Abstract
Background
There are few studies of atrial fibrillation (AF) outside of North America or Europe. The aim of the present study was to assess the prevalence, incidence, management and outcomes of patients with new atrial fibrillation, in a large contemporary cohort (2004–2012) of adult patients.
Methods and Results
The Clalit Health Services (CHS) computerized database of 2 420 000 adults, includes data of community clinic visits, hospital discharge records, medical diagnoses, medications, medical interventions, and laboratory test results. The prevalence of AF on January 1, 2004 was 71 644 (3%). Prevalence and incidence of AF increased with age and was higher in men versus women. During the study period (2004–2012) 98 811 patients developed new non‐valvular AF (mean age −72, 50% women, 46% with cardiovascular disease, 6% with prior stroke). The rate of persistent warfarin use (dispensed for >3 months in a calendar year) was low (25.7%) and it increased with increasing stroke risk score. Individual Time in Therapeutic Range (TTR) among warfarin users was 42%. The incidence rate of ischemic stroke and death increased with age. The rate of stroke increased from 2 per 1000 person years in patients with CHA2DS2_VASC SCORE of 0, to 58 per 1000 person years in those with a score of 9.
Conclusions
In the present study the prevalence and incidence of AF, stroke, and death were comparable to those reported in Europe and North America. The low use of anticoagulation calls for measures to increase adherence to current treatment recommendations in order to improve outcomes.
Keywords: anticoagulants, atrial fibrillation, epidemiology, stroke
Introduction
Atrial fibrillation (AF) is the most common arrhythmia worldwide with a reported prevalence of 0.4% to 2%1 and a significant anticipated increase over the next several decades.2–5 The incidence and prevalence of AF are higher in men,2–3,6 and increase with age.3,6
AF is associated with an increased risk of stroke7–8 death,7,9–11 heart failure,10 a reduced quality of life and with significant healthcare expenditures12 incurred by treating this condition and its associated complications.
Most of the epidemiological data of the prevalence, incidence and consequences of AF are derived from North America and Europe.6–7,6–18 The rate of AF is different among different ethnic populations such as Indo‐Asians19 and African Americans3,20–21 and varies considerably in different geographical areas of the globe2,22–23 with wide variation not only in the prevalence of AF2,22–23 but also in management and outcomes.2,22–23 Of note, studies that used mostly hospitalization‐based diagnoses of AF showed higher rates of AF22–23 and stroke22 as compared with community studies. Studies that include patients diagnosed with atrial fibrillation at the hospital setting as well as those diagnosed in the community may provide more comprehensive and complete data of the distribution, management, and outcomes of this condition.
There are few data derived from large community studies from non‐North American or European countries on the epidemiology, management, and outcome of patients with AF.
The aim of the present study was to define the prevalence, incidence, management, and outcome of AF in a contemporary cohort of patients.
Methods
Study Database and Population
Clalit Health Services (CHS) is, the largest health care maintenance organization (HMO) in Israel, with about 4 217 000 insured citizens that provides care to >50% of the adult population over the age of 21 and to >60% of adults older than 65 years of age. The system is characterized by extremely low annual turnover of <1%.
A historical prospective cohort study was conducted using the Clalit Health Services (CHS) database. Since 1998, with increasing comprehensiveness, CHS's information is kept in a central computerized data warehouse that includes demographic data, clinical diagnoses (based on hospital discharge diagnoses, primary care physician diagnoses, and specialist outpatient clinic diagnoses), laboratory data results, medical treatment, and medications (including date of prescription and quantity and time of medication dispensed). For the purpose of this study, all of the CHS members on January 1, 2004, age 21 and older were included in the registry for prevalence rate assessment.
Eight years incidence rate was assessed for Clalit members starting at January 1, 2004. Outcomes were extracted for 8 years of study period, between January 1, 2004 and December 31, 2011 for new AF cases during this follow‐up period. Patients with diagnoses of severe mitral or aortic valve disease based on ICD9 codes or with any previous valve surgery or previous diagnosis of rheumatic heart disease prior to the diagnosis of AF were excluded from the study.
Variables Definitions
Atrial fibrillation was defined according to the International Classification of Diseases (ICD 9 diagnoses 427.31‐3). Any patient designated with one of those codes at least once on either hospital discharge, outpatient clinic visits, or during family physician clinic visit during the study period was considered a case of atrial fibrillation. Seventy‐four percent of our final study population was designated with the diagnosis of AF for 2 or more times. We chose to include any patient that was given the diagnosis to create an inclusive database, including early and milder cases of paroxysmal AF as well, and avoid underrepresentation of these earlier forms of AF, since recent data suggests that even asymptomatic AF has prognostic importance. For the purpose of the present analysis we did not differentiate cases of atrial flutter (427.32) from AF. Given the frequent overlap between flutter and AF and difficulties in ascertaining the accuracy of differential diagnostic coding and given similarities in treatment we elected not to distinguish fibrillation and flutter. Based on the database we could not distinguish reliably between cases of paroxysmal and non‐paroxysmal atrial fibrillation.
Prevalence and Incidence
Prevalence was calculated based on the number of patients with the diagnosis of AF (ICD 9 codes 427.31‐3) on January 1, 2004 divided by the total number of patients alive at that time (n=2 414 282). Incidence was calculated in the population, after excluding any patients with a preexisting diagnosis of AF, based on the number of new AF cases during the time period January 1, 2004 to December 31, 2011 divided by the number of persons at risk during that time and given as number per person year at risk. Only patients with at least 3 consecutive years of enrollment in Clalit health services prior to the index date of new AF diagnosis were included, to exclude misclassification of preexisting AF cases as new cases. To provide a detailed and comprehensive description of the epidemiology of AF in Israel analyses are presented by gender and age.
Concomitant Medical Conditions and Medical Treatment
Medical conditions preexisting the index date were recorded. Medical diagnoses given during hospitalizations, by primary care providers or by specialists, are coded based on ICD‐9 codes and an internal coding system is utilized to integrate, cross check, and validate both hospital and community diagnostic coding. Laboratory values are also available through this central database as are data relating to medical treatment. Since CHS pays for the medical treatment prescribed for its patients there are data as to start date, stop date, and duration of medical treatment. For the purpose of the present study, medication was considered as received regularly when a prescription medication was dispensed for >3 months on a calendar year during the study period. Similarly, Warfarin treatment was defined when a patient with AF received a prescription for Warfarin and the prescription was dispensed for >3 months in a calendar year after the index date of AF diagnosis. We used this restrictive definition since we have shown previously that this is more effective in detecting noncompliance than standard dispensary based counts.24 In addition we chose the >3 months use as cut‐off, since medications in CHS are usually dispensed for 3 months (a quarter) and dispensing for >3 months marks a second visit for collection of medications and hence a higher likelihood of persistent use of a given medication.24
Outcome Data
The primary outcomes that were systematically collected are all‐cause death, ischemic stroke, myocardial infarction (MI), and the combined end point of death, MI, and ischemic stroke. New incidence of MI was defined by an incident hospitalization that contained the relevant hospital discharge ICD‐9 code (codes 410.00 to 410.92). In the few cases of missing discharge diagnosis, values were imputed based on combinations of strong supportive information from multiple other sources in the data warehouse. (ie, acute primary angioplasty, post‐MI referral to cardiac rehabilitation). New incidence of ischemic stroke case was defined by the presence of an incident hospitalization that contained the relevant hospital discharge ICD‐9 code (codes 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91). In case of a missing discharge diagnosis for an index hospitalization, values were imputed based on documentations of ischemic stroke ICD‐9 code in the community clinic electronic health records or a new documentation of ischemic stroke diagnosis in a dedicated CHS chronic disease registry. All‐cause mortality data were obtained from the Ministry of Interior. Mean follow‐up time was 48.8 months.
Statistical Analyses
Incidence and prevalence of AF are reported per 100 000 person‐year at risk separately in men and women by age groups. Differences in crude and age‐adjusted prevalence and incidence rates between genders were assessed by the Mantel‐Haenszel chi‐square test. Differences across age groups were assessed by using a linear regression of log‐transformed values on time in each age group, and testing the interaction of age and gender.
Individual time in therapeutic range (TTR) was calculated among patients treated with Warfarin based on Rosendaal's linear model.25 Briefly, all of the INR test results, values, and dates, were extracted for each individual patient, creating an individual time series of INR level. Based on Rosendaal's method linear interpolation was used to determine the times at which the patient entered or exited the therapeutic range (ie, INR levels 2 to 3). The times of range entry were subtracted from the subsequent times of exit to establish the time within range for a single period. These periods were then summed to establish total time within range. Differences between normally distributed continuous or categorical variables were assessed by using the Student t test and the chi‐square tests, respectively.
In patients with incident AF, Kaplan‐Meier survival analysis was used to create survival curves and describe the risk of ischemic stroke or death over time, following the index date of AF diagnosis. All statistical analyses were performed using the SPSS statistical software version 20 (SPSS Inc, Chicago, IL) and in R version 2.14.2 (R Foundation for Statistical Computing).
The study was approved by the central ethics institutional review board of CHS.
Results
Prevalence and Incidence of AF
Prevalence of AF
There were 71 644 patients with AF out of 2 414 282 patients on January 1, 2004 giving an overall prevalence of 3.0% in the adult population older than 21 years.
Age and gender specific prevalence (per 100 000 persons) are shown in Figure 1. The crude prevalence rates of AF were 2.91% in men and 3.01% in women. Age adjusted rates were 3.19% (95% CI; 3.16 to 3.23) in men, and 2.79% (95% CI; 2.77 to 2.83) in women (P<0.001). The prevalence of atrial fibrillation was higher in men as compared with women at any age group (Figure 1), and it increased in both men and women with increasing age. In men it increased from 170.0 per 100 000 patients at risk (0.2%) in those <35 years of age, to 15 184 per 100 000 patients at risk (15.2%) in those >85 years of age. The corresponding prevalence rates in women were 150 (0.15%) per 100 000 in women <35 years of age and 12 577 (12.6%) per 100 000 in women >85 years of age (Figure 1). The increase in prevalence across age groups was highly significant in men and women (P<0.001).
Figure 1.
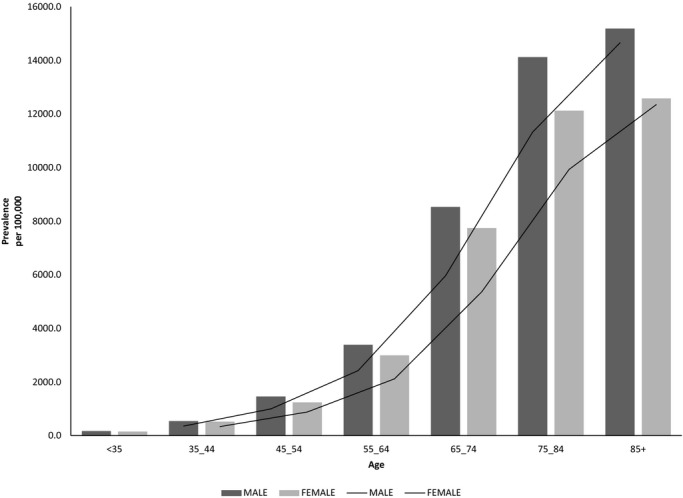
Prevalence of atrial fibrillation in January 1, 2004, according to age and gender.
Baseline Characteristics of Incident AF Cases
After excluding the prevalent AF cases among the remaining AF‐free adult population (n=2 342 638) we were able to identify between January 1, 2004 to December 31, 2011, 116 637 patients with incident new AF who met the inclusion criteria. After excluding patients with significant valvular disease and previous valve surgery (n=17 826) we remained with a cohort of 98 811 patients with incident new non‐valvular AF (Figure 2).
Figure 2.
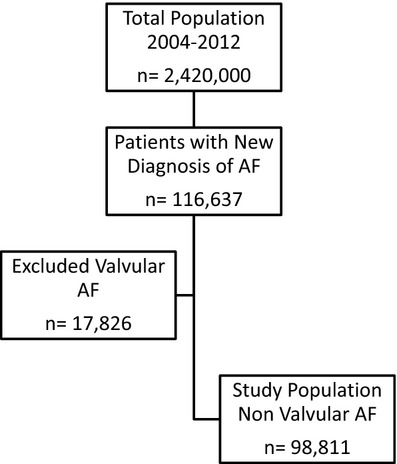
Study population of incident atrial fibrillation cases between 2004 and 2012.
Age‐ and gender‐specific incidence rates are given in Figure 3. The incidence (per 10 000 person year at risk) of AF was 635 in men as compared with 575 in women. Corresponding age‐adjusted incidence rates were 699 (95% CI, 693 to 704) in men as compared with 529 (95% CI, 524 to 533) in women P<0.0001. The incidence was significantly higher among men at any age group and it increased from 47.7 at age <35 to 4427.6 in men >85 years of age and from 36.7 at age <35 to 3790 in women >85 years of age (Figure 3). The increase in incidence across age groups was highly significant in men and women (P<0.001).
Figure 3.
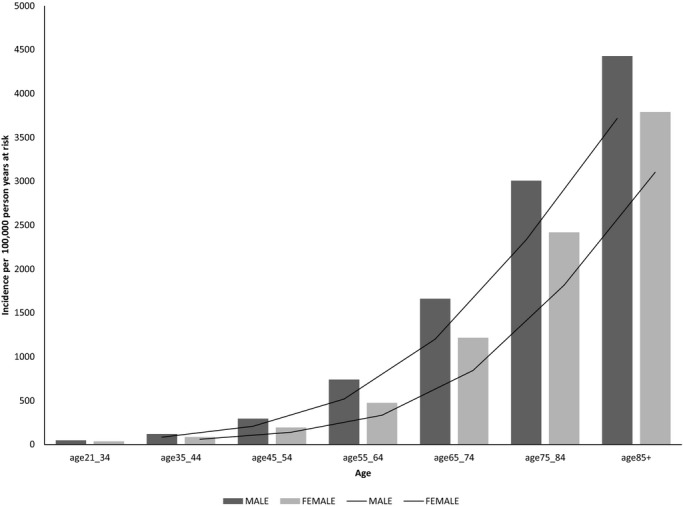
Incidence of atrial fibrillation 2004–2012 according to age and gender.
There were 49 275 (49.8%) women and 49 536 (51.2%) men. The mean age was 72 years (70 in men and 74 in women). Other clinical and baseline characteristics are given in Table 1. The most common comorbid conditions were hypertension, diabetes mellitus, smoking, and previous coronary heart disease. There were 6080 patients (6.2%) with previous ischemic stroke. As compared with the total CHS adult population patients with AF were significantly older, and had a significantly higher rate of other comorbidities (Table 1).
Table 1.
Baseline Clinical Characteristics of Patients With Incident Non Valvular Atrial Fibrillation From 2004–2012 and of the Clalit Health Services Adult (>21 Years of Age) Population
| Incident AF (n=98 811) | General Population (n=2 641 200) | |
|---|---|---|
| Age, mean (SD) | 72 (14) | 44 (16) |
| Female, n (%) | 49 275 (50%) | 1 379 342 (52%) |
| Medical conditions, n (%) | ||
| Hypertension | 79 755 (81%) | 539 714 (20%)* |
| Diabetes mellitus | 33 175 (34%) | 341 066 (13%)* |
| Myocardial infarction | 8655 (9%) | 45 476 (2%)* |
| Ischemic heart disease | 40 984 (42%) | 218 569 (8%)* |
| Ischemic stroke | 6080 (6%) | 41 732 (2%)* |
| Peripheral arterial disease | 10 873 (11.0%) | 65 237 (2%)* |
| Previous CABG | 3661 (4%) | 26 016 (1%)* |
| Cardio vascular disease | 45 793 (46%) | 256 100 (10%)* |
| Heart failure | 21 202 (22%) | 77 368 (3%)* |
| Smoker | 37 901 (38%) | 532 418 (20%)* |
Cardiovascular disease‐ includes ischemic heart disease, previous stroke or TIA, and peripheral arterial disease. AF indicates atrial fibrillation; CABG‐coronary artery bypass graft surgery; TIA, transient ischemic attack.
P<0.01.
Medical Treatment Among Patients With Incident AF
Table 2 provides details as to other concomitant cardiac medications given to the patients with AF. The most commonly used medications were beta‐blockers, angiotensin‐converting enzyme inhibitors, and calcium‐channel blockers. Antiarrhythmic use was relatively uncommon as compared with other medications and the most common antiarrhythmic was Amiodarone. Digoxin was used in 6.2% of the patients. Among the general CHS adult population use of medications was significantly lower as compared with the AF population (Table 2).
Table 2.
Concomitant Medical Treatment in Patients With Incident AF and in the Clalit Health Services Adult (>21 Years of Age) Population
| Incident AF (n=98 811) | General Population (n=2 641 200) | |
|---|---|---|
| Amiodarone | 13 000 (13%) | 21 445 (1%)* |
| Propafenone | 6797 (7%) | 13 172 (0%)* |
| Flecainide | 1750 (2%) | 3236 (0%)* |
| Sotalol | 1089 (1%) | 2857 (0%) |
| ACE inhibitors | 61 917 (63%) | 462 248 (18%)* |
| ARBs | 15 873 (16%) | 102 337 (4%)* |
| Dihydropiridines CCB | 43 947 (45%) | 280 358 (11%)* |
| Non dihydropiridines CCB | 9737 (10%) | 31 633 (1%)* |
| Beta‐blockers | 63 329 (64%) | 393 466 (15%)* |
| Digoxin | 6245 (6%) | 10 895 (0%)* |
ACE indicates angiotensin converting enzyme, AF, atrial fibrillation; ARB, angiotensin II receptor blocker; CCB, calcium channel blockers.
P<0.01.
Distribution of Risk and Warfarin Treatment
Warfarin was used within 90 days of the index diagnosis in 22.8% of our study population and the rate increased to 26.3% within the first year after the index diagnosis. Persistent use of >3 months of treatment was observed in 25.7% of the patients with incident AF. Persistent use of Warfarin was 29.7% in 2004 to 26.4% in 2011 (P<0.01).
The breakdown of patients according to their CHADS2 and CHA2DS2_VASC scores and rate of Warfarin use in each category are shown in Table 3.
Table 3.
Distribution of CHADS2 and CHA2DS2_VASC Scores Risk Categories and Rate of Persistent Treatment With Warfarin
| Low Risk (Score 0) | Intermediate Risk (Score 1) | High Risk (Score ≥2) | |
|---|---|---|---|
| CHADS2 | 12 296 (12.4%) | 22 806 (23.1%) | 63 709 (64.5%) |
| Warfarin treatment, n (%) | 1659 (13.5%) | 6107 (26.8%) | 17 662 (27.7%) |
| CHA2DS2_VASC | 4300 (4.4%) | 8868 (9.0%) | 85 643 (86.7%) |
| Warfarin treatment, n (%) | 452 (10.5%) | 1459 (16.5%) | 23 517 (27.5%) |
Warfarin was regularly used during the study period in only 25 428 patients (25.7%). The rates of Warfarin use increased with increasing CHADS2 and CHA2DS2_VASC risk score categories (Table 3).
The time in therapeutic range‐ TTR (INR between 2 to 3) among those that were treated with warfarin in our cohort was 42% of the time. Among those treated with warfarin, only 40.8% of the patients had a TTR >50% of the time (Figure 4), and 43% of time was spent with an INR <2 and 16% of time with an INR value >3.
Figure 4.
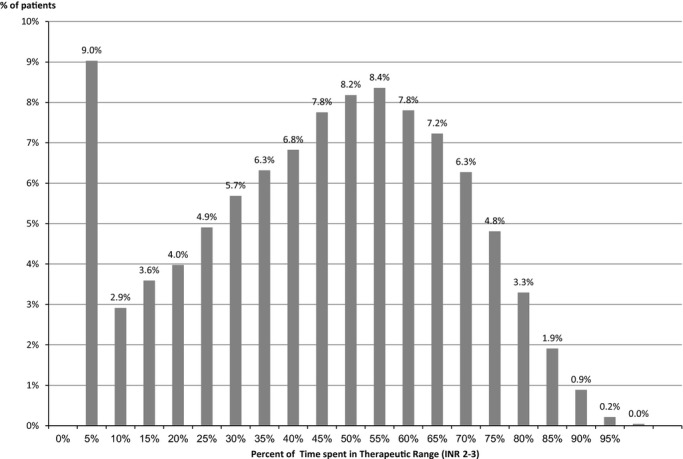
Distribution of time in therapeutic range among incident atrial fibrillation patients treated with warfarin.
On multivariate analysis, we found that hypertension, diabetes, age >65, and increased CHADS2 and CHA2DS2_VASC risk scores were independent predictors for use of warfarin.
Outcome
Over an average follow‐up time of 48.8 months the incidence rate (per 1000 person‐year at risk) of ischemic stroke increased from a rate of 3.88 among those aged <55 at diagnosis to 16.4 in those aged 75 to 84 (Figure 5). Likewise the rate of death was directly related to age (Figure 6). Similar trends were seen in men and women. The rate of stroke increased from 2 (per 1000 person‐year at risk) in patients with CHA2DS2_VASC SCORE of 0, to 58 in those with a score of 9 and from 3.5 per 1000 person‐year in patients with a CHADS2 score of 0, to 55 in those with a score of 6. Figure 7 shows the risk of death (Figure 7A) and stroke (Figure 7B) over time in the AF incident cases.
Figure 5.
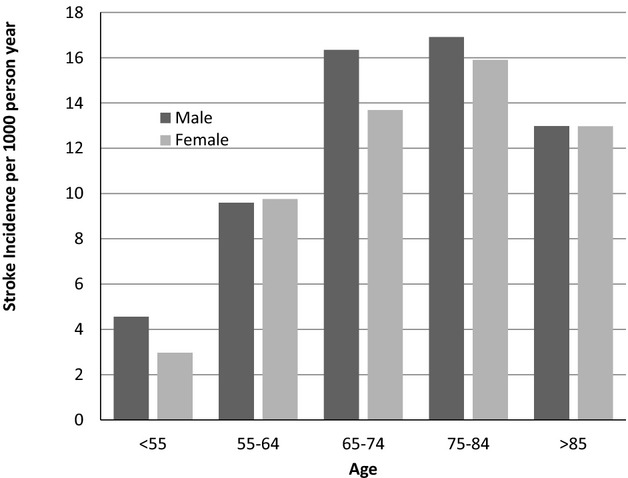
Incidence of stroke among incident atrial fibrillation patients according to age and gender.
Figure 6.
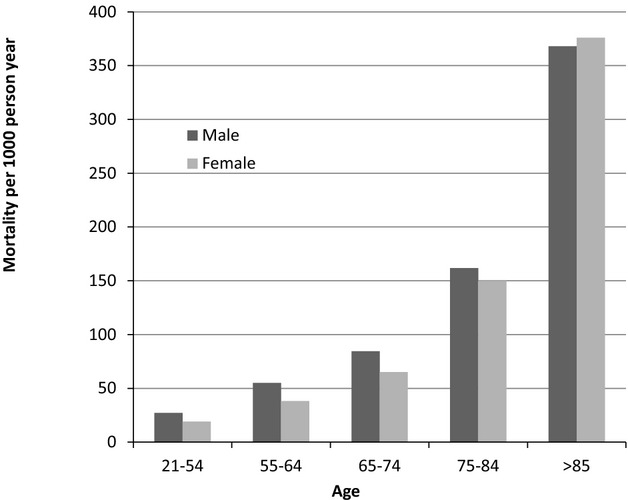
Death rate among incident atrial fibrillation patients according to age and gender.
Figure 7.
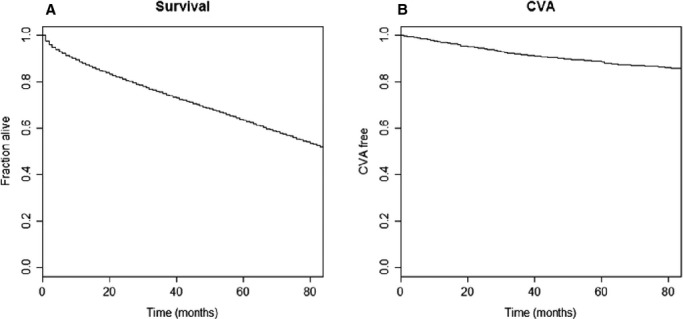
Survival curves describing the risk of death (A) and ischemic stroke (CVA‐cerebrovascular accident) (B) over time in patients with incident atrial fibrillation (AF).
Discussion
The present study provides data of the incidence, prevalence, management, and outcome of a large contemporary cohort of patients with incident atrial fibrillation from outside North America or Europe. Our data are unique in the sense that they provide robust contemporary epidemiological data of a very large population from the community as well as patients that were diagnosed during hospitalization. There is data captured from diagnoses collected in the community and integration with hospital discharge diagnoses. Thus, the data are comprehensive and cover the entire spectrum of possible sources of information. The present analysis provides data not only of the extent of atrial fibrillation, but also data about antithrombotic management and its quality (ie, TTR) as well as outcome data in terms of stroke and death.
Prevalence
The overall prevalence of AF was close to 3%, very similar to that reported recently in Sweden (3.2%)26 in a study that was similar to the present study. It was conducted over a similar time period (2009, 2010), and in a population similar to ours in respect to age (persons older than 20 years of age) and it included hospital as well as community diagnoses. Similar prevalence rates were reported in recent contemporary community studies from the USA3,5,21 and Europe.18,27 ranging from 1% in studies that included patients from the community and that included people as young as 20 years of age3,5 and up to 5.5% in studies that included hospital‐based diagnoses and older patients.18,21,27 In the present study, the prevalence of AF increased with age to as high as 15% in men and 12% in women older than 85. The prevalence rates of AF in most studies worldwide were positively associated with age and were higher in men as compared with women across age groups2,5,18,21–22,21–28. There are few large‐scale community studies outside the US and Europe that report the prevalence of AF and these differ significantly in reported rates.2,22–23 Differences in prevalence rates across studies were related to geographical region and demographic origin but also to populations studied (ie, stroke survivors, patients in cardiology wards or general in‐patients wards, or from the community). Reported prevalence rates are lower in community studies ranging between 0.1% to 4%22 as compared with hospitalization records studies ranging from 2.8% to 14%,22–23and up to 28% to 50% among hospitalized stroke survivors.23 The reported prevalence rates in the present study, which integrates community and hospital sources, are in the higher range of previously reported rates from the community globally and should assist policymakers in prioritizing resources towards management of AF as a significant health problem.
Incidence
The incidence of AF was directly related to age, it was 0.5 and 0.4/1000 person‐years in men and women aged <35 and increasing to 44 and 38/1000 person‐year in men and women >85 years of age. The incidence of AF was higher in men across all age groups. The reported incidence rates in the present study are similar to those reported recently in contemporary community studies from the United States ranging from as low as 0.7 (per 1000 person‐year) in younger patients to 47 among the elderly20 and it increased from 13 among those 65 to 69 years of age (per 1000 person‐year) to 69 among those >90 in a study that included Medicare beneficiaries >65.21 Similar rates were reported in Olmsted County, Minnesota,4,20–21 the Rotterdam study,18 and Germany.27 Studies of the incidence of AF outside the United States and Europe are limited and the reported incidence varied considerably.2,22 Our study is unique in the size and stability of the general population studied, the comprehensiveness of our data sources, and the ability to integrate the incidence data with treatment practices. The reported incidence rates of AF and the association with age and male gender are in agreement with the findings of a recent a global survey of AF.2
Comorbid Conditions
The most common comorbidities associated with AF in our study cohort were hypertension, diabetes mellitus, coronary heart disease, and smoking. These associated conditions were also reported in other series of patients with AF3–5,27,29and probably explain the similar prevalence and incidence rates of AF reported in our study cohort as compared with other studies. These associated conditions emphasize the complexity of care of patients with atrial fibrillation and the need to treat other comorbid conditions. In particular, hypertension was present in 80% of our study cohort, reflecting its importance not only as a comorbidity that increases the risk of adverse outcomes but also as an important underlying cause of AF.
Patient's Risk of Thromboembolism
In the present study 64.5% of the patients were in CHADS2 score ≥2 (high‐risk group) and 86.7% were categorized as high risk according to CHA2DS2_VASC risk score. Similar rates of high‐risk category according to CHADS2 were recently reported in patients with permanent AF by Chiang et al in a recent international survey,29 in the Global Anticoagulant Registry in the FIELD (GARFIELD) study,30 in participants of the SPORTIF III and V trials (67% and 94.2%),31 in the BAFTA trial of elderly patients with AF (69% and 100%),32 and in a recent European survey.33 Slightly lower rates of patients with CHADS2 and CHA2DS2_VASC scores ≥2 (47% and 79.7%) were reported by Olesen et al in a Danish registry based on hospital discharge diagnoses34 and in a recent study from Alberta, Canada (50% and 78.4%).35 Differences in study populations as well as different definitions of comorbidities and different data sources (ie, community diagnoses versus hospital‐based diagnoses) could account for these small differences.
The rate of chronic use of oral anticoagulants (OAC), primarily warfarin, was low in the present study with an overall rate of persistent use of 25.7% in our entire group and a higher rate of OAC use (27.7%) in higher‐risk patients as compared with low‐risk patients (10.5%) (Table 3). Patients with higher‐risk scores were more likely to be treated with warfarin and accordingly age >65, hypertension and diabetes were independently associated with use of OAC.
There is inconsistency in reported use of OAC among AF patients from different parts of the globe ranging between as low as 2% to 3% and high as 88%.22–23,36 Some of the differences are related to geographic region, some to the baseline risk of patients. Studies that were hospital based included mainly high‐risk patients. It seems that the use of OAC is higher in Western Europe and North America as opposed to other regions. The overall reported rate of OAC use in AF patients ranged from 70% to 80% and it was around 90% in patients at highest risk of stroke in studies from Europe.33,36–37 In contrast, other studies reported lower rates of OAC use. In a large cohort of AF patients from Sweden the rate of warfarin use was significantly lower at 40%,38 which was somewhat higher than the rate in the present study. The definition of OAC use in that study relied on collection at pharmacy within 3 months before or after the index diagnosis without taking into account subsequent use or discontinuation. We used a restrictive definition of regular OAC users, that required at least 4 dispenses of prescribed OAC in the pharmacy during a calendar year to be counted as an OAC user, a methodology that we have shown is more effective in detecting noncompliance than standard dispensary based counts.24 This difference in definition of “chronic OAC use” could account for the difference between these studies and ours. Similarly the rate of OAC use was 50% to 54% among different risk categories in a recent study from Alberta, Canada significantly lower than that reported in Europe.39 In contrast to our analysis that study provided information of warfarin use only in patients older than 65 years of age and defined users as those that were prescribed (but not necessarily filled or collected the prescription) OAC during the 90 day period after the index diagnosis date. Similar rates of OAC use were reported in the GARFIELD study30 and in a study from Scotland that used prescribing information and showed a warfarin rate of use of 42%.28 A recent study from the United States showed that the rate of persistent use of warfarin among AF patients ranged from 24% to 39% a rate that is similar to that reported in the present analysis—and the median length of stay on warfarin ranged from 135 to 222 days depending on the definitions used.40 In a recent global registry of patients with AF the rate of warfarin use was 30% overall, with a wide range of use in different parts of the globe with lowest rates in China and India and highest in North America and Western Europe.41 Despite that, the rate of regular OAC use in our cohort was still surprisingly low – a finding that may be related to our utilizing hospital‐based, specialist‐based, and family physician‐based diagnosis. It is possible that since our study included new incident AF cases, some of them, especially those with relatively uncommon paroxysms of AF, may have been perceived as “milder” forms of AF not necessitating chronic OAC use. Such a false notion may lead to under treatment with OACs. Major efforts are needed to study the causes for lack of OAC prescription and use, and to encourage both physicians and patients to use these highly effective medications to decrease and prevent adverse outcomes such as stroke and death. It is possible that with new oral anti‐coagulants the rate of permanent use will increase. But this requires confirmation with further studies.
Many epidemiological and population studies did not have access to INR test results and did not provide these data. Our study enabled us to evaluate all the INR test results the patient had during the study period. The quality of OAC treatment as assessed by the individual TTR in the present study was modest; only 42% among those that were treated with OAC had effective treatment. This rate is in the mid‐range of TTR reported in other randomized and observational studies. The TTR was 48.6% in a recent global registry ranging from 33% to 62% in different parts of the globe.41 A recent study from the United States showed similar rates of TTR ranging from 48% in patients recently started on VKA antagonists, to 57% among those on VKA for >6 months.42 Only 40% of our patients had a TTR >50% of the time. These findings call for measures to improve quality of OAC treatment either by more accessible and convenient INR testing using point‐of‐care assays with warfarin dose adjustment at home or the institutionalization of specialized dedicated OAC clinics. It is possible that by switching to medications with a more predictable effect such as the new direct anti‐thrombins and anti‐Xa inhibitors, this problem will be neutralized. Such interventions could improve TTR and potentially reduce the rate of stroke and adverse outcome of patients with AF.
Outcome
The rates of stroke and of death were directly related to age. Similar mortality rates and association with age were recently reported by Andersson et al.11 In that study as well as in the Framingham Heart Study, the Women's Health Study, and in several other studies, AF was associated with increased mortality risk as compared with counterparts without AF, even after accounting for other comorbidities.9,17,43
The incidence rate of ischemic stroke was related to age in both men and women, increasing until the age of 84 and decreasing slightly thereafter in both genders (Figure 5). This decrease in patients >85 years of age, was probably related to competing mortality risks, which showed a sharp increase in incidence rate in both men and women with AF >85 years of age. The rates of ischemic stroke in the present study are comparable to those reported in the Loire Valley Atrial Fibrillation Project44 in Denmark,45 in the United States,46 and in Canada47 but are somewhat lower than those reported in other studies mostly from Europe.38,48 Several factors may explain our lower reported rates. First, we excluded the diagnosis of transient ischemic attacks (TIA) and included only patients diagnosed with ischemic strokes. Secondly our analysis included patients who were on OAC as well those not on OAC. In addition, our rates of ischemic strokes in this contemporary cohort could be lower than those reported previously as a result of decline in the incidence of stroke overtime as repeatedly reported possibly due to improved anticoagulation and control of other risk factors such as hypertension.45,49–50 An alternative explanation may be the greater comprehensiveness of our database. We, by design included patients diagnosed with AF from the community – some of them have never been hospitalized and probably represent lower risk patients. This case definition will include less morbid patients and possibly less adverse outcomes such as strokes. We cannot entirely exclude underreporting or under documentation of ischemic strokes despite regular inspections and validation of our database by using algorithms designed to minimize such occurrences.
Limitations
Several limitations are worth discussing. First, any large‐scale epidemiological database that relies on diagnoses is prone to errors made by either community or hospital physicians. Although an independent adjudication of morbid outcomes was not done in our particular study population, such errors are kept to a minimum in our integrated database by periodical assessment and corroboration of diagnoses and of patients' charts to assess reliability and routine algorithm‐driven comparisons and cross checks of hospital and community coding.
Reliable differentiation between paroxysmal, persistent, and permanent AF could not be performed and we did not distinguish between atrial flutter and atrial fibrillation. In addition, we did not report data on aspirin use since many patients use aspirin as an over‐the‐counter medication and these data were not available or recorded systematically.
Despite these limitations we believe the strength of our report is based on the size of the database, the comprehensive nature of healthcare coverage in Israel assuring widespread access and participation, low annual patient turnover, our inclusion of the new cases of non‐valvular AF, access to all laboratory tests and access to all hospitalizations, and medical treatment, thus providing a unique set of data that can provide both baseline data at time of diagnosis and also management as well as outcome.
Conclusions
The prevalence and incidence of AF in the present contemporary cohort was similar to that reported in other observational studies. Patients in our cohort had similar comorbidities and outcome as that reported in other studies.
However, the use of OAC in our population was low as compared with other studies and the TTR was also relatively low. Despite that, the rate of stroke and mortality rates were similar to those reported by other epidemiological studies. This suggests that our broader definition base incorporates a wider spectrum of milder cases of atrial fibrillation.
Disclosures
Dr Haim received Honoraria from Pfizer, Bayer, Rafa Medical, Sanofi‐Aventis, and Medtronic and participated in Advisory Boards of Pfizer and Rafa‐Medical. Dr Haim also received and unrestricted research grant from Sanofi‐Aventis.
References
- Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey J‐Y, Ponikowski P, Rutten FHGuidelines ECfP.Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck‐Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Reviewers D, Agladze V, Aliot E, Balabanski T, Blomstrom‐Lundqvist C, Capucci A, Crijns H, Dahlöf B, Folliguet T, Glikson M, Goethals M, Gulba DC, Ho SY, Klautz RJM, Kose S, McMurray J, Perrone Filardi P, Raatikainen P, Salvador MJ, Schalij MJ, Shpektor A, Sousa J, Stepinska J, Uuetoa H, Zamorano JL, Zupan I. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Europace. 2010; 12:1360-1420. [DOI] [PubMed] [Google Scholar]
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014; 129:837-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001; 285:2370-2375. [DOI] [PubMed] [Google Scholar]
- Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TSM. Secular trends in incidence of atrial fibrillation in Olmsted county, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006; 114:119-125. [DOI] [PubMed] [Google Scholar]
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009; 104:1534-1539. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994; 271:840-844. [PubMed] [Google Scholar]
- Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998; 82:2N-9N. [DOI] [PubMed] [Google Scholar]
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991; 22:983-988. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998; 98:946-952. [DOI] [PubMed] [Google Scholar]
- Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of left ventricular dysfunction. J Am Coll Cardiol. 1998; 32:695-703. [DOI] [PubMed] [Google Scholar]
- Andersson T, Magnuson A, Bryngelsson I‐L, Frøbert O, Henriksson KM, Edvardsson N, Poçi D. All‐cause mortality in 272 186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long‐term case–control study. Eur Heart J. 2013; 34:1061-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011; 4:313-320. [DOI] [PubMed] [Google Scholar]
- Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol. 1994; 74:236-241. [DOI] [PubMed] [Google Scholar]
- Lip GY, Golding DJ, Nazir M, Beevers DG, Child DL, Fletcher RI. A survey of atrial fibrillation in general practice: the West Birmingham Atrial Fibrillation Project. Br J Gen Pract. 1997; 47:285-289. [PMC free article] [PubMed] [Google Scholar]
- Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997; 96:2455-2461. [DOI] [PubMed] [Google Scholar]
- Ryder KM, Benjamin EJ. Epidemiology and significance of atrial fibrillation. Am J Cardiol. 1999; 84:131R-138R. [DOI] [PubMed] [Google Scholar]
- Stewart S, Hart CL, Hole DJ, McMurray JJ. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med. 2002; 113:359-364. [DOI] [PubMed] [Google Scholar]
- Heeringa J, van der Kuip DAM, Hofman A, Kors JA, van Herpen G, Stricker BHC, Stijnen T, Lip GYH, Witteman JCM. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006; 27:949-953. [DOI] [PubMed] [Google Scholar]
- Lip GY, Bawden L, Hodson R, Rutland E, Snatchfold J, Beevers DG. Atrial fibrillation amongst the Indo‐Asian general practice population. The West Birmingham Atrial Fibrillation Project. Int J Cardiol. 1998; 65:187-192. [DOI] [PubMed] [Google Scholar]
- Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009; 158:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries: 1993–2007. Circ Cardiovasc Qual Outcomes. 2012; 5:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012; 142:1489-1498. [DOI] [PubMed] [Google Scholar]
- Nguyen TN, Hilmer SN, Cumming RG. Review of epidemiology and management of atrial fibrillation in developing countries. Int J Cardiol. 2013; 167:2412-2420. [DOI] [PubMed] [Google Scholar]
- Singer SR, Hoshen M, Shadmi E, Leibowitz M, Flaks‐Manov N, Bitterman H, Balicer RD. EMR‐based medication adherence metric markedly enhances identification of nonadherent patients. Am J Manag Care. 2012; 18:e372-e377. [PubMed] [Google Scholar]
- Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993; 69:236-239. [PubMed] [Google Scholar]
- Björck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population‐based study. Stroke. 2013; 44:3103-3108. [DOI] [PubMed] [Google Scholar]
- Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, Maywald U, Bauersachs R, Breithardt G. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013; 15:486-493. [DOI] [PubMed] [Google Scholar]
- Murphy NF, Simpson CR, Jhund PS, Stewart S, Kirkpatrick M, Chalmers J, MacIntyre K, McMurray JJV. A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart. 2007; 93:606-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CE, Naditch‐Brule L, Murin J, Goethals M, Inoue H, O'Neill J, Silva‐Cardoso J, Zharinov O, Gamra H, Alam S, Ponikowski P, Lewalter T, Rosenqvist M, Steg PG. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real‐life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. 2012; 5:632-639. [DOI] [PubMed] [Google Scholar]
- Kakkar AK, Mueller I, Bassand J‐P, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Lip GYH, Mantovani LG, Turpie AGG, van Eickels M, Misselwitz F, Rushton‐Smith S, Kayani G, Wilkinson P, Verheugt FWAfor the GRI. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One. 2013; 8:e63479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010; 41:2731-2738. [DOI] [PubMed] [Google Scholar]
- Hobbs FD, Roalfe AK, Lip GY, Fletcher K, Fitzmaurice DA, Mant J. Performance of stroke risk scores in older people with atrial fibrillation not taking warfarin: comparative cohort study from BAFTA trial. BMJ. 2011; 342:d3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, Schmitt J, Zamorano JL. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC guidelines on atrial fibrillation: primary results of the prevention of thromboemolic events–European registry in atrial fibrillation (PREFER in AF). Europace. 2014; 16:6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp‐Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011; 342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: the risk–treatment paradox in patients with newly diagnosed non‐valvular atrial fibrillation. Heart. 2011; 97:2046-2050. [DOI] [PubMed] [Google Scholar]
- Volterrani M, Iellamo F, Rosano G, Guarini P, Pusineri E, Bonassi S, Chimini C, Zacca F, Proto C. Anticoagulation in “real world” patients with atrial fibrillation in Italy: results from the ISPAF (indagine sicoa paziente con fibrillazione atriale) survey study. Int J Cardiol. 2013; 168:4729-4733. [DOI] [PubMed] [Google Scholar]
- Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, Oliveira MM, Mairesse G, Crijns HJ, Simantirakis E, Atar D, Kirchhof P, Vardas P, Tavazzi L, Maggioni AP. A prospective survey in European Society of Cardiology member countries of atrial fibrillation management: baseline results of Eurobservational Research Programme Atrial Fibrillation (EORP‐AF) Pilot General Registry. Europace. 2014; 16:308-319. [DOI] [PubMed] [Google Scholar]
- Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012; 33:1500-1510. [DOI] [PubMed] [Google Scholar]
- Pilote L, Eisenberg MJ, Essebag V, Tu JV, Humphries KH, Leung Yinko SS, Behlouli H, Guo H, Jackevicius CA. Temporal trends in medication use and outcomes in atrial fibrillation. Can J Cardiol. 2013; 29:1241-1248. [DOI] [PubMed] [Google Scholar]
- Zalesak M, Siu K, Francis K, Yu C, Alvrtsyan H, Rao Y, Walker D, Sander S, Miyasato G, Matchar D, Sanchez H. Higher persistence in newly diagnosed nonvalvular atrial fibrillation patients treated with dabigatran versus warfarin. Circ Cardiovasc Qual Outcomes. 2013; 6:567-574. [DOI] [PubMed] [Google Scholar]
- Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P, Zhu J, Jansky P, Sigamani A, Morillo CA, Liu L, Damasceno A, Grinvalds A, Nakamya J, Reilly PA, Keltai K, Van Gelder IC, Yusufali AH, Watanabe E, Wallentin L, Connolly SJ, Yusuf S. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE‐LY Atrial Fibrillation Registry. Circulation. 2014; 129:1568-1576. [DOI] [PubMed] [Google Scholar]
- Dlott JS, George RA, Huang X, Odeh M, Kaufman HW, Ansell J, Hylek EM. National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation. Circulation. 2014; 129:1407-1414. [DOI] [PubMed] [Google Scholar]
- Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Tsang TSM. Mortality trends in patients diagnosed with first atrial fibrillation: a 21‐year community‐based study. J Am Coll Cardiol. 2007; 49:986-992. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Fauchier L, Vourc'h P, Andres CR, Taillandier S, Halimi JM, Lip GY. Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. J Am Coll Cardiol. 2013; 61:2079-2087. [DOI] [PubMed] [Google Scholar]
- Frost L, Vukelic Andersen L, Vestergaard P, Husted S, Mortensen LS. Trends in risk of stroke in patients with a hospital diagnosis of nonvalvular atrial fibrillation: National Cohort Study in Denmark, 1980–2002. Neuroepidemiology. 2006; 26:212-219. [DOI] [PubMed] [Google Scholar]
- McManus DD, Hsu G, Sung SH, Saczynski JS, Smith DH, Magid DJ, Gurwitz JH, Goldberg RJ, Go ASStudy ftCRNP. Trends in risk of stroke in patients with a hospital diagnosis of nonvalvular atrial fibrillation: National Cohort Study in Denmark, 1980–2002. J Am Heart Assoc. 2013; 2:e00569410.1161/JAHA.112.00569423525446 [Google Scholar]
- Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Behlouli H, Pilote L. Sex differences in stroke risk among older patients with recently diagnosed atrial fibrillation. JAMA. 2012; 307:1952-1958. [DOI] [PubMed] [Google Scholar]
- Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the congestive heart failure, hypertension, age >75, diabetes mellitus, and prior stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008; 156:57-64. [DOI] [PubMed] [Google Scholar]
- Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Seward JB, Bailey KR, Iwasaka T, Tsang TSM. Time trends of ischemic stroke incidence and mortality in patients diagnosed with first atrial fibrillation in 1980 to 2000: report of a community‐based study. Stroke. 2005; 36:2362-2366. [DOI] [PubMed] [Google Scholar]
- Olsson LG, Swedberg K, Lappas G, Stewart S, Rosengren A. Trends in stroke incidence after hospitalization for atrial fibrillation in Sweden 1987 to 2006. Int J Cardiol. 2013; 167:733-738. [DOI] [PubMed] [Google Scholar]


