Abstract
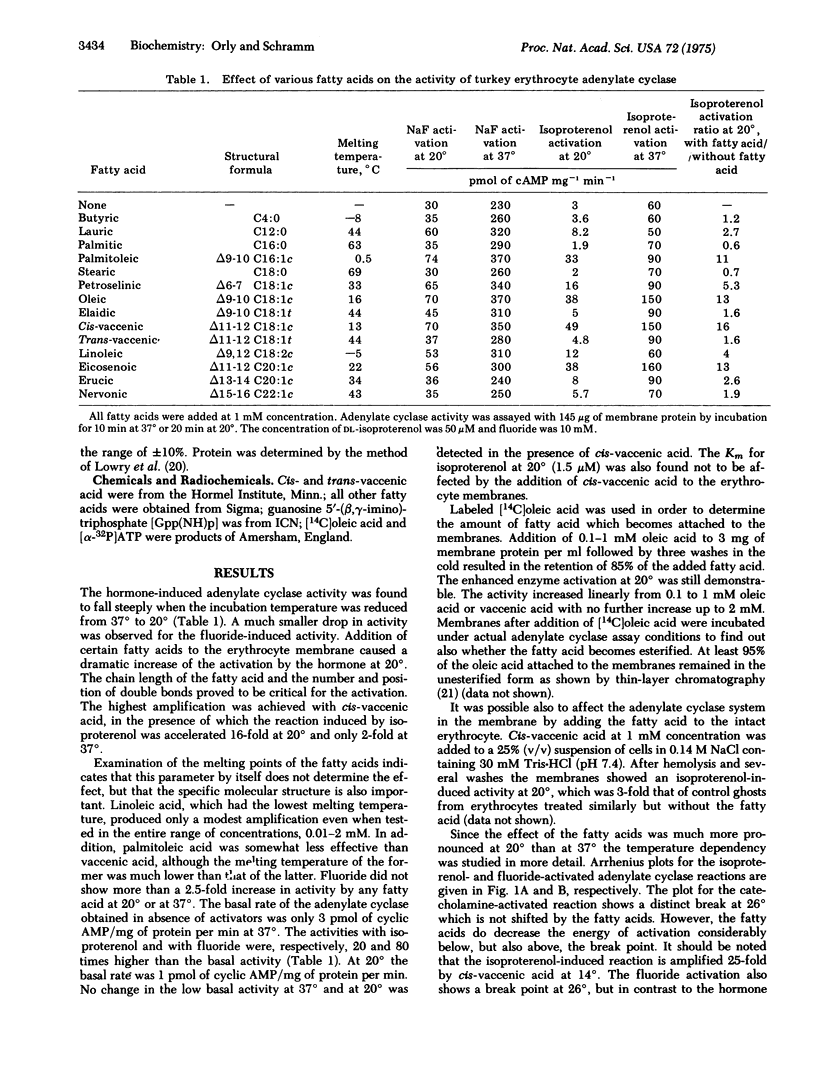
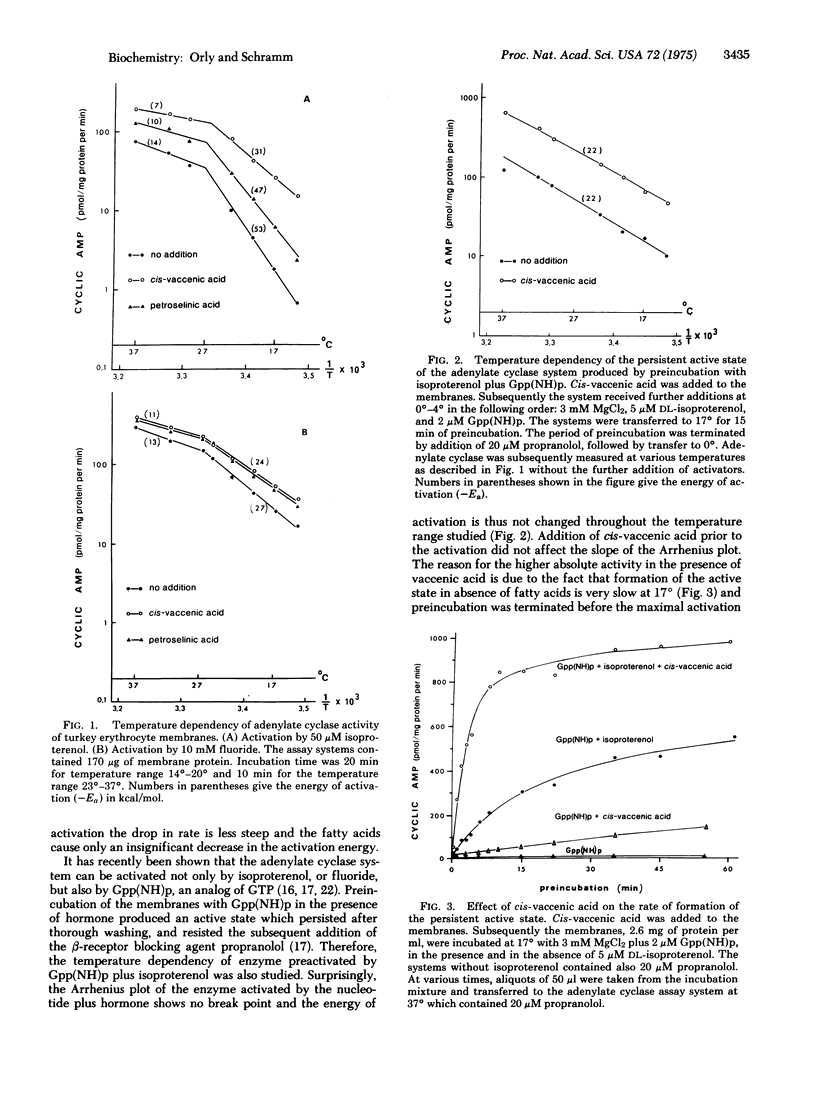
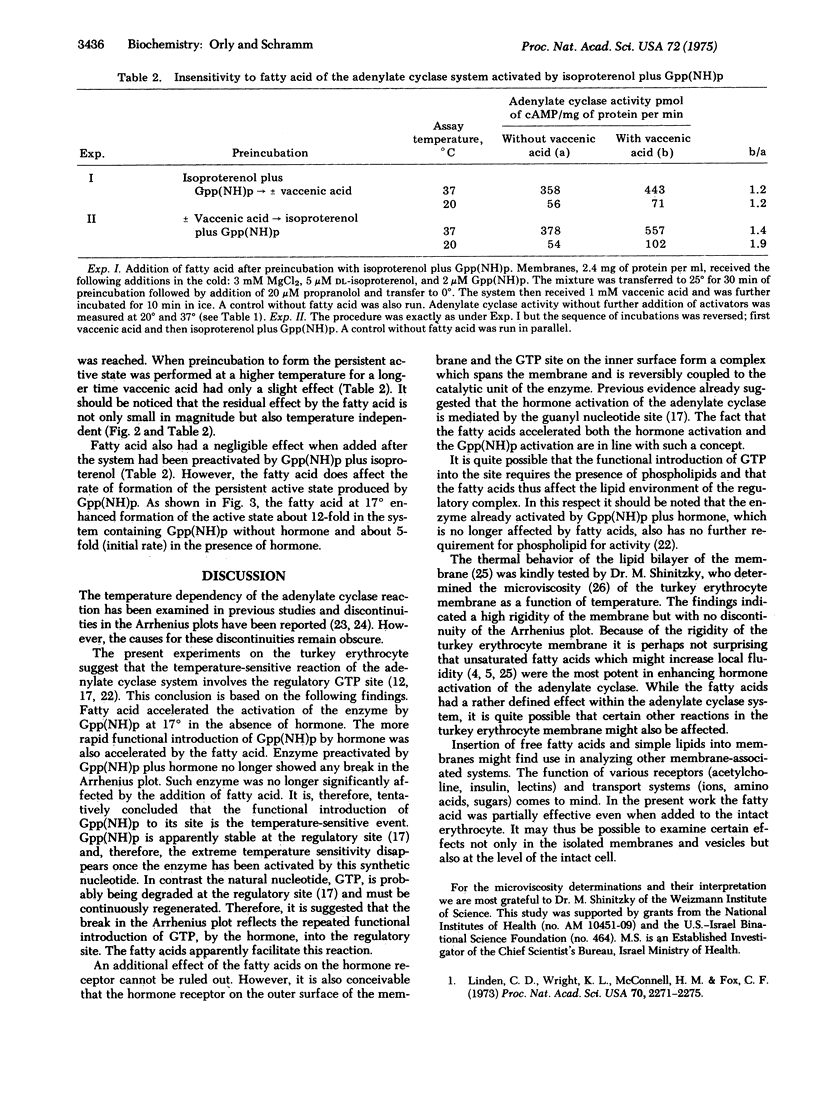
Activation of the adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1[ from turkey erythrocytes by isoproterenol decreased precipitously below 26 degrees. Certain unsaturated fatty acids enhanced the activation by isoproterenol up to 25-fold at reduced temperatures. The fatty acid also enhanced the formation of a persistent active state of the enzyme which was produced by preincubation with guanosine 5'-(beta,gamma-imino)triphosphate [Gpp(NH)p]. Once the enzyme had been activated by Gpp(NH)p plus isoproterenol the reaction rate was no longer as temperature sensitive and the fatty acid had little effect. The synthetic Gpp(NH)p apparently substituted for the natural GTP, which is known to play a regulatory role in the adenylate cyclase system. The findings suggest that the function of GTP which is mediated by the hormone is the temperature-sensitive event which is enhanced by the fatty acid. The use of free fatty acid to probe membrane-associated reactions in intact cells and in isolated membrane preparations is proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Fisher D., Tampion W., Lucy J. A. The fusion of erythrocytes by fatty acids, esters, retinol and alpha-tocopherol. Biochem J. 1973 Sep;136(1):147–155. doi: 10.1042/bj1360147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni B., Shinitzky M., Livne A. Dynamics of erythrocyte lipids in intact cells, in ghost membranes and in liposomes. Biochim Biophys Acta. 1974 Jun 26;348(3):438–441. doi: 10.1016/0005-2760(74)90223-9. [DOI] [PubMed] [Google Scholar]
- Horwitz A. F., Hatten M. E., Burger M. M. Membrane fatty acid replacements and their effect on growth and lectin-induced agglutinability. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3115–3119. doi: 10.1073/pnas.71.8.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner P. W., Keirns J. J., Bitensky M. W. A temperature-sensitive change in the energy of activation of hormone-stimulated hepatic adenylyl cyclase. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1785–1789. doi: 10.1073/pnas.70.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leray F., Chambaut A. M., Hanoune J. Role of GTP in epinephrine and glucagon activation of adenyl cyclase of liver plasma membrane. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1385–1391. doi: 10.1016/0006-291x(72)90866-2. [DOI] [PubMed] [Google Scholar]
- Levey G. S. Restoration of glucagon responsiveness of solubilized myocardial adenyl cyclase by phosphatidylserine. Biochem Biophys Res Commun. 1971 Apr 2;43(1):108–113. doi: 10.1016/s0006-291x(71)80093-1. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973 Jul 3;12(14):2625–2634. doi: 10.1021/bi00738a012. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Pohl S. L., Krans H. M., Kozyreff V., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. VI. Evidence for a role of membrane lipids. J Biol Chem. 1971 Jul 25;246(14):4447–4454. [PubMed] [Google Scholar]
- Raz A., Livne A. Differential effects of lipids on the osmotic fragility of erythrocytes. Biochim Biophys Acta. 1973 Jun 22;311(2):222–229. doi: 10.1016/0005-2736(73)90269-1. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rosen O. M., Rosen S. M. Properties of an adenyl cyclase partially purified from frog erythrocytes. Arch Biochem Biophys. 1969 May;131(2):449–456. doi: 10.1016/0003-9861(69)90417-2. [DOI] [PubMed] [Google Scholar]
- Rottem S., Cirillo V. P., de Kruyff B., Shinitzky M., Razin S. Cholesterol in mycoplasma membranes. Correlation of enzymic and transport activities with physical state of lipids in membranes of Mycoplasma mycoides var. capri adapted to grow with low cholesterol concentrations. Biochim Biophys Acta. 1973 Nov 16;323(4):509–519. doi: 10.1016/0005-2736(73)90159-4. [DOI] [PubMed] [Google Scholar]
- Rubalcava B., Rodbell M. The role of acidic phospholipids in glucagon action on rat liver adenylate cyclase. J Biol Chem. 1973 Jun 10;248(11):3831–3837. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schramm M., Eisenkraft B., Barkai E. Cold-induced leakage of amylase from the zymogen granule and sealing of its membrane by specific lipids. Biochim Biophys Acta. 1967 Feb 1;135(1):44–52. doi: 10.1016/0005-2736(67)90006-5. [DOI] [PubMed] [Google Scholar]
- Schramm M., Rodbell M. A persistent active state of the adenylate cyclase system produced by the combined actions of isoproterenol and guanylyl imidodiphosphate in frog erythrocyte membranes. J Biol Chem. 1975 Mar 25;250(6):2232–2237. [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Smolowe A. F., Sullivan R. C., Barclay M. Separation of lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1965 Oct 4;106(2):386–396. doi: 10.1016/0005-2760(65)90047-0. [DOI] [PubMed] [Google Scholar]
- de Kruyff B., van Dijck P. W., Godlbach R. W., Demel R. A., van Deenen L. L. Influence of fatty acid and sterol composition on the lipid phase transition and activity of membrane-bound enzymes in Acholeplasma laidlawii. Biochim Biophys Acta. 1973 Dec 22;330(3):269–282. doi: 10.1016/0005-2736(73)90232-0. [DOI] [PubMed] [Google Scholar]



