Abstract
Purpose
Nephrolithiasis has been increasingly reported in bottlenose dolphins, with all cases to date being ammonium urate nephrolithiasis.
Materials and Methods
A case-control study was conducted in dolphins with and without evidence of nephrolithiasis, aiming to identify biomarkers and risk factors associated with stone formation in a managed population. Dolphins were sampled in both fasting and postprandial states in order to study the effect of dietary factors on serum and urinary biochemistry. Urine was continuously collected over a 6-hr period via catheter and divided into three 2-hour collections, with a bolus fish meal given after completion of the first collection. Blood was sampled at the beginning of the fasting period and end of the postprandial period.
Results
There were no significant differences in serum and urine chemistries and acid base profiles between dolphins with and without stones, at baseline or postprandially, suggesting that case and control animals in this study represent a continuum of stone risk. In analyses combining the case and control dolphins in a single cohort, we noted significant postprandial increases in urinary uric acid, sulfate and net acid excretion, accompanied by increased urinary ammonium excretion and a commensurate rise in urine pH. The supersaturation index of ammonium urate increased postprandially by more than twofold.
Conclusion
These findings suggest that dolphins are susceptible to ammonium urate nephrolithiasis at least in part because a high dietary load of acid and purines results in a transient but marked increase in the urinary supersaturation of the sparingly soluble ammonium urate salt.
Keywords: ammonium urate, nephrolithiasis, risk factors
INTRODUCTION
Ammonium urate (NH4U) nephrolithiasis associated with morbidity has been reported in managed bottlenose dolphins (Tursiops truncatus), but research efforts evaluating the pathophysiological and physicochemical mechanisms of nephrolithiasis in dolphins have been limited 1, 2.
NH4U nephrolithiasis is rare in humans in the developed world, with up to 0.55% of stones containing predominantly NH4U 3. Clinical conditions associated with NH4U nephrolithiasis include diarrheal states due to inflammatory bowel disease, ileostomy, and laxative abuse 4-6. In these conditions, the relative abundance of urinary ammonium accompanied by decreased urinary sodium and potassium shifts the balance of urinary urate salts from the relatively soluble Na+-urate and K+-urate toward the sparingly soluble NH4U 7. The higher prevalence of NH4U nephrolithiasis in some developing countries in the present day, and historically in preindustrial Europe, has been attributed to a poor, cereal-based diet combined with inadequate fluid intake, as well as to recurrent untreated urinary tract infections 8. However, none of these conditions apply to dolphins with NH4U nephrolithiasis 1, 2.
No prior study in dolphins has specifically investigated the potential role of diet in NH4U stone risk. We hypothesized that bolus fish meals, naturally high in protein and purines 9, may predispose dolphins to stone formation through a combination of elevated urinary uric acid and physiologic amplification of urinary ammonium excretion 10. Furthermore, we hypothesized that the postprandial urinary milieu may be different in stone-forming versus non-stone forming dolphins. To test these hypotheses, we conducted a detailed study of 8 managed dolphins, including 4 with and 4 without evidence of kidney stones.
METHODS
Study population
Eight sexually mature bottlenose dolphins were selected for this study (2 male and 2 female stone formers; 2 male and 2 female body weight-matched non-stone formers). Non-stone-formers were defined as dolphins with no history of renal azotemia (BUN > 59mg/dL, Creatinine > 1.8mg/dL) within the past 10 years and no evidence of nephrolithiasis upon ultrasound examination at the time of the study. Stone-formers were defined as dolphins with a history of renal azotemia within the past 10 years and sonographic evidence of nephrolithiasis at the time of the study. The mean age was 29 years (range, 14-38 yrs), and the mean weight was 187kg (range, 155-251kg). All dolphins were housed in ocean enclosures and fed high-quality frozen-thawed fish, according to hazard analysis and critical control points guidelines for food inspection.
All study animals are owned and cared for by the U.S. Navy Marine Mammal Program. The Secretary of Navy Instruction 3900.41G directs that Navy marine mammals be provided the highest quality care. The Navy Marine Mammal Program is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and adheres to the national standards of the United States Public Health Service Policy on the Humane Care and Use of Laboratory Animals and the Animal Welfare Act. Animals included in this study were assigned an attending veterinarian specific to the project and samples were collected under an Animal Care and Use Protocol (#96-2011) approved by a Navy Institutional Animal Care and Use Committee and Navy Bureau of Medicine.
Study Protocol
On the day prior to sampling, study animals were fed their routine diets [Table 1] consisting of a mean of 7.1kg (range, 5.5-8.2kg) of frozen-thawed fish. Dolphins with kidney stones were receiving fresh water orally to prevent renal azotemia, therefore fresh water was administered via a small orogastric feeding tube (at 1% of body mass) to all study animals to eliminate hydration therapy as a confounding variable. After 12-hour overnight fast, all animals received light sedation with diazepam (0.12-0.20mg/kg orally) or midazolam (0.06-0.08mg/kg intramuscularly).
Table 1.
Macronutrient composition of morning meals fed to bottlenose dolphins (Tursiops truncatus)
| Nutrients | Total | Per kg body weight |
|---|---|---|
| Total calories (kcal) | 3,253 ± 732 | 188 ± 28 |
| Protein (g) | 453 ±132 | 2.5 ± 0.9 |
| Fat (g) | 184 ± 43 | 1.0 ± 0.3 |
| Carbohydrates (g) | 1 ± 1 | 0 |
Once sedate, animals were placed in right lateral recumbency. The genital slit was flushed with sterile water and surrounding skin cleaned with alcohol. An 8.5 French × 60cm multipurpose drainage catheter (Cook Medical, Bloomington, IN) was used for urinary catheterization of males. For females, either a 10.2 French × 45cm multipurpose drainage catheter (Cook Medical) or a 10 French × 90cm Foley catheter (MILA International, Erlanger, KY) was placed. The bladder was drained and an air-tight catheter collection bag was connected to the catheter for continuous urine collection. A baseline blood sample was immediately collected from a periarterial venous rete (PAVR) in either the caudal peduncle or a fluke blade using a Vacutainer® needle (Rutherford, NJ) or a butterfly needle attached to Vacutainer® holder, respectively.
Animals were placed in fleece-lined stretchers and suspended in water-filled containers for added comfort. Baseline fasting urine was collected from hours 0-2. Animals were then fed one-third of their daily ration of fish (range, 1.8-2.7kg) consisting of one-third herring and two-thirds capelin [Table 1]. Two 2-hour urine samples were collected postprandially from hours 2-4 and 4-6. At the completion of each sampling period, an aliquot of urine was collected in a 1-3cc syringe for pH and PCO2. The remainder of the urine was transported to the laboratory for processing on wet ice. A second blood sample was obtained at the end of the 6-hour study, and animals were returned to their ocean enclosures.
Imaging
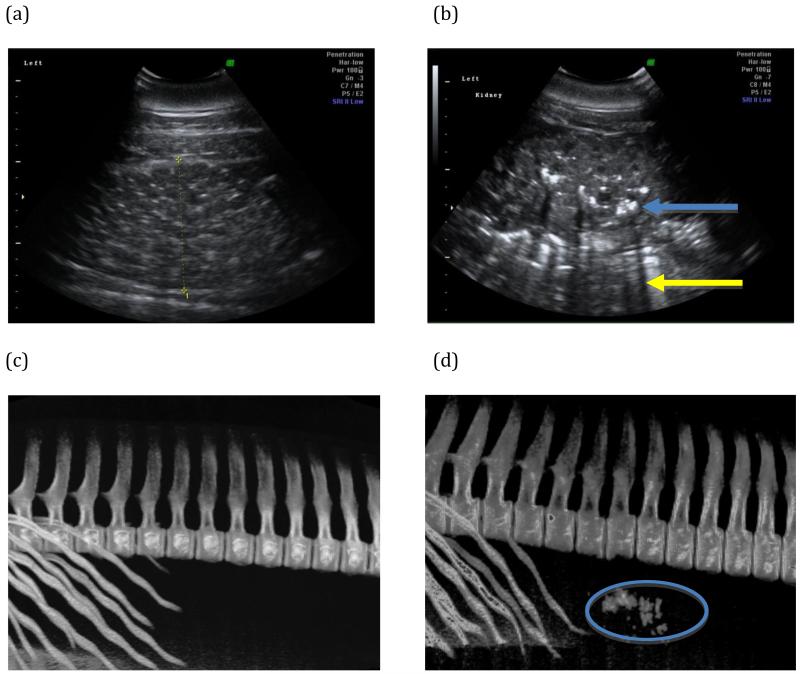
Renal ultrasound examinations were performed in real-time, B-mode either in water or on land. Portable ultrasound units (Voluson i, GE Healthcare, Waukesha, WI; and M-Turbo, Sonosite, Bothell, WA) with 2-5MHz variable frequency and curvilinear transducers were utilized. The GE ultrasound unit was equipped with Z800 video glasses (eMagin, Bellevue, WA) and the Sonosite unit with Cinemizer video glasses (Carl Zeiss, Oberkochen, Germany). Both kidneys were examined for evidence of nephrolithiasis in both dorsal and transverse planes. Nephrolithiasis was defined as hyperechoic foci within the renal parenchyma with distinct acoustic shadows [Figure 1].
Figure 1.
Representative renal images from study animals. (a) Dorsal plane renal sonogram of the left kidney from an animal with no stones. (b) Dorsal plane renal sonogram of the left kidney of an animal with a heavy stone burden. Hyperechoic foci (blue arrow) with acoustic shadowing (yellow arrow) are characteristic of calculi on ultrasound examination. (c) Representative three-dimensional reconstruction of CT data that include the caudal ribs and lumbar vertebral bodies, evaluated specifically for mineral density in the region of the kidneys. No mineral densities were detected in this non-stone former. (d) Numerous mineral densities in the kidneys were detected in the reconstructed CT data of a stone-forming dolphin (oval).
Three case dolphins were transported to the Naval Medical Center San Diego’s computed tomography (CT) facility [Figure 1]. Sedation was induced with an intramuscular dose of midazolam (0.04-0.08mg/kg), and dolphins were continuously monitored by veterinary personnel during the CT examination using GE Lightspeed RT16 helical CT scanner (GE Healthcare, Waukesha, WI). Non-contrast helical images were obtained during a normal prolonged end-inspiratory breath hold using contiguous 1.25 mm slices.
Analytical procedures and calculations
Urine processing
A NOVA Critical Care Xpress blood gas analyzer (Waltham, MA) was utilized to measure pH and PCO2 within 5 minutes of sample collection. Urine pH was measured by electrode within 30 minutes. The remainder of the urine was partitioned into aliquots, stored on ice, and used for the measurement of urinary sodium, potassium, calcium, magnesium, phosphorus, uric acid, oxalate, citrate, chloride, sulfate, ammonium (NH4+) and creatinine (Cr) using standard methods 11. Urinary titratable acidity (TA) and net acid excretion (NAE) were calculated as previously described 10, 12 ENREF 12. Urinary bicarbonate was calculated from urinary pH and PCO2 10.
Urinary supersaturation of stone forming salts (calcium oxalate, brushfire, uric acid and NH4+U) were calculated using the Joint Expert Speciation System (JESS) program 13.
Serum processing
For serum chemistry, blood samples were collected in serum separator tubes, chilled on ice for 30 minutes, centrifuged within 2 hours, and serum samples were sent on ice to Antech Diagnostics (San Diego, CA) for biochemistry. Insulin resistance was calculated from fasting glucose and insulin measurements using the homeostasis model assessment for insulin resistance (HOMA-IR)14.
Statistical analysis
Mixed linear model repeated measures analysis was conducted to compare pre-meal and post-meal measurements with dolphin modeled as a random effect. Pairwise comparisons were assessed by least square means contrasts derived from the mixed models. To compare responses between stone-formers and non-stone-formers, a between-group factor was included in the mixed linear model. Positively skewed variables were log-transformed prior to analysis. All tests were two-tailed with p<0.05 considered significant. Statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Imaging
Control animals had no evidence of nephrolithiasis by ultrasound (Figure 1a) or CT examination (Figure 1c). Nephrolithiasis was confirmed in all case animals by ultrasound (Figure 1b). Stone burden was categorized as mild (<5 stones), moderate (5-15 stones), or severe (>15 stones). Of the case animals, one had a moderate stone burden and three were severe. However, sonographic stone counts may underestimate stone burden, as the acoustic shadows created by nephrolithiasis may obscure any stones in the path of the shadow. CT confirmed stone burden in the three case animals examined (Figure 1d).
Serum and urine biochemical profiles in cases vs. controls
In analyses of case versus control dolphins, there were no significant differences in serum electrolytes, urate, creatinine, BUN, insulin and HOMA-IR, at baseline or after feeding. There were no significant differences in any measured or calculated urinary parameter, at baseline and postprandially, including urinary ammonium, urate, pH, net acid excretion and supersaturation index of NH4U.
Serum and urine biochemical profiles in the combined cohort
Serum biochemical profiles in fasting and postprandial states are shown in Table 2, and urinary biochemical, acid-base and physicochemical profiles are shown in Table 3.
Table 2.
Serum biochemical profiles. Data are presented as mean ± standard deviation. Statistical significance for Postprandial vs. Fasting is indicated by *p < 0.05 and **p < 0.01.
| Fasting (N = 8) |
2nd Postprandial (N = 8) |
|
|---|---|---|
|
|
||
| Na, meq/L | 154 ± 2 | 155 ± 3 |
| K, meq/L | 3.8 ± 0.3 | 4.0 ± 0.2* |
| Cl, meq/L | 118 ± 2 | 116 ± 2 |
| CO2, meq/L | 25 ± 4 | 25 ± 2 |
| BUN, mg/dL | 46 ± 8 | 54 ± 10* |
| Creatinine, mg/dL | 1.4 ± 0.2 | 1.5 ± 0.2** |
| BUN/Cr | 34.5 ± 8.1 | 36.6 ± 11.3 |
| Uric Acid, mg/dL | 0.3 ± 0.3 | 0.8 ± 0.4 |
| Ca, mg/dL | 8.9 ± 0.4 | 9.2 ± 0.6 |
| P, mg/dL | 4.6 ± 0.3 | 5.4 ± 0.8* |
| Mg, mg/dL | 1.7 ± 0.1 | 1.7 ± 0.1 |
| Protein, g/dL | 7.0 ± 0.5 | 7.2 ± 0.7 |
| Albumin, g/dL | 5.1 ± 0.1 | 5.2 ± 0.2 |
| Globulin, g/dL | 1.9 ± 0.5 | 2.0 ± 0.6 |
| Albumin/Globulin | 2.8 ± 0.6 | 2.8 ± 0.8 |
| Glucose, mg/dL | 118 ± 15 | 113 ± 15 |
| ALT, IU/L | 50 ± 16 | 49 ± 15 |
| AST, IU/L | 325 ± 127 | 275 ± 147 |
| Alkaline Phosphatase, IU/L | 255 ± 58 | 232 ± 94 |
| GGT, IU/L | 35 ± 11 | 35 ± 13 |
| Bilirubin, mg/dL | 0.1 ± 0.1 | 0.1 ± 0.1 |
| Cholesterol, mg/dL | 242 ± 57 | 242 ± 52 |
| Triglycerides, mg/dL | 108 ± 102 | 136 ± 38 |
| Insulin, mU/L | 2.4 ± 2.5 | 5.2 ± 5.0 |
| HOMA-IR | 0.33 ± 0.34 | 0.70 ± 0.67 |
Table 3.
Urinary biochemical, acid-base and physicochemical profiles. Data are presented as mean ± standard deviation. Statistical significance for Postprandial vs. Fasting is indicated by *p < 0.05, **p < 0.01 and †p < 0.001.
| Fasting (N = 8) |
1st Postprandial (N = 8) |
2nd Postprandial (N = 8) |
|
|---|---|---|---|
|
|
|||
|
Urinary Biochemical
Profiles | |||
| Total Volume, L/2hr | 0.211 ± 0.136 | 0.311 ± 0.193 | 0.379 ± 0.227** |
| Na, meq/2hr | 15 ± 11 | 24 ± 33 | 28 ± 48 |
| K, meq/2hr | 16 ± 8 | 19 ± 11 | 24 ± 13** |
| Ca, mg/2hr | 8 ± 12 | 16 ± 28 | 9 ± 17 |
| Mg, mg/2hr | 22 ± 23 | 33 ± 32 | 19 ± 23 |
| P, mg/2hr | 139 ± 94 | 155 ± 104 | 169 ± 131 |
| Uric Acid, mg/2hr | 33 ± 12 | 85 ± 48† | 164 ± 94† |
| Oxalate, mg/2hr | 9 ± 7 | 10 ± 8 | 9 ± 6 |
| Cl, meq/2hr | 28 ± 18 | 47 ± 37* | 49 ± 56 |
| Creatinine, mg/2hr | 341 ± 116 | 429 ± 120** | 403 ± 138* |
| FE Uric Acid | 0.63 ± 0.36 | 0.89 ± 0.53 | |
| FE HCO3 × 103 | 0.36 ± 0.14 | 1.53 ± 1.71* | |
| Urinary Acid Base Profiles | |||
| pH | 5.94 ± 0.15 | 6.16 ± 0.29* | 6.15 ± 0.31* |
| NH4+, meq/2hr | 13 ± 6 | 21 ± 13* | 24 ± 14** |
| Titratable Acidity, meq/2hr | 3.3 ± 2.3 | 3.2 ± 2.3 | 3.2 ± 2.1 |
| HCO3, meq/2hr | 0.2 ± 0.1 | 0.6 ± 0.6 | 1.1 ± 1.7 |
| Citrate, meq/2hr | 0.050 ± 0.031 | 0.063 ± 0.047 | 0.061 ± 0.053 |
| Net Acid Excretion, meq/2hr | 15.9 ± 8.4 | 23.1 ± 13.7* | 26.7 ± 13.8** |
| SO4, mmol/2hr | 9 ± 4 | 10 ± 5 | 14 ± 9** |
| NH4+/Total Cations, % | 27.9 ± 9.4 | 35.7 ± 17.1* | 37.0 ± 12.6* |
| Urinary Physicochemical Profiles | |||
| SI CaOx | 1.43 ± 1.13 | 1.81 ± 2.49 | 0.56 ± 0.60* |
| SI Brushite | 0.49 ± 0.58 | 0.50 ± 0.63 | 0.24 ± 0.33* |
| SI Uric Acid | 0.58 ± 0.18 | 0.69 ± 0.39 | 1.11 ± 0.52 |
| SI NH4 Urate | 6.04 ± 2.85 | 10.13 ± 3.37† | 15.17 ± 4.831† |
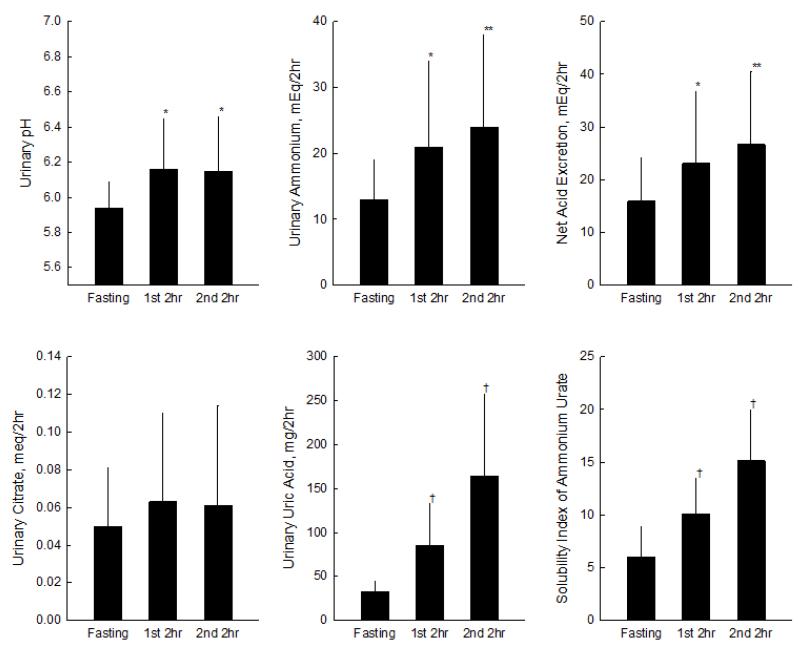
Compared with fasting, urinary uric acid in the final postprandial urine collection increased by approximately five-fold (P<0.001) [Table 3 and Figure 2]. There were also marked increases in urinary ammonium (P=0.002), pH (P=0.04), sulfate (P=0.001), and NAE (P=0.002) [Table 3 and Figure 2], but no significant changes in urinary titratable acidity, bicarbonate or citrate. Importantly for NH4U stone risk, the percentage of total urinary cations represented by ammonium (NH4+/total cations) increased significantly (P=0.01) after the meal [Table 3], and the supersaturation index (SI) of NH4U more than doubled (P<0.0001) [Table 3 and Figure 2].
Figure 2.
Urinary biochemical and physiochemical profiles. Data presented as mean ± standard deviation. Statistical significance from Fasting indicated by * p < 0.05, ** p < 0.01 and † p < 0.001.
While differences from fasting were numerically greatest in the final postprandial urine collection, the postprandial rise in urinary uric acid, ammonium, pH, NAE, NH4+/total cations and SI NH4U was statistically significant as early as during the first 2 hours after the meal [Table 3 and Figure 2].
DISCUSSION
There are several key findings from this study of eight well-characterized dolphins from a managed population. First, the high acid and purine load provided by bolus fish feeding resulted in a marked postprandial increase in urinary ammonium and uric acid, with a corresponding increase in the supersaturation of NH4U. We postulate that such transient postprandial spikes in NH4U supersaturation are a key contributor to NH4U stone risk in managed dolphins. Second, there were no differences in serum and urinary parameters between dolphins with and without kidney stones, in both fasting and postprandial states, suggesting that all animals in the study represent a biochemical and physicochemical continuum, with other unmeasured factors (such as putative promoters and inhibitors of stone formation) also contributing to stone risk. Finally, to the best of our knowledge, this is the first study to describe in detail the effects of feeding on serum and urine biochemical and physicochemical parameters in bottlenose dolphins.
Comparison with human physiology and pathophysiology
Numerous human studies have examined the physiological and physicochemical effects of high animal protein diets alone, and of a combined high protein and low carbohydrate diet 10, 15-18. The metabolism of sulfur-rich dietary animal protein can confer a notable endogenous acid load through oxidation of sulfur to sulfate, generating excess protons 19. Previous studies have shown that such diets increase the risk of kidney stone formation from hypercalciuria, hyperuricosuria, hypocitraturia, and high urinary acidity; all of these factors increase the predisposition for crystallization of calcium oxalate and uric acid 20, 21. A recent study in uric acid stone formers has shown that urinary pH fell following lunch and dinner without notable alterations in urinary ammonium excretion rate 11. In contrast, the present study showed a significant postprandial rise in urinary ammonium in dolphins, likely driven by the dietary acid load, as evidenced by the significant postprandial rise in urinary sulfate. The amplified ammonium response allowed the study dolphins to effectively buffer secreted protons and maintain acid-base balance, effectively raising urinary pH (because of the increased buffer capacity represented by urinary ammonium, with a pKa of 9.2) 22, 23. Such increase in urinary net acid excretion accompanied by a rise in urinary pH attributable to robust increase in ammonium excretion has also been described in humans subjected to experimental chronic NH4Cl loading 22. This robust ammonium excretion was associated with an increased fraction of NH4+/ total cations, and with a significant rise in the supersaturation of NH4U, from 6.0±2.9 to 15.2±4.8. For comparison, SI NH4U in one study of non-stone forming humans fed a standard metabolic diet was 1.5±0.9 (unpublished data). The preponderance of urinary ammonium over other urinary cations in the study dolphins is analogous to conditions reported in humans with NH4U stones 4-6, 24.
Physicochemical considerations
Previous studies have shown that high urinary levels of both ammonium and urate ions are essential for the precipitation of NH4U25,26. The NH4U salt is the most insoluble urate species in the urinary environment. The thermodynamic solubility product of NH4U of 0.36×10−5M2 is 8 times lower than that of sodium urate (2.79×10−5M2) and 27 times lower than that of potassium urate (9.63×10−5M2)7. Moreover, an experimental physicochemical study showed that when urate levels are kept constant with varying pH, a lower amount of ammonium is needed for precipitation at higher pH values 25. Postprandial changes in urinary biochemistry demonstrated in this study, including significant rises in urinary ammonium, pH and uric acid, likely underpin the enhanced propensity for NH4U stone formation in dolphins.
Potential roles of diet composition and feeding patterns
The incidence, prevalence and composition (if applicable) of kidney stones in wild dolphins are unknown. We have recently reported that none of 39 studied free-ranging dolphins from Sarasota Bay, Florida had nephrolithiasis, but these animals were significantly younger than managed dolphins with stones 27. We have also reported that managed dolphins are more likely to have hypocitraturia 2, possibly reflective of dietary differences between managed and free-ranging populations. Managed dolphins eat 3-4 meals per day of high-quality, frozen-thawed fish and squid from several species, while free-ranging dolphins feed more frequently and prey on a wider range of marine species 2, 27. Further studies are imperative to establish whether these differences in dietary composition and feeding patterns contribute to stone risk in managed dolphins.
Potential role of uricase
In most mammals, ingested purines are converted to uric acid and then further to allantoin through a series of enzymatic reactions that occur primarily in the peroxisomes of hepatic cells ENREF 28. Humans and higher primates lack functional uricase, the enzyme that catalyzes conversion of uric acid to allantoin, thus making uric acid the final product of purine metabolism in these species 28. Furthermore, some monkeys harbor mutations in the promoter region of uricase, and have functional uricase but reduced uricase activity compared with other mammals 29. In this study, we noted a marked postprandial increase in urinary uric acid in dolphins, without changes in the fractional excretion (and thus tubular reabsorption) of uric acid, suggesting that high purine loads may overwhelm hepatic uricase. Future studies of uricase gene structure and activity in dolphins, including contemporaneous measurement of serum and urinary uric acid and allantoin, are imperative.
Strengths and limitations
This was the first study to examine the effects of diet on serum and urine parameters and kidney stone risk in dolphins. Key strengths include the well-characterized study subjects (including high-quality clinical imaging), comprehensive biochemical measurements, controlled feeding, and accurately timed, complete urine collections obtained via catheterization. There are also several limitations, mostly inherent to the difficulty of conducting such a detailed study in large ocean-dwelling mammals. First, while the lack of differences between case and control dolphins may imply that cases and controls are both at risk for nephrolithiasis, it could also be attributable to the small sample size. Second, study conditions including fluid intake and activity level may not be representative of the conditions that existed in study dolphins during stone formation, and also not representative of conditions in the wild. Third, another potential mechanism for the postprandial rise in urinary pH is the phenomenon of postprandial alkaline tide, characterized by a simultaneous rise in serum and urine pH following gastric acid secretion 30. We have shown that fractional urinary bicarbonate excretion increased postprandially in dolphins, but this change was not associated with a rise in serum CO2 concentration. It is possible that the lack of more frequent blood sampling may have precluded our ability to detect simultaneous changes in serum CO2 and urinary pH. Finally, although urinary supersaturation is an important physicochemical parameter, it is not singularly sufficient to predict stone risk. The balance between potential urinary promoters and inhibitors of stone formation may play an important role in NH4U stone formation, and may differ between case and control dolphins. However, although predicted to exist, no inhibitors or promoters of NH4U precipitation have been identified to date. Future research in this area is of vital importance.
CONCLUSION
The present study clearly demonstrates that bolus fish feeding results in a marked increase in urinary ammonium, uric acid and pH in dolphins, creating a urinary milieu that is favorable for the precipitation of the sparingly soluble ammonium urate salt. However, the apparent lack of biochemical and physicochemical differences between animals with and without stones supports the hypothesis that supersaturation of ammonium urate is not the sole determinant of ammonium urate stone formation, and that potential promoters and inhibitors of crystallization may also play a role. These findings constitute a firm foundation for future research aimed at identifying such promoters and inhibitors, investigating the role and activity of uricase, and devising logical strategies for the treatment and prevention of stone formation in dolphins.
ACKNOWLEDGEMENTS
The authors are grateful to the Office of Naval Research for funding this work (ONR Award N000141110203) and to the Navy Marine Mammal Program for their support. Drs. Sakhaee and Bobulescu were supported by NIH grants R01-DK081423 and K01-DK090282. Special thanks to Sacha Stevenson, Celeste Parry, Mark Baird, Veronica Cendejas, Dr. John Traversi, and Dr. Carolina Le-Bert for critical veterinary and technical support, to Kathy Hill, Paulette Padalino, and Kevin Carlin for laboratory support, and to Ashlei L. Johnson for editorial assistance. Elaine Allen, Brenda Bauer, and Druann Price provided innovative solutions for collecting urine via catheter under voluntary control in the dolphin. Randall Dear was instrumental in ensuring proper behavioral conditioning of animals to facilitate sampling and provided technical oversight for animal procedures.
Footnotes
Publisher's Disclaimer: DISCLAIMER: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
References
- 1.Venn-Watson S, Smith CR, Johnson S, et al. Clinical relevance of urate nephrolithiasis in bottlenose dolphins Tursiops truncatus. Dis Aquat Organ. 2010;89(2):167–77. doi: 10.3354/dao02187. [DOI] [PubMed] [Google Scholar]
- 2.Venn-Watson SK, Townsend FI, Daniels RL, et al. Hypocitraturia in common bottlenose dolphins (Tursiops truncatus): assessing a potential risk factor for urate nephrolithiasis. Comp Med. 2010;60(2):149–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Daudon M, Donsimoni R, Hennequin C, et al. Sex- and age-related composition of 10 617 calculi analyzed by infrared spectroscopy. Urological research. 1995;23(5):319–26. doi: 10.1007/BF00300021. [DOI] [PubMed] [Google Scholar]
- 4.Leaf DE, Bukberg PR, Goldfarb DS. Laxative abuse, eating disorders, and kidney stones: a case report and review of the literature. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;60(2):295–8. doi: 10.1053/j.ajkd.2012.02.337. [DOI] [PubMed] [Google Scholar]
- 5.Soble JJ, Hamilton BD, Streem SB. Ammonium acid urate calculi: a reevaluation of risk factors. J Urol. 1999;161(3):869–73. doi: 10.1016/s0022-5347(01)61794-4. [DOI] [PubMed] [Google Scholar]
- 6.Buyukgebiz B, Arslan N, Ozturk Y, et al. Complication of short bowel syndrome: an infant with short bowel syndrome developing ammonium acid urate urolithiasis. Pediatr Int. 2003;45(2):208–9. doi: 10.1046/j.1442-200x.2003.01689.x. [DOI] [PubMed] [Google Scholar]
- 7.Pak C, Holt K, Britton F, Peterson R, Crother C, Ward D. Assesment of pathogenetic role of uric acid, monpotassium urate, monoammonium urate, monosodium urate in hyperuricosuric calcium oxalate nephrolithiasis. Miner Electrolyte Metab. 1980;4:130–6. [Google Scholar]
- 8.Klohn M, Bolle JF, Reverdin NP, et al. Ammonium urate urinary stones. Urological research. 1986;14(6):315–8. doi: 10.1007/BF00262382. [DOI] [PubMed] [Google Scholar]
- 9.Venn-Watson SK, Ridgway SH. Big brains and blood glucose: common ground for diabetes mellitus in humans and healthy dolphins. Comp Med. 2007;57(4):390–5. [PubMed] [Google Scholar]
- 10.Reddy ST, Wang CY, Sakhaee K, et al. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002;40(2):265–74. doi: 10.1053/ajkd.2002.34504. [DOI] [PubMed] [Google Scholar]
- 11.Cameron M, Maalouf NM, Poindexter J, et al. The diurnal variation in urine acidification differs between normal individuals and uric acid stone formers. Kidney Int. 2012;81(11):1123–30. doi: 10.1038/ki.2011.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kok DJ, Poindexter J, Pak CY. Calculation of titratable acidity from urinary stone risk factors. Kidney Int. 1993;44(1):120–6. doi: 10.1038/ki.1993.221. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers A, Allie-Hamdulay S, Jackson G. Therapeutic action of citrate in urolithiasis explained by chemical speciation: increase in pH is the determinant factor. Nephrol Dial Transplant. 2006;21(2):361–9. doi: 10.1093/ndt/gfi211. [DOI] [PubMed] [Google Scholar]
- 14.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 15.Ball D, Maughan RJ. Blood and urine acid-base status of premenopausal omnivorous and vegetarian women. Br J Nutr. 1997;78(5):683–93. doi: 10.1079/bjn19970187. [DOI] [PubMed] [Google Scholar]
- 16.Lemann J., Jr. Relationship between urinary calcium and net acid excretion as determined by dietary protein and potassium: a review. Nephron. 1999;81(Suppl 1):18–25. doi: 10.1159/000046294. [DOI] [PubMed] [Google Scholar]
- 17.Breslau NA, Brinkley L, Hill KD, et al. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. The Journal of clinical endocrinology and metabolism. 1988;66(1):140–6. doi: 10.1210/jcem-66-1-140. [DOI] [PubMed] [Google Scholar]
- 18.Sakhaee K, Maalouf NM, Sinnott B. Clinical review. Kidney stones 2012: pathogenesis, diagnosis, and management. The Journal of clinical endocrinology and metabolism. 2012;97(6):1847–60. doi: 10.1210/jc.2011-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tschope W, Ritz E. Sulfur-containing amino acids are a major determinant of urinary calcium. Miner Electrolyte Metab. 1985;11(3):137–9. [PubMed] [Google Scholar]
- 20.Robertson WG, Heyburn PJ, Peacock M, et al. The effect of high animal protein intake on the risk of calcium stone-formation in the urinary tract. Clin Sci (Lond) 1979;57(3):285–8. doi: 10.1042/cs0570285. [DOI] [PubMed] [Google Scholar]
- 21.Fellstrom B, Danielson BG, Karlstrom B, et al. The influence of a high dietary intake of purine-rich animal protein on urinary urate excretion and supersaturation in renal stone disease. Clin Sci (Lond) 1983;64(4):399–405. doi: 10.1042/cs0640399. [DOI] [PubMed] [Google Scholar]
- 22.Madison LL, Seldin DW. Ammonia excretion and renal enzymatic adaptation in human subjects, as disclosed by administration of precursor amino acids. The Journal of clinical investigation. 1958;37(11):1615–27. doi: 10.1172/JCI103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wrong O, Davies HE. The excretion of acid in renal disease. Q J Med. 1959;28(110):259–313. [PubMed] [Google Scholar]
- 24.Dick WH, Lingeman JE, Preminger GM, et al. Laxative abuse as a cause for ammonium urate renal calculi. J Urol. 1990;143(2):244–7. doi: 10.1016/s0022-5347(17)39923-8. [DOI] [PubMed] [Google Scholar]
- 25.Bowyer RC, McCulloch RK, Brockis JG, et al. Factors affecting the solubility of ammonium acid urate. Clin Chim Acta. 1979;95(1):17–22. doi: 10.1016/0009-8981(79)90331-0. [DOI] [PubMed] [Google Scholar]
- 26.Teotia M, Sutor DJ. Crystallisation of ammonium acid urate and other uric acid derivatives from urine. Br J Urol. 1971;43(4):381–6. doi: 10.1111/j.1464-410x.1971.tb12056.x. [DOI] [PubMed] [Google Scholar]
- 27.Smith CR, Venn-Watson S, Wells RS, et al. Comparison of Nephrolithiasis Prevalence in Two Bottlenose Dolphin (Tursiops truncatus) Populations. Frontiers in endocrinology. 2013;4:145. doi: 10.3389/fendo.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Advances in chronic kidney disease. 2012;19(6):358–71. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oda M, Satta Y, Takenaka O, et al. Loss of urate oxidase activity in hominoids and its evolutionary implications. Molecular biology and evolution. 2002;19(5):640–53. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 30.Niv Y, Asaf V. Abolition of postprandial alkaline tide in arterialized venous blood of duodenal ulcer patients with cimetidine and after vagotomy. Am J Gastroenterol. 1995;90(7):1135–7. [PubMed] [Google Scholar]