Abstract
Background
An important number of patients with idiopathic dilated cardiomyopathy have dramatically improved left ventricular function with optimal treatment; however, little is known about the evolution and long‐term outcome of this subgroup, which shows apparent healing. This study assesses whether real healing actually exists in dilated cardiomyopathy .
Methods and Results
Persistent apparent healing was evaluated among 408 patients with dilated cardiomyopathy receiving tailored medical treatment and followed over the very long‐term. Persistent apparent healing was defined as left ventricular ejection fraction ≥50% and indexed left ventricular end‐diastolic diameter ≤33 mm/m2 at both mid‐term (19±4 months) and long‐term (103±9 months) follow‐up. At mid‐term, 63 of 408 patients (15%) were apparently healed; 38 (60%; 9% of the whole population) showed persistent apparent healing at long‐term evaluation. No predictors of persistent apparent healing were found. Patients with persistent apparent healing showed better heart transplant–free survival at very long‐term follow‐up (95% versus 71%; P=0.014) compared with nonpersistently normalized patients. Nevertheless, in the very long term, 37% of this subgroup experienced deterioration of left ventricular systolic function, and 5% died or had heart transplantation.
Conclusions
Persistent long‐term apparent healing was evident in a remarkable proportion of dilated cardiomyopathy patients receiving optimal medical treatment and was associated with stable normalization of main clinical and laboratory features. This condition can be characterized by a decline of left ventricular function over the very long term, highlighting the relevance of serial and individualized follow‐up in all patients with dilated cardiomyopathy, especially considering the absence of predictors for long‐term apparent healing.
Keywords: cardiac remodeling, dilated cardiomyopathy, heart failure
Introduction
Idiopathic dilated cardiomyopathy (DCM) is a rare primary heart muscle disease with genetic, infective, and autoimmune etiologies that is characterized by progressive loss of cardiomyocytes and progression to end‐stage cardiac failure.1 It generally affects relatively young patients with low comorbidity profiles and, theoretically, long life expectancy.2 Consequently, DCM represents a peculiar model of heart failure (HF) with substantial differences from other etiologies (ie, ischemic, hypertensive, and valvular heart disease) that more commonly affect the elderly population. The prognosis of DCM, considered ominous in the past,3 has improved progressively over the past 2 decades as the result of an integrated strategy based on evidence‐based therapy, early diagnosis, and structured follow‐up.4–5 Although some patients with DCM are still projected to have a severe outcome soon after diagnosis, most now show favorable long‐term survival, usually associated with left ventricular (LV) reverse remodeling.5–6 Recently, persistent recovery of LV function during long‐term follow‐up has been demonstrated in >70% of patients with HF from different etiologies that initially improved with β‐blockers7; however, the real prevalence and exhaustive long‐term characterization of apparently healed patients under medical treatment, particularly in the specific setting of DCM, remain unknown. Few data are reported in the literature, which is limited to small and not optimally treated populations.8
In the present study, we analyzed the prevalence of persistent normalization of LV function and dimension in a broad cohort of patients with DCM receiving optimal medical treatment. We carefully described the clinical and instrumental evolution during very long‐term regular follow‐up to assess whether a real healing phenomenon might exist in this progressive disease. Furthermore, we sought to identify the early predictors of this peculiar condition.
Methods
In this observational retrospective study, we specifically investigated patients with DCM with persistent apparent healing conditions (defined in Study Design) among those consecutively enrolled in the Heart Muscle Disease Registry of Trieste, a database from a tertiary referral center for cardiomyopathies and HF, from January 1988 to December 2003. The follow‐up ended at the time of death or urgent (status I) heart transplant (HTx) or at December 31, 2011, in the absence of main events; therefore, the study population had potential clinical follow‐up of at least 8 years. Informed consent was obtained from all subjects under the institutional review board policies of the Trieste Hospital administration. All patients underwent a structured serial clinical and instrumental follow‐up evaluation at the Cardiomyopathy Clinic of the Cardiovascular Department of Trieste at 6 months (range: 3 to 8), 12 months (range: 9 to 18), and 24 months (range: 19 to 36) and then every 2 years or according to specific clinical needs.
DCM diagnosis was defined according to the World Health Organization criteria.9 Patients with LV systolic dysfunction (LV ejection fraction [LVEF] <50%) at baseline evaluation in the absence of known causes were included in the registry. Exclusion criteria were history of blood pressure >160/100 mm Hg, >50% obstruction of a major coronary artery branch (at coronary angiography), alcohol intake >100 g/day, advanced systemic disease affecting short‐term prognosis, pericardial diseases, congenital heart diseases, HF secondary to chronic lung disease, and biopsy‐proven active myocarditis. Persistent, high‐rate, supraventricular arrhythmias were considered as an exclusion criterion when documented in the 6 months before enrollment; however, patients with persistent LV systolic dysfunction 6 months after the resolution of the arrhythmia were enrolled in the registry and included in the analysis. All familial DCM cases fulfilled the published criteria.10
At enrollment, all patients underwent an accurate clinical history interview, a complete physical examination, blood sampling for laboratory tests, 12‐lead electrocardiogram, and echocardiographic and Doppler evaluation. Coronary angiography was performed in patients aged >35 years with cardiovascular risk factors and/or without familial history of DCM. Until 1996, all patients underwent endomyocardial biopsy to exclude active myocarditis according to the Dallas criteria.11 Thereafter, biopsy was performed in patients with recent‐onset HF refractory to conventional therapy, severe LV systolic dysfunction, and/or unexplained life‐threatening ventricular arrhythmias and a clinical history suggesting active myocarditis in the absence of marked LV dilation and LV bundle‐branch block.12–13
According to our internal protocol since 1988, after careful clinical stabilization on an optimal dose of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers, all patients without contraindications or hemodynamic impairment (85% of study population) were treated with β‐blockers (metoprolol tartrate and, later, carvedilol or bisoprolol) and received diuretics and digitalis if clinically indicated. Daily dosages of ACE inhibitors and β‐blockers are reported as equivalent to enalapril and carvedilol, respectively (enalapril‐equivalent dosages: captopril ×3.75 mg; lisinopril ×1 mg; carvedilol‐equivalent dosages: metoprolol/2 mg, bisoprolol ×10)14 and refer to the end of the titration period (generally 1 to 3 months after enrollment). Moreover, according to preliminary data from our registry and the published evidence on secondary prevention of sudden death,15–17 the use of an implanted cardioverter‐defibrillator for primary prevention started in 1998 for patients with DCM who matched high‐risk criteria for sudden death (persistent LVEF ≤35% and New York Heart Association [NYHA] classes II and III despite optimal treatment). Device implantation for cardiac resynchronization therapy started in 2005, after publication of the CARE‐HF trial.18
Echocardiographic Study
Comprehensive M‐mode, 2‐dimensional, and Doppler echocardiographic studies were performed at baseline and at mid‐term and long‐term follow‐up. Systolic and diastolic functions were evaluated according to international guidelines.19 Right ventricular areas and fractional area contraction and the end‐systolic left atrial area were measured with the same approach. Mitral regurgitation was semiquantitatively graded considering the regurgitant jet area at color Doppler imaging and/or the vena contracta width.20 Mitral regurgitations with jet area >4 cm2 and vena contracta ≥0.4 cm were considered significant.
The transmitral flow velocity curve was obtained by pulsed Doppler imaging, positioning the sample volume between the tips of the mitral leaflets; the LV filling pattern was classified as restrictive in the presence of E‐wave deceleration time <120 ms or E/A ≥2 associated with E‐wave deceleration time ≤150 ms.21 For patients with atrial fibrillation, only data from the E wave were considered. All measurements were obtained from the mean of 3 beats for the patients with sinus rhythm and 5 beats for those with atrial fibrillation. Chamber diameters, areas, and volumes were normalized for body surface area. Reproducibility of Doppler echocardiographic data in our echo laboratory has been published previously.21
Study Design
Apparent healing was defined as the combined presence at mid‐term follow‐up (mean 19±4 months) of normal LVEF (≥50%) and normal indexed LV end‐diastolic diameter (≤33 mm/m2). Apparent healing was considered persistent if the normalization of both LV function and dimension were maintained at long‐term follow‐up (mean 103±9 months).
The primary outcome measure was either death or urgent HTx. The indication for HTx was considered in patients with refractory HF requiring inotropic treatment and/or mechanical support (status I). Information regarding the end points was obtained directly from the patient, from the patient's physician, or from the register of death for the municipality of residence.
Statistical Analysis
Summary statistics of clinical and instrumental variables were expressed as mean and SD or percentage, as appropriate. Comparisons between groups were made using the ANOVA test with continuous variables and the Brown‐Forsythe statistic if the assumption of equal variances did not hold; the chi‐square test was calculated for discrete variables. General survival for death or HTx was calculated by the Kaplan–Meier method, and the log‐rank test was used to assess differences among groups. To find prognostic factors for the persistent apparent healing condition, univariable logistic regression models were estimated. All calculations were performed using IBM SPSS 19.0 for Windows (IBM Corp) and R statistical software version 2.15.0 (R Foundation).
Results
Persistent Apparent Healing: Prevalence and Characterization
The apparent healing condition was found in 63 patients, representing 15% of the initial population of 408 patients with DCM with available baseline and follow‐up data. Before enrollment, only 45% of patients were treated with ACE inhibitors or angiotensin receptors blockers and 16% were treated with β‐blockers; after our first evaluation, they were optimally treated with ACE inhibitors (or angiotensin receptor blockers) and β‐blockers (95% with the equivalent dosage of enalapril of 18±12 mg/day and 85% with the equivalent dosage of carvedilol of 46±25 mg/day, respectively) without significant differences between apparently healed patients at mid‐term and the non‐apparently healed patients at mid‐term. Optimal medical treatment was maintained over follow‐up (90% and 81% were treated with ACE inhibitors and β‐blockers, respectively, after mid‐term follow‐up).
Apparently healed patients showed significantly better long‐term survival (P<0.001) than patients who were not apparently healed and alive at mid‐term (42 patients died or underwent urgent HTx before mid‐term follow‐up) (Figure 1).
Figure 1.
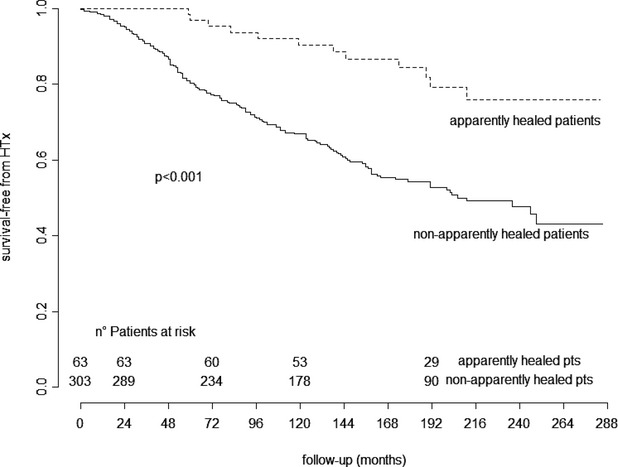
Kaplan–Meier curves for very long‐term heart transplant‐free survival of patients who were apparently healed and not apparently healed and alive at mid‐term. Dotted lines represent apparently healed patients; solid lines represent patients who were not apparently healed. HTx indicates heart transplant.
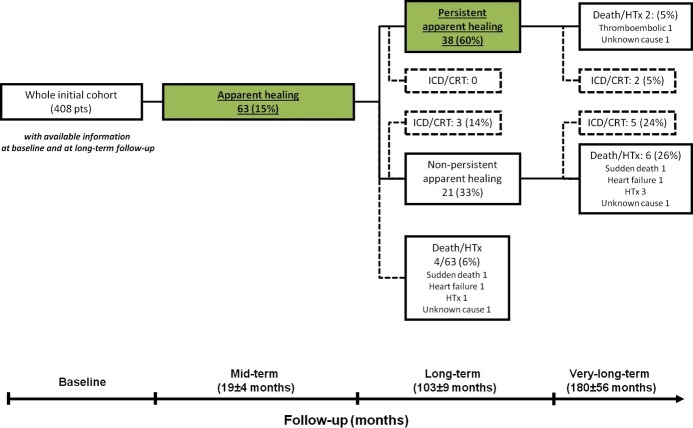
Persistent apparent healing at long‐term follow‐up was detected in 38 of 63 patients (60% of those apparently healed; 9% of the whole population). Before the long‐term evaluation, 4 of 63 patients (10%) died (1 death for HF, 1 sudden death, 1 death for unknown cause) or underwent HTx (1 case) (Figure 2). Patients with persistent long‐term recovery of LVEF and LV end‐diastolic diameter less frequently presented significant mitral regurgitation at baseline compared with the nonpersistently apparently healed patients (17% versus 45%, respectively; P=0.022). No other baseline and mid‐term clinical and laboratory differences emerged between the 2 groups (Table 1). At univariate analysis, no baseline and mid‐term parameters emerged as predictors of persistent apparent healing at long‐term follow‐up (Table 2); therefore, subsequent multivariate analyses were not performed.
Figure 2.
Flowchart of the long‐term evolution of the study population. All analyzed patients underwent a complete echocardiographic evaluation at each follow‐up. CRT indicates cardiac resynchronization therapy; HTx, heart transplant; ICD, implantable cardioverter‐defibrillator.
Table 1.
Baseline and mid‐term Clinical and Laboratory Characteristics of Persistent Apparently Healed Vs Nonpersistently Apparently Healed Patients
| Persistently Apparently Healed Patients (n=38) | Nonpersistently Apparently Healed Patients (n=21) | P Value | |
|---|---|---|---|
| Parameters at baseline | |||
| Age at diagnosis, y | 42±13 | 42±3 | 0.940 |
| Male, % | 74 | 81 | 0.401 |
| Familial DCM, % | 16 | 24 | 0.449 |
| Duration of HF, months | 5±11 | 9±17 | 0.20 |
| SBP, mm Hg | 127±14 | 132±16 | 0.215 |
| Heart rate, beats/min | 80±18 | 77±12 | 0.442 |
| NYHA classes III to IV, % | 24 | 19 | 0.681 |
| Serum Hb, g/dL | 14.0±1.6 | 14.6±1.6 | 0.286 |
| Serum creatinine, mg/dL | 1.10±0.19 | 1.13±0.17 | 0.700 |
| Sinus rhythm, % | 90 | 100 | 0.133 |
| LBBB, % | 13 | 25 | 0.256 |
| LAAI, cm2/m2 | 12±4 | 12±4 | 0.580 |
| LVEF, % | 36±10 | 36±13 | 0.901 |
| LVEDDI, mm/m2 | 33±4 | 33±4 | 0.957 |
| LVEDVI, mL/m2 | 84±27 | 94±39 | 0.308 |
| RFP, % | 18 | 30 | 0.315 |
| Significant MR, % | 17 | 45 | 0.022 |
| β‐blockers after first evaluation, % | 84 | 86 | 0.878 |
| ACEi/ARBs after first evaluation, % | 82 | 95 | 0.142 |
| Parameters at mid‐term follow‐up | |||
| SBP, mm Hg | 127±14 | 126±9 | 0.775 |
| Heart rate, beats/min | 64±9 | 66±9 | 0.562 |
| NYHA class II (vs NYHA class I), % | 23 | 18 | 0.666 |
| Serum Hb, g/dL | 13.0±1.7 | 15.3±0.5 | 0.52 |
| Sinus rhythm, % | 94 | 94 | 1.00 |
| LBBB, % | 11 | 11 | 1.00 |
| LAAI, cm2/m2 | 11±3 | 11±2 | 0.725 |
| LVEF, % | 53±3 | 54±4 | 0.649 |
| LVEDDI, mm/m2 | 29±4 | 28±3 | 0.755 |
| LVEDVI, mL/m2 | 61±14 | 66±18 | 0.277 |
| RFP, % | 0 | 6 | 0.153 |
| Significant MR, % | 0 | 6 | 0.147 |
| β‐blockers after midterm evaluation, % | 82 | 81 | 0.953 |
| ACEi/ARBs after midterm evaluation, % | 90 | 90 | 0.908 |
ACEi indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; Hb, haemoglobin; HF, heart failure; LAAI, indexed left atrial area; LBBB, left bundle‐branch block; LVEDDI, indexed left ventricular end‐diastolic diameter; LVEDVI, indexed left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NYHA, New York Heart Association; RFP, restrictive filling pattern; SBP, systolic blood pressure.
Table 2.
Univariable Analysis: Baseline and Mid‐term Predictors of a Persistent Apparent Healing Condition
| OR | CI 95% | P Value | |
|---|---|---|---|
| Parameters at baseline | |||
| Age (for 1‐year increase) | 0.999 | 0.966 to 1.032 | 0.940 |
| Male sex | 0.646 | 0.232 to 1.797 | 0.403 |
| HF duration (for 1‐month increase) | 0.982 | 0.949 to 1.016 | 0.296 |
| NYHA class (for 1‐class increase) | 1.034 | 0.634 to 1.684 | 0.894 |
| SBP (for 1‐mm Hg increase) | 0.985 | 0.954 to 1.018 | 0.364 |
| LVEF (for 1‐unit increase) | 0.993 | 0.951 to 1.038 | 0.767 |
| LAAI (for 1‐cm2/m2 increase) | 0.964 | 0.848 to 1.083 | 0.533 |
| LVEDDI (for 1‐mm/m2 increase) | 0.997 | 0.983 to 1.011 | 0.683 |
| LVEDVI (for 1‐mL/m2 increase) | 1.016 | 0.918 to 1.124 | 0.761 |
| RFP | 0.835 | 0.284 to 2.454 | 0.744 |
| Significant MR | 0.375 | 0.129 to 1.089 | 0.071 |
| LBBB | 0.640 | 0.195 to 2.100 | 0.461 |
| β‐blockers | 1.231 | 0.396 to 3.825 | 0.720 |
| ACEi/ARBs | 0.403 | 0.108 to 1.495 | 0.174 |
| Parameters at midterm follow‐up | |||
| NYHA class (for 1‐class increase) | 1.354 | 0.435 to 4.220 | 0.601 |
| SBP (for 1‐mm Hg increase) | 0.995 | 0.956 to 1.035 | 0. 799 |
| LVEF (for 1‐unit increase) | 0.948 | 0.881 to 1.021 | 0.158 |
| LAAI (for 1‐cm2/m2 increase) | 0.904 | 0.740 to 1.104 | 0. 321 |
| LVEDDI (for 1‐mm/m2 increase) | 1.071 | 0.924 to 1.241 | 0.644 |
| LVEDVI (for 1‐mL/m2 increase) | 0.994 | 0.963 to 1.025 | 0.686 |
| Significant MR | 0.811 | 0.384 to 1.715 | 0.584 |
| LBBB | 1.094 | 0.252 to 4.740 | 0.905 |
ACEi indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; HF, heart failure; LAAI, indexed left atrial area; LBBB, left bundle‐branch block; LVEDDI, indexed left ventricular end‐diastolic diameter; LVEDVI, indexed left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NYHA, New York Heart Association; OR, odds ratio; RFP, restrictive filling pattern; SBP, systolic blood pressure.
Long‐Term Temporal Trends of Main Clinical and Laboratory Features
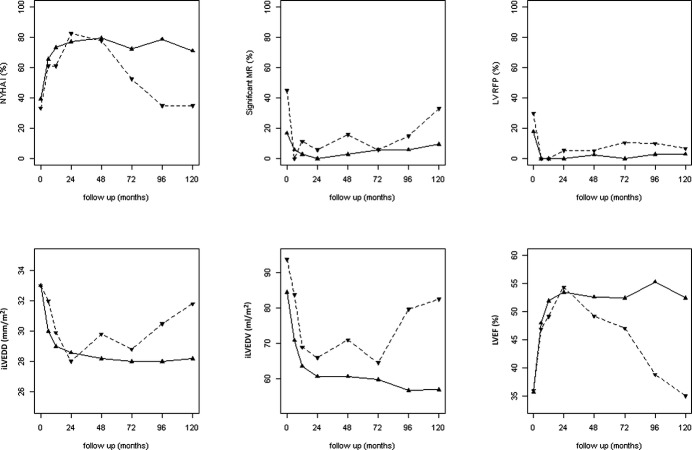
Figure 3 shows the longitudinal trends of main clinical and laboratory features (ie, patients in NYHA class I, LVEF, indexed LV end‐diastolic diameter, indexed LV end‐diastolic volume, significant mitral regurgitation, LV restrictive filling pattern) during the structured long‐term follow‐up in the 38 persistently apparently healed and 21 nonpersistently normalized patients. In the first subgroup, all parameters reached normalization at 24 months and were maintained at long‐term evaluation. Conversely, nonpersistently normalized patients satisfied the apparent healing criteria at mid‐term but later showed progressive worsening of clinical and echocardiographic parameters, usually starting from the fifth year of follow‐up, with the exception of LVEF, which dramatically decreased after the 24th month of follow‐up.
Figure 3.
Longitudinal long‐term trends of main clinical and laboratory features in patients who were persistently apparently healed and nonpersistently apparently healed. All analyzed patients underwent a complete echocardiographic evaluation at each follow‐up. Solid lines represent persistently apparently healed patients; broken lines represent nonpersistently apparently healed patients. iLVEDD indicates indexed left ventricular end‐diastolic diameter; iLVEDV, indexed left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVRFP, left ventricular restrictive filling pattern; MR, mitral regurgitation; NYHA, New York Heart Association.
Persistent Apparent Healing Condition: Very Long‐Term Prognostic Assessment
During very long‐term follow‐up of 180±56 months, persistently apparently healed patients showed better outcomes with respect to nonpersistently healed patients (95% versus 71% HTx‐free survival; P=0.014) (Figure 4). Interestingly, at the last echocardiogram, 14 of 38 persistently apparently healed patients (37%) showed systolic dysfunction (LVEF <50%), and 12 (32%) presented increased LV dimensions (LV end‐diastolic diameter >33 mL/m2). At very long‐term follow‐up, 2 of 38 patients with persistent apparent healing (5%) died or underwent HTx (1 thromboembolic death and 1 death from HF), both presenting normal LVEF but increased LV end‐diastolic diameter at the last available echocardiogram compared with 6 of 21 nonpersistently apparently healed patients (29%; 1 death from HF, 1 sudden death, 1 death from unknown cause, 3 HTx). Moreover, at very long‐term follow‐up, 2 of 38 patients (5%) who were persistently apparently healed (at 175±25 months) and 5 of 21 patients (24%) who were nonpersistently apparently healed (at 173±59 months) underwent implanted cardioverter‐defibrillator and/or cardiac resynchronization therapy implantation for severe deterioration of LVEF (Figure 2).
Figure 4.
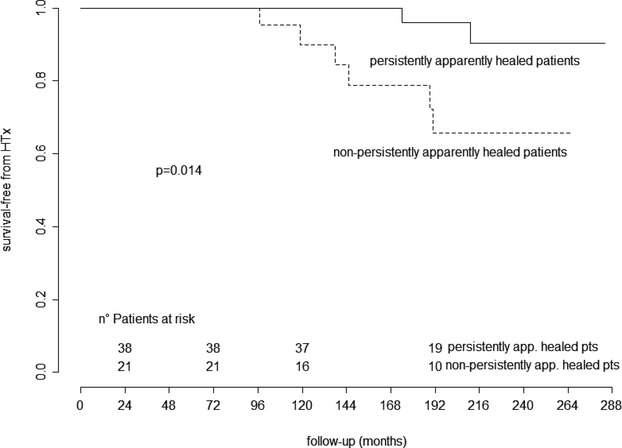
Kaplan–Meier curves for very long‐term heart transplant‐free survival of patients who were persistently apparently healed vs nonpersistently apparently healed and alive at long‐term follow‐up. HTx indicates heart transplant.
Discussion
It is becoming clear that DCM represents not the irreversible consequence of a cardiomyopathic process but rather a dynamic model with evolution that is highly variable and able to be changed by optimized pharmacological and nonpharmacological treatments.5,22–23 Nevertheless, accurate information regarding the long‐term characterization and prognosis of “super‐responders” to optimal treatment in this peculiar model of HF are lacking. This study provides this information. The population of 38 persistently apparently healed patients with DCM is only apparently small; in fact, this group derives from an initial population of 408 patients with DCM of whom 63 (15%) experienced normalized LV function and dimension under optimal medical treatment. To our knowledge, this DCM population is the largest with the longest follow‐up focusing on the recovery of normal LV function and dimension in response to medical treatment.
Persistent Apparent Healing Condition in Long‐Term Follow‐up
After an adequate period of optimal medical therapy (almost 2 years), 15% of patients with DCM in our study showed apparent healing and more favorable long‐term outcomes. These findings are only partially surprising because they are consistent with the well‐known effectiveness of ACE inhibitors, β‐blockers, and aldosterone antagonists in inducing LV reverse remodeling in more than one third of patients with DCM at mid‐term5,24 and subsequently higher long‐term survival directly related to the amount of LV systolic function and improvement in size (Figure 1). Moreover, this study probably underestimated the magnitude of the apparent healing phenomenon. To assess the characterization of persistent long‐term apparent healing in DCM, enrollment ended in 2003. Consequently, only a minority (2%) of the whole initial population underwent cardiac resynchronization therapy implantation in the first 2 years of follow‐up; however, the possibility of LVEF normalization in a subgroup of patients with DCM after cardiac resynchronization therapy implantation is widely described in literature, and future studies are needed to characterize this subgroup of patients during long‐term follow‐up.25
The most surprising and novel results of the present study concern the long‐term characterization of the apparent healing phenomenon in DCM, in particular, (1) the demonstration of long‐term persistence (at least 8 years; mean follow‐up: 18056 months) of an apparent healing condition in 60% of normalized patients at mid‐term, representing almost 10% of the whole DCM population; (2) the evidence in these patients of a concomitant persistent normalization of other important clinical and echocardiographic features mostly induced by long‐term optimal medical therapy; (3) the absence of baseline and mid‐term predictors of a persistent apparent healing condition; and (4) the need for continuous, individualized, and probably lifelong follow‐up and optimal treatment also for persistently normalized patients with DCM, considering the high frequency (>30%) of deterioration of LV size and systolic function and non‐negligible rates (5%) of death or HTx at very long‐term follow‐up in this subgroup. In our opinion, the latter results support the absence of real healing in DCM. In this sense, the apparent healing condition appears to be a phenomenon driven by optimal medical treatment rather than by natural healing (there is no active myocarditis in our population). There is a need for further imaging and molecular studies26–27 to investigate the unexplored mechanisms underlying the possibly real healing phenomenon in DCM.
Long‐Term Temporal Trends of Main Clinical and Laboratory Features and Prognostic Assessment
Interestingly, before long‐term evaluation, 24% of patients with an initial apparent healing condition worsened progressively over the course of the disease, and 5% died or underwent HTx. The main clinical and echocardiographic features of these patients showed stable values in the first 3 years after initial normalization, with a subsequent progressive decline. This highlights the frequently degenerative nature of DCM over the long‐term despite pharmacological and nonpharmacological integrated evidence‐based therapy. Notably, LVEF was the first parameter that declined after the initial transient normalization. For this reason, LVEF might represent the most important parameter for periodic assessment of these patients during follow‐up because it could precede the unfavorable evolution of other clinical and instrumental features, such as progressive LV remodeling, significant mitral regurgitation, and severe diastolic dysfunction.
The present study failed to identify predictors of long‐term persistent apparent healing among the more commonly evaluated parameters both at baseline and at mid‐term, as confirmed by the lack of significant differences in clinical and laboratory features for patients who were persistently and nonpersistently apparently healed (Table 2). It is possible that follow‐up of 24 months represents too short a period in which to assess the likelihood of long‐term persistent apparent healing; however, this finding confirms the complexity of prognostic stratification of DCM that has recently emerged in other experiences.28 DCM represents an extremely varied disease in terms of etiopathogenesis, clinical presentation, and evolution. In this sense, accurate and individualized long‐term surveillance over time (probably lifelong) together with administration of optimal medical treatment from initial diagnosis remain the cornerstones for appropriate management of patients with DCM. Consequently, we strongly suggest that a serial echocardiographic assessment of patients with DCM be performed independently from variations in clinical conditions.29 This management could allow identification of patients at higher risk of disease progression and set the correct timing for aggressive nonpharmacolo‐gical interventions. Future studies are needed to investigate the individual genetic background of response to medical treatment.
Notably, no sudden death or implanted cardioverter‐defibrillator interventions were reported in the 8 patients with transient apparent healing who underwent primary‐prevention implanted cardioverter‐defibrillator implantation during long‐term follow‐up (Figure 2). In our opinion, this interesting aspect further highlights the lack of solid criteria for early arrhythmic risk stratification, which represents a major issue in the management of patients with DCM, particularly in the first years of follow‐up.28,30 Future studies focused specifically on this issue are needed.
Study Limitations
This observational retrospective study of long‐term registry data suffers from the common bias of different protocols and treatment; however, the presence of the same inclusion criteria over time at the same institution could represent an advantage for the present analysis. Furthermore, the study population was enrolled in a tertiary referral center for cardiomyopathies and HF, imposing a selection bias with respect to the characteristics of DCM in the general population.
The definitions of mid‐term (about 2 years after enrollment), long‐term (about 8 years after enrollment), and very long‐term (about 15 years after enrollment) follow‐up were arbitrary. There is no specific guideline or clear evidence on this topic, thus we set the timing of follow‐up based on our clinical experience and previous reports.2,5
According to recommendations, endomyocardial biopsy has not been performed systematically in our patients with DCM since 199712; however, we found similar prevalence of apparently healed patients at mid‐term before and after 1996 (19% and 21%, respectively; P=0.765).
Because the enrollment period ended in 2003, before the results of large randomized studies, treatment with aldosterone antagonists was not administered systematically in symptomatic patients (NYHA class II or higher).29
No systematic information on genetic profile, tissue Doppler or speckle tracking echocardiography, cardiac magnetic resonance, and brain natriuretic peptide values was available because many patients were enrolled before these evaluations were performed routinely at our center.
We included HTx in the composite end point even though it is not a fatal event. In our opinion, it remains a major event in the evolution of DCM and has an impact similar to death in the prognostic evaluation of the disease, especially considering that only urgent HTx was performed in our series.
Finally, our population included only patients with DCM; therefore, these results should not be extended to patients with other causes of impaired LVEF, such as hypertensive or ischemic heart disease.
Clinical Implications and Conclusions
In optimally treated DCM, a remarkable number of patients (60% of normalized patients at midterm; 9% of the initial population) experienced persistent apparent healing during long‐term follow‐up. This favorable condition was associated with persistent normalization of main clinical and laboratory parameters, but no early features emerged as being able to predict the stability of the apparent healing condition. Despite the apparent resolution of the disease, a non‐negligible proportion of patients died or had a worsened clinical and instrumental condition over the very long‐term. These patients cannot be considered to be effectively healed and should be carefully and systematically followed and treated over the long‐term to identify early clues of disease progression.
Disclosures
None.
Acknowledgments
We are grateful to “Fondazione Cassa Risparmio Trieste” of Trieste, Italy, for the continuous support of research in cardiology.
References
- 1.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008; 29:270-276. [DOI] [PubMed] [Google Scholar]
- 2.Merlo M, Pivetta A, Pinamonti B, Stolfo D, Zecchin M, Barbati G, Di Lenarda A, Sinagra G. Long‐term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: changing mortality over the last 30 years. Eur J Heart Fail. 2014; 16:317-324. [DOI] [PubMed] [Google Scholar]
- 3.Franciosa JA, Wilen M, Ziesche S, Cohn JN. Survival in men with severe chronic left ventricular failure due to either coronary heart disease or idiopathic dilated cardiomyopathy. Am J Cardiol. 1983; 51:831-836. [DOI] [PubMed] [Google Scholar]
- 4.Di Lenarda A, Secoli G, Perkan A, Gregori D, Lardieri G, Pinamonti B, Sinagra G, Zecchin M, Camerini F. Changing mortality in dilated cardiomyopathy. Br Heart J. 1994; 72:S46-S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merlo M, Pyxaras S, Pinamonti B, Barbati G, Di Lenarda A, Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011; 57:1468-1476. [DOI] [PubMed] [Google Scholar]
- 6.Brenyo A, Barsheshet A, Kutyifa V, Ruwald A‐C, Rao M, Zareba W, Pouleur A‐C, Knappe D, Solomon SD, McNitt S, Huang DT, Moss AJ, Goldenberg I. Predictors of spontaneous reverse remodeling in mild heart failure patients with left ventricular dysfunction. Circ Heart Fail. 2014; 7:565-572. [DOI] [PubMed] [Google Scholar]
- 7.De Groote P, Fertin M, Duva Pentiah A, Goéminne C, Lamblin N, Bauters C. Long‐term functional and clinical follow‐up of patients with heart failure with recovered left ventricular ejection fraction after ß‐blocker therapy. Circ Heart Fail. 2014; 7:434-439. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura Y, Hoshikawa‐Nagai E, Kubo T, Yamasaki N, Furuno T, Kitaoka H, Takata J, Sugiura T, Doi Y. Left ventricular reverse remodeling in long‐term (>12 years) survivors with idiopathic dilated cardiomyopathy. Am J Cardiol. 2013; 111:106-110. [DOI] [PubMed] [Google Scholar]
- 9.Richardson P, McKenna W, Bristow M, Maisch B. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996; 93:841-842. [DOI] [PubMed] [Google Scholar]
- 10.Mestroni L, Maisch B, McKenna WJ, Schwartz K, Charron P, Rocco C, Tesson F, Richter A, Wilke A, Komajda M. Guidelines for the study of familial dilated cardiomyopathies. Collaborative Research Group of the European Human and Capital Mobility Project on Familial Dilated Cardiomyopathy. Eur Heart J. 1999; 20:93-102. [DOI] [PubMed] [Google Scholar]
- 11.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ, Olsen EG, Schoen FJ. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987; 1:3-14. [PubMed] [Google Scholar]
- 12.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Eur Heart J. 2007; 28:3076-3093. [DOI] [PubMed] [Google Scholar]
- 13.Anzini M, Merlo M, Sabbadini G, Barbati G, Finocchiaro G, Pinamonti B, Salvi A, Perkan A, Di Lenarda A, Bussani R, Bartunek J, Sinagra G. Long‐term evolution and prognostic stratification of biopsy‐proven active myocarditis. Circulation. 2013; 128:2384-2394. [DOI] [PubMed] [Google Scholar]
- 14.Poole‐Wilson PA, Swedberg K, Cleland JGF, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp‐Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003; 362:7-13. [DOI] [PubMed] [Google Scholar]
- 15.Kuck KH, Cappato R, Siebels J, Rüppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000; 102:748-754. [DOI] [PubMed] [Google Scholar]
- 16.Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, Mitchell LB, Green MS, Klein GJ, O'Brien B. Canadian Implantable Defibrillator Study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000; 101:1297-1302. [DOI] [PubMed] [Google Scholar]
- 17.Wyse DG, Friedman PL, Epstein AE. A comparison of antiarrhythmic‐drug therapy with implantable defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997; 337:1576-1583. [DOI] [PubMed] [Google Scholar]
- 18.Cleland JGF, Daubert J‐C, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005; 352:1539-1549. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MSJ, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiograph. J Am Soc Echocardiogr. 2005; 18:1440-1463. [DOI] [PubMed] [Google Scholar]
- 20.Spain MG, Smith MD, Grayburn PA, Harlamert EA, DeMaria AN. Quantitative assessment of mitral regurgitation by Doppler color flow imaging: angiographic and hemodynamic correlations. J Am Coll Cardiol. 1989; 13:585-590. [DOI] [PubMed] [Google Scholar]
- 21.Pinamonti B, Zecchin M, Di Lenarda A, Gregori D, Sinagra G, Camerini F. Persistence of restrictive left ventricular filling pattern in dilated cardiomyopathy: an ominous prognostic sign. J Am Coll Cardiol. 1997; 29:604-612. [DOI] [PubMed] [Google Scholar]
- 22.Hoshikawa E, Matsumura Y, Kubo T, Okawa M, Yamasaki N, Kitaoka H, Furuno T, Takata J, Doi Y. Effect of left ventricular reverse remodeling on long‐term prognosis after therapy with angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers and beta blockers in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2011; 107:1065-1070. [DOI] [PubMed] [Google Scholar]
- 23.Vaillant C, Martins RP, Donal E, Leclercq C, Thébault C, Behar N, Mabo P, Daubert J‐C. Resolution of left bundle branch block‐induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol. 2013; 61:1089-1095. [DOI] [PubMed] [Google Scholar]
- 24.Binkley P. A new Era in the natural history of dilated cardiomyopathy. J Am Coll Cardiol. 2012; 59:776-777. [DOI] [PubMed] [Google Scholar]
- 25.Serdoz LV, Daleffe E, Merlo M, Zecchin M, Barbati G, Pecora D, Pinamonti B, Fantoni C, Lupo P, Di Lenarda A, Sinagra G, Cappato R. Predictors for restoration of normal left ventricular function in response to cardiac resynchronization therapy measured at time of implantation. Am J Cardiol. 2011; 108:75-80. [DOI] [PubMed] [Google Scholar]
- 26.Okada M, Tanaka H, Matsumoto K, Ryo K, Kawai H, Hirata K‐I. Subclinical myocardial dysfunction in patients with reverse‐remodeled dilated cardiomyopathy. J Am Soc Echocardiogr. 2012; 25:726-732. [DOI] [PubMed] [Google Scholar]
- 27.Masci PG, Schuurman R, Andrea B, Ripoli A, Coceani M, Chiappino S, Todiere G, Srebot V, Passino C, Aquaro GD, Emdin M, Lombardi M. Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: a contrast‐enhanced cardiovascular magnetic study. Circ Cardiovasc Imaging. 2013; 6:790-799. [DOI] [PubMed] [Google Scholar]
- 28.Goldberger JJ, Subačius H, Patel T, Cunnane R, Kadish AH. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2014; 63:1879-1889. [DOI] [PubMed] [Google Scholar]
- 29.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte Op Reimer WJM, Vrints C, Wood D, Zamorano JL, Zannad F, Cooney MT, Bax J, Baumgartner H, Ceconi C, Dean V, Fagard R, Funck‐Brentano C, Hasdai D, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu Ba, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Aboyans V, Ezquerra EA, Baigent C, Brotons C, Burell G, Ceriello A, De Sutter J, Deckers J, Del Prato S, Diener H‐C, Fitzsimons D, Fras Z, Hambrecht R, Jankowski P, Keil U, Kirby M, Larsen ML, Mancia G, Manolis AJ, McMurray J, Pajak A, Parkhomenko A, Rallidis L, Rigo F, Rocha E, Ruilope LM, van der Velde E, Vanuzzo D, Viigimaa M, Volpe M, Wiklund O, Wolpert C. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J. 2012; 33:1635-1701. [DOI] [PubMed] [Google Scholar]
- 30.Zecchin M, Merlo M, Pivetta A, Barbati G, Lutman C, Gregori D, Serdoz LV, Bardari S, Magnani S, Di Lenarda A, Proclemer A, Sinagra G. How can optimization of medical treatment avoid unnecessary implantable cardioverter‐defibrillator implantations in patients with idiopathic dilated cardiomyopathy presenting with “SCD‐HeFT criteria?”. Am J Cardiol. 2012; 109:729-735. [DOI] [PubMed] [Google Scholar]