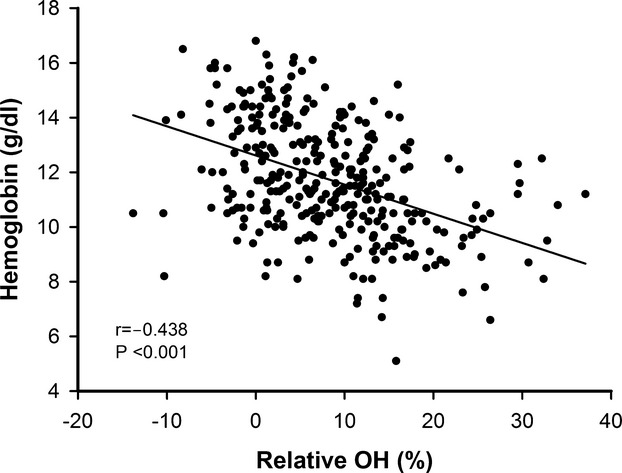
Fluid status, as defined by relative overhydration (OH), was negatively correlated with hemoglobin concentrations (r=−0.438, P<0.001).
We stratified the entire study cohort into 3 groups (no anemia, true anemia, or anemia with excess OH) and examined their relationship with the cardiovascular and renal outcomes in time‐to‐event analyses. During a median follow‐up of 2.2 years, there were 3 cardiovascular end‐point events (2.9%) in the no‐anemia group, 5 (6.1%) in the true‐anemia group, and 37 (26.6%) in the anemia with excess OH group (Table 3). For renal end‐point events, there were 9 (8.6%) in the no‐anemia group, 17 (20.7%) in the true‐anemia group, and 70 (50.4%) in the anemia with excess OH group. By multivariate regression analysis, there was no difference in cardiovascular and renal risks between patients with true anemia and those with no anemia. However, anemia with excess OH remained an independent predictor of the adverse outcomes. Patients with anemia with excess OH had a significantly increased risk of the cardiovascular and renal outcomes relative to those with no anemia. Moreover, the HR was also statistically different for patients with anemia with excess OH (reference group) from those with true anemia (adjusted HR for cardiovascular outcome, 0.31; 95% CI, 0.12 to 0.82; P=0.018, and adjusted HR for renal outcome, 0.42; 95% CI, 0.23 to 0.74; P=0.003, respectively). We also performed multivariate Cox analyses with hemoglobin as a continuous variable. The predictive power of hemoglobin for cardiovascular and renal risks attenuated after adjustment for OH (Table 4).
Table 3.
Hazard Ratios of Different Anemia Groups in Relation to the Cardiovascular and Renal Outcomes
| Anemia Group | Events | Unadjusted | Multivariate Adjusted |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||
| Cardiovascular outcome | |||
| Anemia with excess OH | 37 | 1 | 1 |
| True anemia | 5 | 0.19 (0.08, 0.49)† | 0.31 (0.12, 0.83)† |
| No anemia | 3 | 0.09 (0.03, 0.28)* | 0.24 (0.07, 0.83)† |
| Renal outcome | |||
| Anemia with excess OH | 70 | 1 | 1 |
| True anemia | 17 | 0.32 (0.19, 0.54)* | 0.46 (0.26, 0.81)† |
| No anemia | 9 | 0.11 (0.06, 0.22)* | 0.26 (0.12, 0.55)* |
Multivariate adjusted model: age, cardiovascular disease, diabetes mellitus, systolic blood pressure, estimated glomerular filtration rate, and a urine protein‐to‐creatinine ratio cut at 0.5. HR indicates hazard ratio; OH, overhydration.
*P<0.001, †P<0.05.
Table 4.
Hazard Ratios (HR) of Hemoglobin Concentrations in Relation to the Cardiovascular and Renal Outcomes
| Hemoglobin | Unadjusted | OH Adjusted | OH and Multivariate Adjusted |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Cardiovascular outcome | |||
| 1 g/dL increase | 0.73 (0.64, 0.84)* | 0.81 (0.69, 0.94)† | 0.99 (0.80, 1.21) |
| Renal outcome | |||
| 1 g/dL increase | 0.68 (0.62, 0.76)* | 0.75 (0.67, 0.84)* | 0.96 (0.82, 1.13) |
Multivariate adjusted model: age, cardiovascular disease, diabetes mellitus, systolic blood pressure, estimated glomerular filtration rate, and a urine protein‐to‐creatinine ratio cut at 0.5. OH indicates overhydration.
*P<0.001, †P<0.05.
Discussion
Our data show that fluid status, defined as the OH level, is one of the major determinants of low hemoglobin concentrations in patients with stage 3 to 5 CKD. Anemia in these patients was not only independently related to their impaired renal function, but also to an increased fluid status. Furthermore, anemia with excess OH was associated with more traditional and nontraditional cardiovascular risk factors and a higher risk of cardiovascular morbidity and mortality and CKD progression as compared with true anemia.
Anemia is common among patients with CKD and is known to impair their prognosis. In our study population of CKD stage 3 to 5, the prevalence of anemia was 68%, which is compatible with previously published data showing that 50% to 60% of patients with stage 4 CKD are anemic, and the prevalence of anemia increases to 75% to 92% in patients reaching stage 5 CKD.14–15 Absolute or relative erythropoietin deficiency is involved in the pathophysiology of CKD‐associated anemia. Although treatment with ESAs markedly improved patient‐perceived quality of life and reduced the need for blood transfusions,16 the results from the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR), Cardiovascular risk Reduction by Early Anemia Treatment with Epoetin β (CREATE), and TREAT trials all demonstrated an increased risk of CKD progression or cardiovascular events such as stroke, thrombosis, or death at nearly normal hemoglobin concentrations and higher ESA doses in patients with ND‐CKD.2–4 However, the many confounding factors that accompany CKD may have prevented clinicians from discerning the specific role anemia plays in the poor outcomes.
Fluid retention is a major component of the clinical syndrome of moderate‐to‐advanced CKD. Patients with CKD have similarities to those with heart failure in that both populations frequently retain fluid and have excessively high cardiovascular mortality. Previous data in patients with advanced heart failure have shown that approximately half of the patients with anemia have hemodilution rather than a true decrease in red blood cell mass.6 In a randomized, double‐blind trial, Swedberg et al evaluated the effects of darbepoetin alfa on clinical outcomes in patients with systolic heart failure and anemia.17 Although darbepoetin alfa treatment led to an early and sustained increase in the hemoglobin concentration, the use of darbepoetin alfa did not reduce the risk of the primary outcome of death or hospitalization for worsening heart failure compared with placebo. Moreover, more patients had thromboembolic events in the treatment group than in the placebo group, an observation similar to the findings of the TREAT study. Therefore, the hemoglobin concentration may simply be a surrogate marker for poor prognosis, which indicates ESA resistance caused by inflammation, impaired iron utilization, or fluid retention in patients with CKD or congestive heart failure, rather than a therapeutic target.
Hemodilution was first described in pregnant women and is believed to be an adaptive mechanism. During pregnancy, the maternal plasma volume expands 45% on average to meet the greater needs of the placental circulation. Therefore, hemoglobin concentrations are diluted and the threshold for a diagnosis of anemia, which is defined as hemoglobin <12.0 g/dL in nonpregnant women, is modified to <11.0 g/dL.18 Currently, there is no supporting information on an established hemoglobin concentration below which the risk of maternal mortality increases.19 We hypothesize that anemia in CKD is, at least in part, also an adaptive response to the underlying state of fluid retention, cardiac dysfunction, and arteriosclerosis. Moderate anemia results in reduced blood viscosity and blood volume, which decreases left ventricular afterload and may improve microvascular perfusion in CKD patients.20 From this viewpoint, it seems reasonable that the recently published Kidney Disease Improving Global Outcomes (KDIGO) guideline further reduced the hemoglobin threshold for the initiation of ESA therapy to <10.0 g/dL in ND‐CKD patients.21 Therefore, before ESA therapy to override the anemia of CKD is considered, the potential benefits of reducing blood transfusions and anemia‐related symptoms must be weighed carefully against the potential risks of harm. Otherwise, the sustained dose‐dependent rise in hemoglobin and blood volume would predispose CKD patients to a further increase in vascular resistance and blood pressure, which may aggravate cardiovascular damage and CKD progression.22
The optimal treatment of CKD‐associated anemia must target the underlying mechanisms. In CKD patients, a progressive decline in GFR, activation of the renin–angiotensin–aldosterone system, and superimposed cardiovascular comorbidities contribute to salt and water retention. Using relative OH ≥7% as the threshold of volume overload, we found a prevalence of hemodilution of 63% in the anemic ND‐CKD population. Volume overload, as assessed by bioimpedance spectroscopy devices, has been recognized as an important contributor to the poor cardiovascular or renal outcomes in hemodialysis and ND‐CKD patients.23–24 In addition to the deleterious effects of high blood pressure, circumferential stretch from fluid retention activates endothelial cells, resulting in an increase in proinflammatory cytokines.25–26 In the present study, anemic patients with excess OH had significantly higher levels of interleukin‐6 compared with those with true anemia. Accumulating evidence has shown that inflammation contributes to both the development of CVD and the progression of CKD. The interaction among hemodilution, CVD, and CKD progression is complex. In our study, patients with hemodilution had a higher proportion of comorbid conditions including diabetes mellitus and CVD, which might confound the association between hemodilution and clinical outcomes. After adjustment for potential confounders, hemodilution remained an independent predictor of the adverse outcomes. Our findings have important clinical implications, suggesting that a concomitant meticulous correction of volume overload can be considered to be incorporated into the treatment for CKD anemia. However, our observational study was not able to establish causality, and we caution against translating the results of our study into therapeutic practice. Future therapeutic trials of CKD anemia should attempt to characterize the patients' fluid status.
Study Limitations
The present study has a number of limitations. First, as is the case for any observational study, there remain unknown and unmeasured confounding factors. Second, OH as detected by the BCM comprises an increase of ECW volume in both intra‐ and extravascular spaces. While it is the increase in the intravascular compartment, also known as the plasma volume, that results in hemodilution, the BCM is unable to distinguish between the 2 compartments. However, previous studies have indicated that in situations of volume overload, excess water was retained within both intra‐ and extravascular components, increasing in approximately the same proportion.27 In our study, we excluded patients with frank nephrotic syndrome or chronic inflammatory diseases, which are often associated with vascular leak and disproportionate distribution of the retained fluid. As a result, a relative OH of 15.6% (Table 1) would amount to an increase in plasma volume of 0.5 L, which might explain the difference in hemoglobin between the true anemia group and the anemia with excess OH group. Third, we did not measure red blood cell mass, the “gold standard” for differentiating between true anemia and hemodilution, because red blood cell mass is essentially decreased in CKD patients due to depressed red blood cell production. Accordingly, true anemia may coexist with hemodilution in CKD patients. Identification of CKD patients with hemodilution is clinically important because it is very likely that the treatment strategy for anemia should be different in these patients. Finally, the overall CVD event rate was low, as reflected by a relatively wide CI in the cardiovascular end point. Actually, CKD patients in Taiwan, in contrast to those in Western countries, are more likely to develop end‐stage renal disease than to suffer a CVD event.28 The limited number of CVD events limits the ability to adjust for other potentially important covariates of interest.
Conclusions
Fluid retention appears to be an important factor for the development of low hemoglobin concentrations in patients with CKD. Patients with anemia with excess OH tend to have worse outcomes than patients with true anemia, suggesting that volume overload may serve as an important mechanism contributing to the adverse outcomes in anemic CKD patients. Hence, further research is warranted to clarify whether, instead of increasing erythropoiesis, the correction of volume overload should be the main target to achieve better outcomes in CKD‐associated anemia.
Sources of Funding
This work was supported in part by grants from the National Science Council (NSC 99‐2314‐B‐010‐004‐MY3; NSC 102‐2314‐B‐010‐004‐MY3; NSC 102‐2314‐B‐303‐003; MOST 103‐2314‐B‐303‐005‐MY2), Taipei Tzu Chi Hospital (TCRD‐TPE‐NSC‐102‐08; TCRD‐TPE‐103‐RT‐4), Taipei Veterans General Hospital (V102C‐143; V103C‐001), and from Ministry of Education, Aim for the Top University Plan in Taiwan.
Disclosures
None.
References
- 1.Kovesdy CP, Trivedi BK, Kalantar‐Zadeh K, Anderson JE. Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney Int. 2006; 69:560-564. [DOI] [PubMed] [Google Scholar]
- 2.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan DCHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006; 355:2085-2098. [DOI] [PubMed] [Google Scholar]
- 3.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag ACREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006; 355:2071-2084. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto RTREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009; 361:2019-2032. [DOI] [PubMed] [Google Scholar]
- 5.Hung SC, Lin YP, Tarng DC. Erythropoiesis‐stimulating agents in chronic kidney disease: what have we learned in 25 years? J Formos Med Assoc. 2014; 113:3-10. [DOI] [PubMed] [Google Scholar]
- 6.Androne AS, Katz SD, Lund L, LaManca J, Hudaihed A, Hryniewicz K, Mancini DM. Hemodilution is common in patients with advanced heart failure. Circulation. 2003; 107:226-229. [DOI] [PubMed] [Google Scholar]
- 7.Westenbrink BD, Visser FW, Voors AA, Smilde TD, Lipsic E, Navis G, Hillege HL, van Gilst WH, van Veldhuisen DJ. Anaemia in chronic heart failure is not only related to impaired renal perfusion and blunted erythropoietin production, but to fluid retention as well. Eur Heart J. 2007; 28:166-171. [DOI] [PubMed] [Google Scholar]
- 8.Adlbrecht C, Kommata S, Hülsmann M, Szekeres T, Bieglmayer C, Strunk G, Karanikas G, Berger R, Mörtl D, Kletter K, Maurer G, Lang IM, Pacher R. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body's red cell volume. Eur Heart J. 2008; 29:2343-2350. [DOI] [PubMed] [Google Scholar]
- 9.Hung SC, Kuo KL, Peng CH, Wu CH, Lien YC, Wang YC, Tarng DC. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014; 85:703-709. [DOI] [PubMed] [Google Scholar]
- 10.Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, de Zeeuw D, Ivanovich P, Levey AS, Parfrey P, Remuzzi G, Singh AK, Toto R, Huang F, Rossert J, McMurray JJ, Pfeffer MATrial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Investigators. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010; 363:1146-1155. [DOI] [PubMed] [Google Scholar]
- 11.Chen YR, Yang Y, Wang SC, Chiu PF, Chou WY, Lin CY, Chang JM, Chen TW, Ferng SH, Lin CL. Effectiveness of multidisciplinary care for chronic kidney disease in Taiwan: a 3‐year prospective cohort study. Nephrol Dial Transplant. 2013; 28:671-682. [DOI] [PubMed] [Google Scholar]
- 12.Chamney PW, Wabel P, Moissl UM, Müller MJ, Bosy‐Westphal A, Korth O, Fuller NJ. A whole‐body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr. 2007; 85:80-89. [DOI] [PubMed] [Google Scholar]
- 13.Wieskotten S, Heinke S, Wabel P, Moissl U, Becker J, Pirlich M, Keymling M, Isermann R. Bioimpedance‐based identification of malnutrition using fuzzy logic. Physiol Meas. 2008; 29:639-654. [DOI] [PubMed] [Google Scholar]
- 14.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF. All‐cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462293 adults in Taiwan. Lancet. 2008; 371:2173-2182. [DOI] [PubMed] [Google Scholar]
- 15.Obrador GT, Roberts T, St. Peter WL, Frazier E, Pereira BJ, Collins AJ. Trends in anemia at initiation of dialysis in the United States. Kidney Int. 2001; 60:1875-1884. [DOI] [PubMed] [Google Scholar]
- 16.Eschbach JW, Kelly MR, Haley NR, Abels RI, Adamson JW. Treatment of the anemia of progressive renal failure with recombinant human erythropoietin. N Engl J Med. 1989; 321:158-163. [DOI] [PubMed] [Google Scholar]
- 17.Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJ, O'Connor C, Pfeffer MA, Solomon SD, Sun Y, Tendera M, van Veldhuisen DJRED‐HF Committees; RED‐HF Investigators. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013; 368:1210-1219. [DOI] [PubMed] [Google Scholar]
- 18.Faupel‐Badger JM, Hsieh CC, Troisi R, Lagiou P, Potischman N. Plasma volume expansion in pregnancy: implications for biomarkers in population studies. Cancer Epidemiol Biomarkers Prev. 2007; 16:1720-1723. [DOI] [PubMed] [Google Scholar]
- 19.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000; 71suppl:1280S-1284S. [DOI] [PubMed] [Google Scholar]
- 20.Zarychanski R, Houston DS. Anemia of chronic disease: a harmful disorder or an adaptive, beneficial response? CMAJ. 2008; 179:333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.KDIGO Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. 2012; 2suppl:1-335. [Google Scholar]
- 22.Raine AE. Hypertension, blood viscosity, and cardiovascular morbidity in renal failure: implications of erythropoietin therapy. Lancet. 1988; 1:97-100. [DOI] [PubMed] [Google Scholar]
- 23.Hung SC, Lin YP, Huang HL, Pu HF, Tarng DC. Aldosterone and mortality in hemodialysis patients: role of volume overload. PLoS One. 2013; 8:e57511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai YC, Tsai JC, Chen SC, Chiu YW, Hwang SJ, Hung CC, Chen TH, Kuo MC, Chen HC. Association of fluid overload with kidney disease progression in advanced CKD: a prospective cohort study. Am J Kidney Dis. 2014; 63:68-75. [DOI] [PubMed] [Google Scholar]
- 25.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008; 52:1527-1539. [DOI] [PubMed] [Google Scholar]
- 26.Gnanaraj JF, von Haehling S, Anker SD, Raj DS, Radhakrishnan J. The relevance of congestion in the cardio‐renal syndrome. Kidney Int. 2013; 83:384-391. [DOI] [PubMed] [Google Scholar]
- 27.Kalra PR, Anagnostopoulos C, Bolger AP, Coats AJ, Anker SD. The regulation and measurement of plasma volume in heart failure. J Am Coll Cardiol. 2002; 39:1901-1908. [DOI] [PubMed] [Google Scholar]
- 28.Chiu YL, Chien KL, Lin SL, Chen YM, Tsai TJ, Wu KD. Outcomes of stage 3–5 chronic kidney disease before end‐stage renal disease at a single center in Taiwan. Nephron Clin Pract. 2008; 109:c109-c118. [DOI] [PubMed] [Google Scholar]


