Abstract
Background
Beta‐blockers (BB) are recommended in secondary prevention of acute myocardial infarction (AMI), but adherence to prescription medication is a recognized problem. Most literature on the consequences of poor adherence to prescribed BB is limited by the possibility of “healthy adherer bias” and better‐designed studies have been advocated.
Methods and Results
We investigated the association between adherence to BB prescription and risk of subsequent AMIs using the self‐controlled case series design, which allows improved control of interpersonal confounding, being based on intrapersonal comparisons. From all the 30 089 patients hospitalized for AMI in the years 2009–2011 in an Italian region we selected those that suffered subsequent AMIs at days 31 to 365 from discharge (1328), and then the 1207 that had at least one BB prescription collected at any of the regional pharmacies. Using information on prescriptions, each individual's observation time was then divided into periods exposed or unexposed to BB and the relative AMI incidence rate ratios (IRR) of BB exposure were estimated by conditional Poisson regression. The IRR (rate of recurrent AMI in exposed versus unexposed periods) was 0.79 (95% CI 0.69 to 0.90, P=0.001). Various sensitivity analyses confirmed the robustness to possible failure of assumptions, ie, considering only first recurrences (IRR 0.76, 95% CI 0.66 to 0.88, P<0.001), excluding cardiovascular fatalities (IRR 0.76, 95% CI 0.65 to 0.89, P<0.001), and excluding individuals with long hospital admissions (IRR 0.60, 95% CI 0.43 to 0.83, P=0.002).
Conclusions
Adherence to recommended BB therapy was associated with a 20% reduction of recurrent AMIs, consistently with previous research, but with decreased concerns about healthy‐adherer bias.
Keywords: adrenergic β‐antagonists, bias (epidemiology), medication adherence, myocardial infarction, secondary prevention
Introduction
Cardiovascular disease, of which coronary artery disease is the predominant form, is the number one cause of death globally.1 Experimental research has shown that β‐blockers (BB) can reduce the risk of cardiovascular death or acute myocardial infarction (AMI) by about 30% in post AMI population.2 However, nonadherence to prescription medication is a problem with potentially large public health implications that has received increasing attention in the last years.3–4
A recent systematic review confirmed the benefits of increased medication adherence on coronary artery disease outcomes,5 but at the same time questioned the validity of this conclusion because it found that most of the studies were prone to the “healthy adherer” bias. This bias arises when some individual characteristics difficult to measure and include in the analysis are associated both with the adherence to prescription medication and with the risk of outcomes. In other words, individuals who tend to adhere to prescriptions may reasonably have some other elusive characteristics that lower their risk of adverse cardiac events (eg, healthier lifestyle).6
A study design that offers improved control over confounding arising from variables that are constant within an individual, such as the “healthy adherer effect,” is the self‐controlled case‐series (SCCS).7 SCSS and other case‐only designs are increasingly used in postlicensure pharmacoepidemiological studies because each individual acts as his or her own control, which “eliminates the possibility of interpersonal confounding.”8
For these reasons, we undertook an SCCS study on the association between adherence to BB prescription and risk of recurrent AMI.
Materials and Methods
Setting and Participants
The study was conducted in Emilia‐Romagna, a wealthy (third highest national per capita GDP) region in Italy with 4.5 million inhabitants and an area of 22 000 km2. The region has 61 hospitals admitting acute patients and 14 hospitals performing interventional cardiology.
We identified all of the patients who were admitted to any of the regional hospitals with a diagnosis of AMI in the 4 calendar years of 2009–2012. From these, we selected those who suffered at least another episode of AMI in the 365 days after the discharge from their index admission (observation period). We then excluded from the analysis the cases where AMI recurred within 30 days. This exclusion was made for two reasons. First, sometimes, additional percutaneous coronary interventions (PCI) are staged shortly after discharge and an admission for such a reason could have been classified as a new AMI episode. Second, the assessment of exposure in the first weeks may be faulty because sometimes, before discharge, patients are given a small provision of drugs that is not recorded by the hospital pharmacy (see subsection below for the description of exposure assessment).
Finally, we considered only patients with at least one prescription for BBs in the year after discharge.
According to our institutional rules, neither patient consent nor ethical committee approval was necessary, given the observational, retrospective design of the study and the anonymity of the databases provided to the researchers.
Data Sources, Outcome, and Exposure Assessment
We linked data at an individual level from 3 regionwide longitudinal registries managed by the Emilia‐Romagna Regional Health Agency. The Hospital Discharge Registry contains the usual so‐called administrative information recorded in the charts of all the regional hospitals. The Prescription Drug Registry collects information on dates, dosage, and quantities for every prescription dispensed within the regional boundaries, from both hospital and community pharmacies. Pharmacists record all prescriptions electronically in order to be reimbursed by the government and BBs cannot be sold without prescription. The Demographic Registry holds information on age, sex, date of birth, place of residence, and vital status of all residents in Emilia‐Romagna. A unique patient identifier allowed cross‐linking between the databases.
Through the Hospital Discharge Registry we identified the initial study population, ie, all individuals with an episode of hospital admission with an ICD‐9‐CM code 410.x1 in any position. This coding has been shown to have Sensitivity, Specificity, and Positive Predictive Value of 98%, 90.4%, and 96%, respectively, in the Italian setting.9 The same definition was used for the other MI episodes. From this registry we recorded also all the other clinical information (eg, demographics, dates of events, type of AMI, procedures, etc).
Using the Prescription Drug Registry we identified for each participant all the prescriptions for BB drugs—ie, Anatomical‐Therapeutic‐Chemical‐Classification‐System (ATC) Class C07—collected at any of the regional pharmacies for 1 year after discharge from the index AMI episode. We then computed the number of days covered by BB as the total number of Defined Daily Doses (DDDs) prescribed to each individual. The DDD is a technical unit of measurement, used by the World Health Organization,10 which reflects the average adult dose used for the main indication. If a patient refilled a prescription early, the number of days covered was still calculated according to the DDD of the previous prescription, allowing for stockpiling.
According to the above information each individual's observation time was then divided in periods covered by BBs (ie, exposed) or not covered (ie, unexposed periods). BBs are short‐acting drugs and they do not accumulate, therefore we did not consider any wash out or intermediate‐risk period.
We used the Demographic Registry to record any death occurring during the observation period and their causes. A death was defined to be of cardiovascular origin if any of the following codes were recorded: ICD9‐CM 410‐414, 425‐438, 798‐799; ICD10 I20‐I25, I39‐I52, I60‐I69, R96‐R99.
Statistical Analyses
The self‐controlled case series method relies on intrapersonal comparisons in a population of individuals who have both the outcome and exposure of interest. The rate of events during exposed periods of time is compared with the rate during unexposed time periods. This method removes the potential confounding effect of characteristics that vary between individuals, such as unmeasured risk factors for cardiovascular disease.
We compared the incidence of AMI in the time periods covered and not covered by medication during days 31 to 365 (or earlier in case of death) following discharge from the index AMI hospital admission. Figure 1 provides a graphical display of the study design. The analyses were conducted in the first months of 2014. We estimated the relative incidence rate ratios (IRR) using conditional Poisson regression with Stata software, version 13 (Stata Corp, College Station, TX). Although the observation period is relatively short, we preferred to adjust for possible intrapersonal time‐trends of both adherence (eg, decreasing with time11) and re‐infarction risk (eg, increasing with time/age) by dividing the observation period in 2 semesters and adding this term to the model. In order to assess whether the risk was different in individuals treated or not with PCI,11 we also evaluated in the model an interaction term PCI×exposure.
Figure 1.
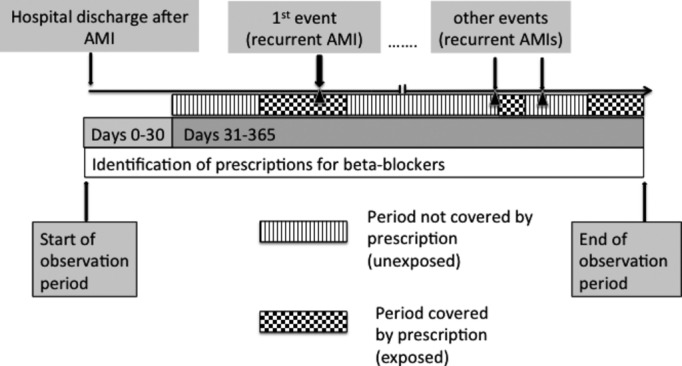
Pictorial description of the study design. AMI indicates acute myocardial infarction.
We conducted several sensitivity analyses in order to test possible violations of the SCCS assumptions. The main limiting assumption is that both the exposure distribution and the observation period must be independent of event times.
One potential violation may occur when events alter in some way the subsequent exposure process. This could be the case if patients tended to improve their adherence after the first AMI recurrence, as it would seem reasonable. Therefore, in analysis 2 we considered as events only first AMI recurrences.
Another potential violation may occur if events increase the mortality rate (as in the case of AMI), because censoring of the observation periods is then event dependent. An adaptation of the method for such situation has been developed.12 Unfortunately, it proved impractical to apply to our data for the large number of crossovers between exposed and unexposed periods. However, the classic SCSS method has been shown to be robust to violations of this assumption13 and it has been previously applied to outcomes that increase mortality.14–15 In any case, in analysis 3, patients who died from cardiovascular diseases were excluded.
Small variations in the timing of refills may exist unrelated to adherence—eg, during hospitalizations, because a hospitalized patient might have received some drug supply undetected by our sources. Because the risk of AMI is also likely to change (eg, surgery is a known risk factor for AMI), this combination could result in bias of unpredictable direction. We addressed this potential source of bias in analysis 4, where we excluded all individuals who had been admitted to the hospital for >5 days (except the AMI admissions) during the observation period.
Finally, we excluded those cases that had suffered from a previous AMI in the 2 years before their index AMI.
Furthermore, to evaluate whether the occurrence of a re‐infarction had any actual influence of the patients’ compliance with medication prescription, we measured the proportion of exposed and unexposed days in the observation period before and after the first re‐infarction. We then compared these proportions with the Wilcoxon signed rank test.
Results
The flow diagram of the study population is shown in Figure 2. We identified 30 089 individuals who had at least one AMI during the study period (2009–2012). Of these, 1777 patients had one or more subsequent AMI episodes during the 365 days following their discharge. After excluding the 449 patients whose subsequent AMI occurred in the first 30 days after discharge from the index admission, 1328 cases remained. Only 1207 had at least one dispensed prescription of BBs and represent the study population.
Figure 2.
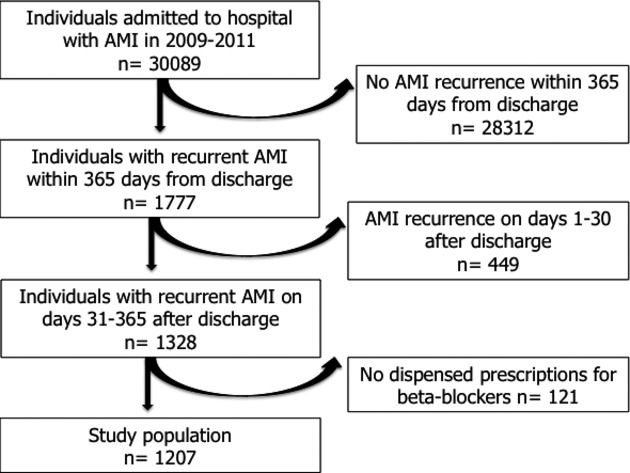
Flow diagram of population selection. AMI indicates acute myocardial infarction.
Table 1 describes this population, together with the slightly different one that was selected for the sensitivity analysis number 3 (ie, without the individuals who died of cardiovascular disease).
Table 1.
Selected Characteristics of the Study Population
| Main Population | Excluding Patients Who Died of Cardiovascular Disease | |
|---|---|---|
| Number of cases | 1207 | 1044 |
| Age (y), median (interquartile range) | 79 (70 to 85) | 78 (70 to 84) |
| Gender (male), n (%) | 752 (62.3) | 667 (63.9) |
| AMI episodes per case in the observation period, n (%) | ||
| 1 | 1059 (87.7) | 926 (88.7) |
| 2 | 126 (10.4) | 101 (9.7) |
| 3 | 14 (1.2) | 10 (1.0) |
| 4 | 8 (0.7) | 7 (0.7) |
| PCI for index AMI, n (%) | 706 (58.5) | 643 (62.0%) |
| Overall mortality, n (%) | 234 (19.4) | 71 (6.8)* |
| Cardiovascular mortality, n (%) | 163 (13.5) | 0 |
| Length of observation period (days), mean±SD | 332.0±82.5 | 355.4±39.3 |
AMI indicates acute myocardial infarction; PCI, percutaneous cardiac intervention; SD, standard deviation.
Noncardiovascular mortality only.
The median age was 79 years and males were predominant (63%). The majority of individuals (88%) experienced only one AMI during the observation period, while the maximum number of AMI recurrences was 4. The overall mortality during the study period was 22%, and 15% was due to cardiovascular causes. Nearly 60% of the population underwent a PCI for their index AMI. After exclusion of cardiovascular fatalities, the patient characteristics were similar.
The overall adherence, in terms of mean percentage of exposed days during the observation period was 48.8%. The mean number of crossovers between exposed and unexposed period was 12.8.
In the main analysis there were 576 and 867 events during exposed (ie, with BB coverage) and unexposed (ie, without BB coverage) periods, respectively. The relative risk (IRR) was 0.79 (95% CI 0.69 to 0.90, P<0.001). These estimates were virtually unchanged when only first events were considered (Table 2, analysis 2) and after the exclusion of cardiovascular deaths (Table 2, analysis 3). Previous PCI had no effect on the risk (IRR of the interaction term PCI×exposure 1.03, 95% CI 0.79 to 1.36, P=0.81).
Table 2.
Main Results and Sensitivity Analyses
| No of Events in Exposed Periods | No of Events in Unexposed Periods | IRR (95% CI) | P Value | |
|---|---|---|---|---|
| Analysis 1* (N=1207) | 610 | 775 | 0.79 (0.69 to 0.90) | <0.001 |
| Analysis 2* (N=1207) | 527 | 680 | 0.76 (0.66 to 0.88) | <0.001 |
| Analysis 3* (N=1044) | 464 | 580 | 0.76 (0.65 to 0.89) | <0.001 |
| Analysis 4* (N=257) | 120 | 130 | 0.60 (0.43 to 0.83) | 0.002 |
CI indicates confidence interval; IRR, incidence rate ratio.
All cases, all events.
Only first events.
Only first events, excluding cardiovascular deaths.
Excluding patients admitted to hospital for longer than 5 days.
After excluding all cases with a hospital admission longer than 5 days (Table 2, analysis 4), the relative risk was 0.60 (95% CI 0.43 to 0.83, P=0.002).
There was no significant difference in the percentage of exposed days before and after the first AMI recurrence (47.1% versus 51.0%, P of the Wilcoxon signed rank test=0.13).
After exclusion of the 50 cases with AMI in the 3 years before their index admission, the estimates did not change (IRR 0.79; 95% CI 0.69 to 0.90).
Discussion
We found that poor adherence to BB prescription medication after hospital discharge for AMI was associated with an increased risk of recurrent AMI. During drug‐covered periods the risk was about 20% lower than during periods not covered, either in patients undergoing PCI for the previous AMI or not. The adopted methodology – SCSS – greatly reduced the possibility that this finding was due to confounding related to unaccounted individual characteristics – such as the “healthy adherer effect” – unlike the majority of the previous literature on the subject.
Our findings are generally consistent with previous research (ie, references 16–21), yet they reinforce the available evidence because they answer the concerns that had been raised over residual confounding and the “critical need for better studies with healthy user controls.”5 This appears particularly opportune at a moment when the importance of nonadherence to prescription medication is increasingly recognized4 and payers and purchasers are starting to apply large resources toward adherence improvement.
We could find only one important study that, contrary to mainstream evidence, questioned the benefit of BB prescription in AMI secondary prevention.22 Two reasons may explain such discrepancy. First, the exposure assessment was less accurate because it was not based on adherence to prescribed therapy but on clinicians’ prescriptions. Therefore, the exposure could have been misclassified in patients poorly adhering to prescribed medication. A second explanation could be that the benefit of BBs decreases with time after the first AMI, as postulated in a recent commentary.23 If so, the longer follow‐up of the above‐mentioned study (43 months versus 12 months here) could explain the smaller benefit. Further research with SCSS methodology and longer follow‐up is needed to clarify this aspect.
The SCSS method requires some strict assumptions, however, all the supplementary analyses we did to test possible violations were reassuring. Moreover, the expected increase of compliance after the first recurrence – a possible source of bias – was minimal and not significant.
The assessment of exposure in this study was based on the collection of medication prescriptions. The use of automated pharmacy databases to assess the exposed time to drug therapy is well established in pharmacoepidemiological research.24 However, it may imply some inevitable inaccuracy, especially when coupled with the SCCS methodology, which is highly sensitive to the definitions of risk periods.6 First, the collection of a prescription at a pharmacy does not imply its regular consumption. Some patients were probably not taking their prescribed BBs during periods we classified as exposed, though this would result only in a reduced effect estimate for drug exposure. Second, the DDD, which we used to calculate the number of days covered by BB is a standard measure, while the actual dosage tailored by the prescribing physician to the individual patient may be different. This would cause some misclassification of exposed time. Because the resulting bias would be in opposite directions according to whether the dosage is higher or lower than DDD, it is likely to be non‐differential, ie, toward the null.
We found that, on average, the percentage of adherence was approximately 49% during the observation time. Low compliance with prescription medication is a recognized problem and our figure is even more disappointing than reported in previous literature. A recently published meta‐analysis25 found an adherence of 62% for BBs in AMI secondary prevention, but the definition of adherence was less stringent (ie, number of patients with at least 75% of days covered over a defined time). Moreover, because our study includes only individuals with at least one recurrence, they are likely to have a lower adherence than the entire post‐AMI population.
One potential limitation of our study is that we could estimate the effect of adherence to β‐blockers only. In periods of nonadherence to BBs, patients were presumably more likely to be unexposed also to other drugs usually recommended for AMI secondary prevention, causing an overestimation of the true effect. However, the risk modifications after crossovers of BB exposure are unlikely to match those of most of the other drugs usually recommended after AMI that have longer induction and effect periods (eg, statins, ASA, diuretics). Furthermore, the relative‐risk reductions brought about by single drugs are not just additive; for example, a recent study showed that the reduction of risk for major cardiovascular events after AMI is 19%, 25%, and 24% for statins, BBs, and ACE inhibitors, respectively, but it is only 36% for the combination of all 3.26
Intrapersonal covariance of adherence to BBs with adherence to other risk reducing health behaviors (eg, physical activity, smoking cessation) might also have overestimated the results. However, this potential bias should be minimized by the longer time required by the latter factors before affecting the risk.
Finally, case‐only design – as compared with the classic cohort or case‐control – in essence shift the question from “why me?” to “why now?,”27 which may imply subtle differences. For example, it is possible that people who experience AMI recurrences are systematically older and more severely diseased than the group without recurrences. If these characteristics were also significant effect modifiers, the SCSS risk estimates would not be automatically generalizable to the entire post‐AMI population.
In conclusion, our study showed that BB use was associated with a significant reduction of recurrent AMIs, in substantial agreement with previous literature. The use of the SCSS design that is based on intrapersonal comparisons and reduces interpersonal confounding should give more strength to the available evidence, previously weakened by the possibility of residual confounding.
Disclosures
None.
Acknowledgments
We are deeply thankful to Prof Paddy Farrington and Dr Heather Whitaker for their prompt, kind, and helpful replies to our emails.
References
- 1.World Health Organization. Global Status Report on Non‐Communicable Diseases 2010. Geneva, Switzerland: WHO; 2011. Available at: http://www.who.int/nmh/publications/ncd_report2010/en/. Accessed November 3, 2014. [Google Scholar]
- 2.Yusuf S, Wittes J, Friedman L. Overview of results of randomized clinical trials in heart disease. I. Treatments following myocardial infarction. JAMA. 1988; 260:2088-2093. [PubMed] [Google Scholar]
- 3.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005; 353:487-497. [DOI] [PubMed] [Google Scholar]
- 4.Zullig LL, Peterson ED, Bosworth HB. Ingredients of successful interventions to improve medication adherence. JAMA. 2013; 310:2611-2612. [DOI] [PubMed] [Google Scholar]
- 5.Bitton A, Choudhry NK, Matlin OS, Swanton K, Shrank WH. The impact of medication adherence on coronary artery disease costs and outcomes: a systematic review. Am J Med. 2013; 126:357.e7-357.e27. [DOI] [PubMed] [Google Scholar]
- 6.Dormuth CR, Patrick AR, Shrank WH, Wright JM, Glynn RJ, Sutherland J, Brookhart MA. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009; 119:2051-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self‐controlled case series method. Stat Med. 2006; 25:1768-1797. [DOI] [PubMed] [Google Scholar]
- 8.Nordmann S, Biard L, Ravaud P, Esposito‐Farèse M, Tubach F. Case‐only designs in pharmacoepidemiology: a systematic review. PLoS One. 2012; 7:e49444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perugini E, Maggioni AP, Boccanelli A, Di Pasquale G. Epidemiology of acute coronary syndromes in Italy. G Ital Cardiol (Rome). 2010; 11:718-729. [PubMed] [Google Scholar]
- 10.ATC/DDD Index. WHO Collaborating Centre for Drug Statistics Methodology; 2006. Available at: http://www.whocc.no/atcddd. Accessed November 3, 2014.
- 11.Maio V, Marino M, Robeson M, Gagne JJ. Beta‐blocker initiation and adherence after hospitalization for acute myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2011; 18:438-445. [DOI] [PubMed] [Google Scholar]
- 12.Farrington CP, Whitaker HJ, Hocine MN. Case series analysis for censored, perturbed or curtailed post‐event exposures. Biostatistics. 2009; 10:3-16. [DOI] [PubMed] [Google Scholar]
- 13.Farrington CP, Whitaker HJ. Semiparametric analysis of case series data (with discussion). Appl Stat. 2006; 55:553-594. [Google Scholar]
- 14.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004; 351:2611-2618. [DOI] [PubMed] [Google Scholar]
- 15.Douglas IJ, Smeeth L. Exposure to antipsychotics and risk of stroke: self controlled case series study. BMJ. 2008; 337:a1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher EJ, Viscoli CM, Horwitz RI. The relationship of treatment adherence to the risk of death after myocardial infarction in women. JAMA. 1993; 270:742-744. [PubMed] [Google Scholar]
- 17.Kleiner SA, Vogt WB, Gladowski P, DeVries A, Levin G, Antonucci C, Fong J. Beta‐blocker compliance, mortality, and reinfarction: validation of clinical trial association using insurer claims data. Am J Med Qual. 2009; 24:512-519. [DOI] [PubMed] [Google Scholar]
- 18.Soumerai SB, McLaughlin TJ, Spiegelman D, Hertzmark E, Thibault G, Goldman L. Adverse outcomes of underuse of beta‐blockers in elderly survivors of acute myocardial infarction. JAMA. 1997; 277:115-121. [PubMed] [Google Scholar]
- 19.Krumholz HM, Radford MJ, Wang Y, Chen J, Heiat A, Marciniak TA. National use and effectiveness of beta‐blockers for the treatment of elderly patients after acute myocardial infarction: National Cooperative Cardiovascular Project. JAMA. 1998; 280:623-629. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes AW, Madhavan SS, Amonkar MM. Evaluating the effect on patient outcomes of appropriate and inappropriate use of beta‐blockers as secondary prevention after myocardial infarction in a Medicaid population. Clin Ther. 2005; 27:630-645. [DOI] [PubMed] [Google Scholar]
- 21.Wei L, Flynn R, Murray GD, MacDonald TM. Use and adherence to beta‐blockers for secondary prevention of myocardial infarction: who is not getting the treatment? Pharmacoepidemiol Drug Saf. 2004; 13:761-766. [DOI] [PubMed] [Google Scholar]
- 22.Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto S, Ohman EM, Cannon CP, Smith SC, Zeymer U, Hoffman EB, Messerli FH, Bhatt DLREACH Registry Investigators. β‐Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012; 308:1340-1349. [DOI] [PubMed] [Google Scholar]
- 23.Danchin N, Laurent S. Are beta‐blockers truly helpful in patients with CAD? Nat Rev Cardiol. 2013; 10:11-12. [DOI] [PubMed] [Google Scholar]
- 24.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006; 15:565-574. [DOI] [PubMed] [Google Scholar]
- 25.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta‐analysis on 376,162 patients. Am J Med. 2012; 125:882-887. [DOI] [PubMed] [Google Scholar]
- 26.Choudhry NK, Glynn RJ, Avorn J, Lee JL, Brennan TA, Reisman L, Toscano M, Levin R, Matlin OS, Antman EM, Shrank WH. Untangling the relationship between medication adherence and post‐myocardial infarction outcomes: medication adherence and clinical outcomes. Am Heart J. 2014; 167:51-58. [DOI] [PubMed] [Google Scholar]
- 27.Maclure M. ‘Why me?’ versus ‘why now?’—differences between operational hypotheses in case‐control versus case‐crossover studies. Pharmacoepidemiol Drug Saf. 2007; 16:850-853. [DOI] [PubMed] [Google Scholar]


