Abstract
There is a significant body of evidence demonstrating that radiation therapy (XRT) enhances the effect of immune therapy. However, the precise mechanisms by which XRT potentiates the immunotherapy of cancer remain elusive. Here, we report that XRT potentiates the effect of immune therapy via induction of autophagy and resultant trafficking of mannose-6-phopsphate receptor (MPR) to the cell surface. Irradiation of different tumor cells caused substantial up-regulation of MPR on the cell surface in vitro and in vivo. Down-regulation of MPR in tumor cells with shRNA completely abrogated the combined effect of XRT and immunotherapy (CTLA4 antibody) in B16F10-bearing mice without changes in the tumor-specific responses of T cells. Radiation-induced MPR up-regulation was the result of redistribution of the receptor to the cell surface. This effect was caused by autophagy with redirection of MPR to autophagosomes in a clathrin-dependent manner. In autophagosomes, MPR lost its natural ligands, which resulted in subsequent trafficking of empty receptor(s) back to the surface. Together, our data demonstrated a novel mechanism by which XRT can enhance the effect of immunotherapy and the molecular mechanism of this process.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1573-4) contains supplementary material, which is available to authorized users.
Keywords: Radiation therapy, Immune therapy, CTLs, Autophagy, Melanoma
Introduction
Multiple strategies to induce antitumor immune responses have shown promise in preclinical studies, but few have been successfully brought to the clinic [1–3]. These applications have mainly been limited to immunogenic tumors like melanoma or renal cell carcinoma and, even then, have benefited only a subset of patients. In this context, combining immunotherapy with conventional chemotherapy or radiation therapy (XRT) appears as an attractive option.
Ionizing radiation and chemotherapeutic agents have been traditionally considered immunosuppressive [4–7]. Surprisingly, during a number of phase I and II trials, the clinical benefits of combining chemotherapy with immunotherapy have been noted and are now being tested in clinical settings [8, 9]. Modern XRT is highly conformal to the tumor site, and although some evidence indicates a tolerogenic effect of XRT [10, 11], localized radiation is generally anticipated to avoid systemic immune suppression. Efforts to combine XRT with immunotherapy in preclinical and clinical studies include intratumoral administration of DCs, treatment with CTLA-4, PD-L1 or CD137 antibody, TLR agonists, or tumor vaccines [12–19]. The reduced therapeutic efficacy of XRT in mice deficient in the normal T cell repertoire has long suggested the critical role of T cells in such antitumor responses [20]. Activation of these tumor-specific T cells requires transfer of antigens from dying tumor cells to DCs [21, 22]. Macrophages expressing iNOS have also been implicated in orchestrating CTL activity in the tumor microenvironment following ionizing radiation [23]. The concept of immunogenic cell death from various classes of chemotherapy and ionizing radiation has been proposed [24, 25]. Release of damage-associated molecular pattern (DAMP) molecules by ionizing radiation has been associated with such immunogenic apoptosis [25, 26].
However, it remains unclear how chemotherapy and XRT could potentiate the effect of immune therapy in real setting of immune-suppressive environment often associated with conventional therapy. We have recently suggested a novel mechanism by which chemotherapy could sensitize tumor cells to CTLs mediated by the cation-independent mannose-6-phosphate receptor (MPR) [27, 28]. MPR is responsible for the binding and uptake of various mannose-6-phosphate-containing molecules. One of them is insulin-like growth factor II (IGFII), hence the other name of the receptor—insulin-like growth factor II receptor (IGFIIR). MPR shuttles its cargo (ligands) between the cell surface membrane and intracellular compartments where it transports the ligand–receptor complex from the trans-golgi network (TGN) to endosomes [29]. In addition, MPR can bind to granzyme B (GrzB) released by CTLs [30–32]. Up-regulation of MPR on the tumor cell surface by chemotherapy renders tumor cells more susceptible to CTL killing by making the tumor cells permeable to GrzB produced by activated CTLs [27, 28]. Moreover, this bypasses the strict requirement for CTL recognition of specific antigens on all tumor cells. This is distinct from other described mechanisms as it does not rely on enhanced immunogenicity for potentiating immunotherapy and sensitizes tumor cells not expressing specific antigens to the effector T cells. Up-regulation of MPR in response to chemotherapy has been associated with autophagy. However, the molecular mechanism of this effect remains unclear. Ionizing radiation has previously been demonstrated to elicit autophagy in multiple types of tumors [33, 34]. If our concept is correct, up-regulation of MPR should represent the universal mechanism by which conventional cytotoxic therapy of cancer synergizes with immune therapy, and XRT should cause an effect similar to that of chemotherapy. In this study, we tested this hypothesis and tried to elucidate the mechanism of MPR up-regulation.
Materials and methods
Mice and tumor models
Animal protocols were approved by the University of South Florida Animal Care and Use Committee. Female C57BL/6J (B6, H-2b) mice were purchased from the NCI (Frederick, MD). Pmel transgenic mice that carry T cell receptor specific for the mouse gp100-derived peptide were obtained from The Jackson Laboratory (Bar Harbor, ME). Murine B16F10 melanoma, mammary carcinoma 4T1, and Lewis lung cancer (LLC) cells were purchased from the American Type Culture Collection (ATCC) after 2009. The cells were maintained in RPMI 1640 or DMEM media containing 10 % FBS.
Reagents
Antibodies against cation-independent MPR, clathrin heavy chain, and LAMP2 were purchased from Abcam. CD45-PE, CD4-PerCP, CD8α-PE, and antirabbit IgG-Alexa647 antibodies were obtained from BD Bioscience. 3MA was purchased from Sigma. Cytosolic and membrane protein fractions were extracted using the Qproteome Cell Compartment kit (Qiagen). CTLA4 antibody was purchased from BioXcell.
Transfection of cells
B16F10 cells were stably transfected with control short hairpin RNA (shRNA), mpr, atg5, or beclin1 shRNA vectors incorporating the puromycin resistance gene for subsequent selection (Mission, Sigma-Aldrich) using a Geneporter 2 kit (Genlantis). B16F10 cells were transfected with clathrin siRNA using the Nucleofector Kit C (Lonza; Program X-05 on Amaxa) and 30 nmol/L of different clathrin siRNAs or with 30 nmol/L of scrambled siRNA. The cells were resuspended in Dulbecco’s Modified Eagle’s Media medium and rested for 48 h before treatment with ionizing radiation or paclitaxel (taxol, TAX).
Detection of MPR
Cell surface MPR was detected by flow cytometry as described earlier [28]. Dead cells were gated out from the live population by FSC/SSC profile and 7AAD staining.
Treatment protocol
C57BL/6 mice were inoculated with 0.5 × 106 tumor cells/mouse in the hindlimb. After 14 days, the tumors were measured and mice were randomized to the treatment groups. Mice were treated 3 times with 100 µg CTLA4 mAb every other day. The day after the first injection of CTLA4 mAb, the hindlimb was treated with 15 Gy (in vivo assay for combined radiation therapy and CTLA4 abrogation) or 10 Gy to 30 Gy (in vivo assay for MPR staining) of X-ray radiation with 320 kV photons using X-RAD orthovoltage X-ray machine from Precision X-ray Inc. The rest of the body was shielded with lead.
Western blotting
Cells were lysed, and samples (40 μg protein per lane) were subjected to electrophoresis on 7 % (MPR), 10 % (clathrin), or 12 % (LC3) SDS-polyacrylamide gel followed by transfer to a polyvinylidene difluoride membrane. Membranes were blocked overnight with 5 % BSA and then incubated with appropriate primary antibodies overnight at 4 °C followed by incubation with antigoat IgG HRP-conjugated antibody (Santa Cruz) for 1 h at room temperature. The bands were visualized by ECL Western plus kit (GE healthcare). To confirm equal loading of protein, the membrane were also probed for cadherin, tubulin, or β-actin.
Confocal microscopy
B16F10 melanoma cells were grown on the cover slip, and once adherent, cells were treated with 20–40 Gy ionizing radiation, washed, and cultured in vitro for 24 h. Cells were fixed with 4 % paraformaldehyde for 30 min, blocked with 10 % goat serum for 30 min, and then labeled with primary MPR antibody, followed by goat antirabbit Alexa 647 antibody (invitrogen). The cells were imaged with a Leica TCS SP5 laser scanning confocal microscope through a 63X/1.40NA Plan Apochromat oil immersion objective lens (Leica Microsystems). Diode laser lines of 405 and 555 nm were applied to excite the samples. An acousto-optical beam splitter was used to collect peak emission photons sequentially to minimize cross talk between fluorochromes.
CTL assay
The CFSE CTL assay was performed as previously described [52]. Briefly, B16F10 control shRNA and mpr shRNA cells were either left untreated or radiated with 20 Gy. The following day, control shRNA cells were labeled with 10 µM CFSE and mpr shRNA cells were labeled with 1 µM CFSE. 2 × 105 control shRNA and mpr shRNA cells were mixed in 1:1 ratio and cultured with 6 × 105 activated effector cells (Pmel splenocytes) for 6 h. The cells were then stained with DAPI and CD45 PE and analyzed on LSRII flow cytometer. Activated effector cells were prepared by incubating splenocytes from Pmel transgenic mice with 1 µM peptide (KVPRNQDWL) for 72 h.
Clonogenic assay
The clonogenic assay was performed as follows. B16F10 were stably transfected with shRNA for MPR or control shRNA were suspended in DMEM containing 10 % fetal bovine serum in a concentration of 10.000 cells/mL in 6-well plates. Test wells were exposed to 20 Gy external radiation (XRT) in triplicates using a MARK-1 Ce137 irradiator. Immediately after, 10.000, 1.000, 100, and 10 cells were plated per well in complete growth media. After 10 days, colonies visible in the wells were stained with crystal violet and counted using Image Pro 7.0 software. The surviving factor (SF) was calculated according to the formula SF = no. of colonies formed after treatment/(no. of cells seeded × PE) where PE = (no. of colonies formed/no. of cells seeded) × 100 %.
Scatchard assay
Human U266 cells were either left untreated or treated overnight with 25nM doxorubicin. Next day, the cells were washed and incubated on ice with serial dilutions of human [125I] IGF-II (Perkin Emler, cat # NEX429005UC) starting with 2.63 nM. To measure non-specific binding, 200-fold molar excess of unlabeled IGF-II were used with three different concentrations of labeled [125I] IGF-II. Cells were incubated at 4 °C for 4 h to achieve equilibrium. The supernatants from each tube were collected and added to CytoScint (MP Biomedical) for measurement of unbound radioactivity. Cells were resuspended in CytoScint for counting bound radioactivity to the cells. Samples were counted on gamma counter (Perkin Elmer) and data analyzed using Scatchard plot analysis. Since the non-treatment group had a very low ratio of bound molecules to the concentration of unbound IGF-II, we had to combine the results of 6 different experiments using inverse weighting given IGF-II (nM), the general form is, . Where the index i is equal to the trial, index j is equal to the IGF-II (nM) concentration, and index k is equal to the current raw data type. In this case, n is equal to 6 and m is equal to 5. The inverse weight is represented with w ijk and is equal to one over its variance () given the index ijk. We can determine the variance because the data collected are count data, meaning it has a poison distribution with the mean equal to the variance. And, x ijk is the count (mean) of the raw data type at the current trial IGF-II (nM) concentration. Inverse weighting results in the new variable y jk which is used for the Scatchard plot analysis. For the treatment group, the ratio of bound molecules to the concentration of unbound IGF-II was sufficiently high that we could perform calculations without preprocessing the data with inverse weighting.
Statistical analysis
Statistical analysis was conducted using a 2-tailed Student’s t test and GraphPad Prism 5 software (GraphPad Software Inc.), with significance determined at p < 0.05. Analysis of tumor growth curves was conducted, using a 2-way ANOVA test with a Bonferroni posttest.
Results
Radiation therapy potentiates the effect of immunotherapy via up-regulation of MPR
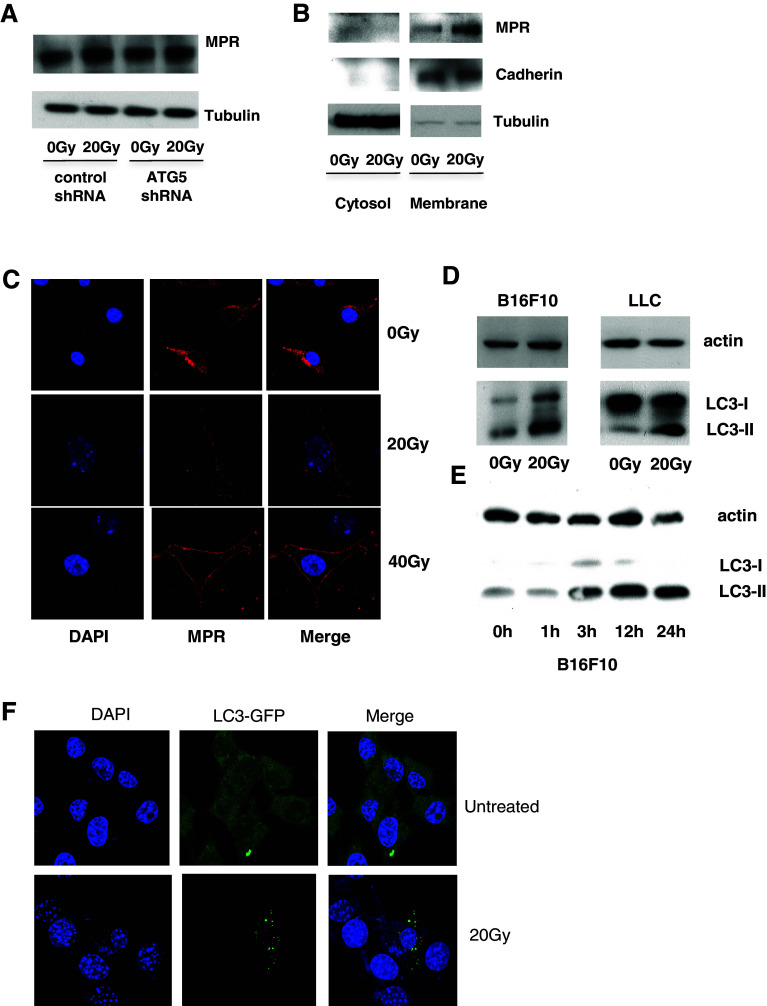
First, we evaluated the effect of radiation on MPR expression in vitro in several murine tumor cell lines. Within 24 h after irradiation with 20 Gy, B16F10 melanoma, LLC lung carcinoma, and 4T1 mammary carcinoma cells exhibited substantial up-regulation of MPR on the cell surface (Fig. 1a). MPR up-regulation was observed in B16F10 melanoma with the radiation dose as low as 10 Gy in vitro (Fig. S1A). The effect of ionizing radiation on MPR expression in vivo was assessed in mice bearing subcutaneous B16F10 tumors. Mice were treated with a single 30 Gy fraction of radiation when tumors reached 1 cm in diameter. Tumors were excised, and MPR expression was evaluated in single-cell suspension by flow cytometry. Up-regulation of MPR on the cell surface was detectable within 24 h following radiation and reached maximal levels by 72 h. It declined to pretreatment levels by day 7 days after irradiation (Fig. 1b). Up-regulation of MPR on the cell surface of tumor cells was also detectable with a single fraction of 10 Gy (Fig. S1B).
Fig. 1.
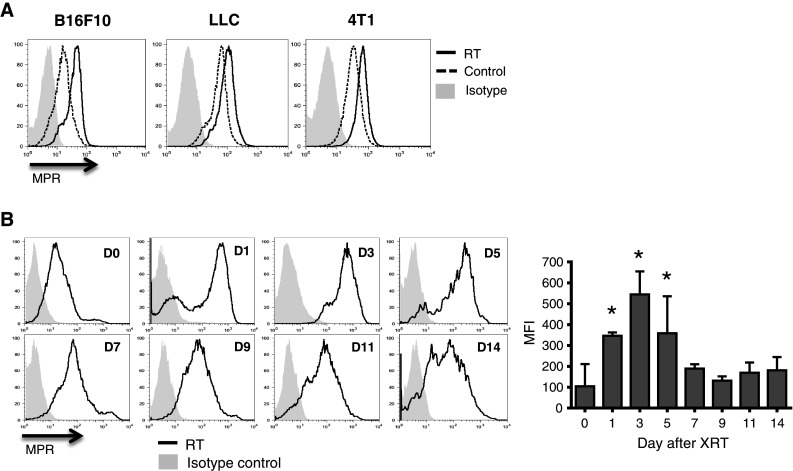
Radiation induces MPR up-regulation in tumor cells. a Up-regulation of MPR in vitro on the surface of the indicated tumors 24 h after irradiation with 20 Gy. Cells were gated on 7AAD-negative population. Typical examples of 3–5 different experiments are shown. b Effects of ionizing radiation in vivo. B16F10 tumor-bearing mice received 30 Gy of local radiation therapy. Tumors were excised at indicated times, and tumor cells were analyzed by flow cytometry. Cells were gated on the CD45−7AAD− population. Representative plots are shown on the left. Cumulative results of MPR expression are shown on the right. Each time point included 2–5 mice. *Statistically significant differences from control (p < 0.05)
The role of MPR in the antitumor effect of combined radiation therapy and immunotherapy was evaluated using B16F10 cells with stable expression of MPR shRNA, which blocked MPR expression (Fig. 2a). Down-regulation of MPR did not affect the viability or growth kinetics of B16F10 cells (Fig S2 and data not shown). We also tested whether down-regulation of MPR had a direct effect on tumor cell viability in response to ionizing radiation in vitro. The number of live MPR shRNA B16F10 cells recovered after irradiation was the same as control shRNA B16F10 cells (Fig. 2b). We then determined whether radiosensitivity of B16F10 cell line was affected by MPR down-regulation. 20 Gy of irradiation had a strong suppressive effect on the clonogenic potential of B16F10 cells (Fig. 2c). This inhibition occurred regardless of MPR silencing by shRNAs (Fig. 2c). Next, the effect of MPR expression on the sensitivity of tumor cells to CTL killing was assessed in vitro as our prior study has demonstrated increased binding of granzyme B released by CTL with up-regulation of MPR on the cell surface [27]. shRNA B16F10 cells and MPR shRNA B16F10 cells were labeled with different amounts of CFSE, mixed at a 1:1 ratio, and cultured for 6 h with activated Pmel T cells that recognize a gp100-derived peptide epitope expressed on B16F10 cells [35]. In non-irradiated cells, no difference in T cell-mediated killing was observed between B16F10 cells transfected with control and MPR shRNA (Fig. 2d). In contrast, when tumor cells were irradiated with 20 Gy, MPR shRNA-transfected tumor cells were substantially less sensitive to CTLs than control shRNA cells (Fig. 2d). To test the combined effect of radiation and immunotherapy in B16F10-bearing mice in vivo, we used repeated administration of CTLA4 mAb and a single 15 Gy fraction of local XRT. Treatment was started when tumors reached 100 mm2. The growth kinetics for B16F10 cells transfected with MPR shRNA was delayed by 3 days in vivo (Fig. 2e). CTLA4 mAb immunotherapy alone did not affect tumor growth in these mice (Fig. 2e). Treatment of mice bearing B16F10 tumors expressing control shRNA with the combination of radiation therapy and CTLA4 mAb showed significantly better (p = 0.0078) tumor growth suppression compared to XRT alone (Fig. 2e, left panel). This effect of combined therapy was completely abrogated in mice bearing tumors expressing MPR shRNA (Fig. 2e, right panel).
Fig. 2.
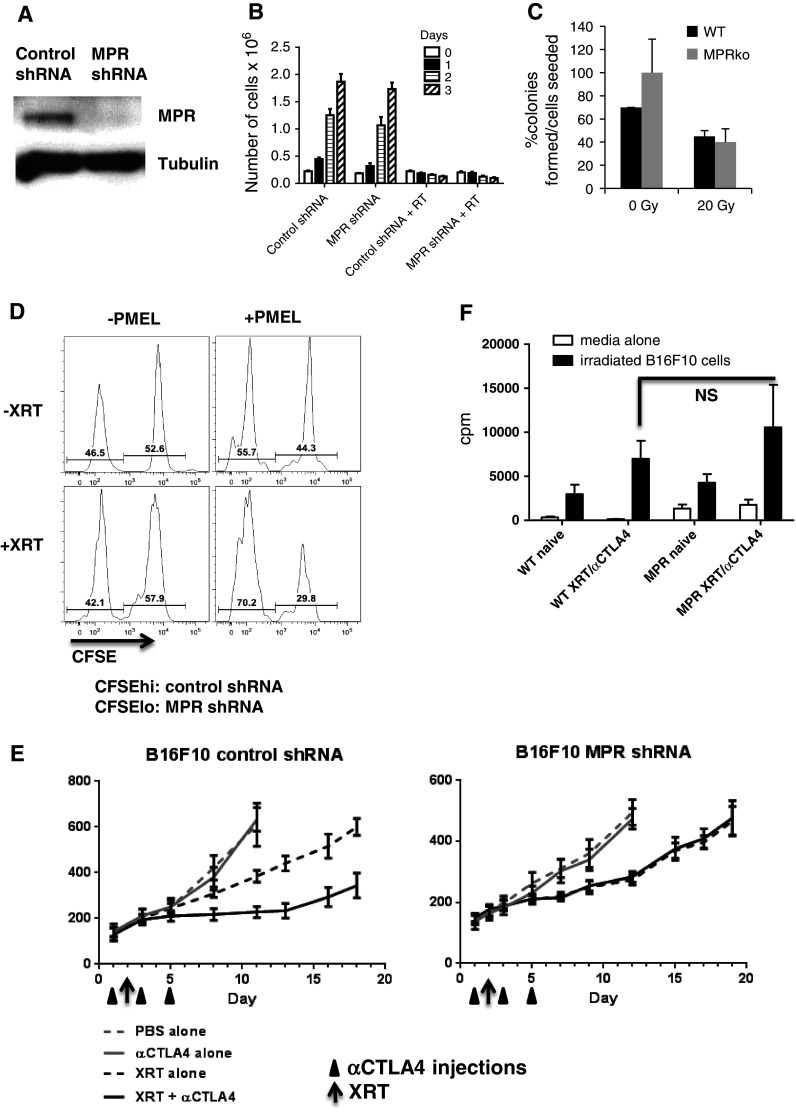
Synergistic antitumor effects of combined radiation therapy and CTLA4 immunotherapy requires MPR. a Down-regulation of MPR in B16F10 cells stably transfected with specific shRNA. b MPR down-regulation does not alter viability of tumor cells after radiation. B16F10 cells were irradiated with 20 Gy, and live cells were counted at different time points. The total number of cells is shown. Cumulative results of three experiments are shown. c MPR down-regulation does not influence clonogenic growth of B16F10 cells. Ten days after seeding, colonies were stained with crystal violet and counted. For each replicate, only wells with the lowest number of cells seeded were counted and results are represented as platting efficiency (PE) for non-radiated cells, i.e. (no. of colonies formed/no. of cells seeded) × 100 (%), and surviving factor (SF), calculated as no. of colonies formed after treatment × 100/(no. of cells seeded × PE) (%), for irradiated groups. Average from 3 replicates is shown. d B16F10 cells transfected with control or MPR shRNA were irradiated with 15 Gy, cultured overnight and then labeled with CFSE. B16F10-control shRNA were labeled with a high concentration of CFSE and B16F10-MPR shRNA with a low concentration of CFSE. Cells were mixed at a 1:1 ratio and incubated with activated PMEL splenocytes at a 1:10 ratio. After 4 h, CSFE fluorescence was measured within the DAPI-negative, CD45-negative population. The data shown are representative of 2 independent experiments. e B16F10 control shRNA or MPR shRNA tumor-bearing mice were treated with 300 µg murine CTLA4 mAb in 3 injections and/or 15 Gy local radiation therapy at indicated time points. Day 0 represents 14–15 days after tumor inoculation. Each group included 5–7 mice. Mean and SEM are shown. *Statistical differences from XRT alone (p < 0.05). f Mice were treated as described in d. Spleens were removed 10 days after starting the treatment. CD3 T cells were purified and restimulated ex vivo with irradiated (200 Gy) B16F10 cells. T cell proliferation was measured by 3[H]-thymidine uptake. Each group included 3 mice. Mean and SEM are shown. CPM from tumor cells alone were subtracted
We investigated the possibility that down-regulation of MPR may have an effect on the immunogenicity of tumors and thus negatively influence the strength of systemic immune responses after combined XRT and CTLA4 mAb immunotherapy. Splenocytes were isolated from tumor-bearing mice 10 days after the treatment and restimulated with irradiated B16F10 cells ex vivo. Combined XRT and CTLA4 mAb caused a significant increase in tumor cell-specific splenocyte proliferation as compared to untreated tumor-bearing mice. However, no differences were found between mice bearing control shRNA and MPR shRNA tumors. When comparing proliferation for splenocytes from mice treated with combined XRT and CTLA4 mAb immunotherapy versus no treatment in vivo, 2.4-fold increase was seen for mice bearing control shRNA tumor versus 2.5-fold increase for MPR shRNA (Fig. 2f). These data indicate that the combined antitumor effect of XRT and immunotherapy depends on up-regulation of MPR expression on the tumor cell surface. This effect was not associated with an enhanced antitumor immune response in mice bearing tumors with blocked expression of MPR.
Autophagy regulates MPR expression on tumor cells
Our previous studies demonstrated that chemotherapy caused redistribution of MPR to the cell surface, and we found that autophagy was associated with this phenomenon [27]. However, the mechanism of this phenomenon remains unclear. We asked whether autophagy is involved in the up-regulation of MPR expression in tumor cells treated with radiation. Radiation did not cause changes in the total amount of MPR in these cells (Fig. 3a). However, when cytosolic and membrane fractions of cells were isolated, a substantial increase in the amount of MPR was found only in the membrane fraction of cells treated with radiation (Fig. 3b). These results suggested that radiation, similar to chemotherapy, caused MPR redistribution to the cell surface. This conclusion was independently confirmed by confocal microscopy demonstrating redistribution of MPR to the cell surface in response to ionizing radiation in a dose-dependent manner (Fig. 3c).
Fig. 3.
Radiation-induced autophagy is associated with MPR up-regulation on tumor cells. a Amount of MPR in B16F10 tumor cells 24 h after 20 Gy irradiation. A Western blot was performed on the whole cell extract. Three experiments with the same results were performed. b Membrane fractions of irradiated B16F10 cells showed increased MPR levels 24 h after irradiation. Three experiments with the same results were performed. c Redistribution of MPR to the surface of B16F10 cells 24 h after irradiation with indicated doses. Cells were analyzed by confocal microscopy. Three experiments with the same results were performed. d Autophagy induction in B16F10 or LLC cells in vitro 24 h after irradiation with 20 Gy. Autophagy was assessed by Western blot. The experiments were repeated twice. e Kinetics of autophagy induction in B16F10 cells. B16F10 cells were irradiated with 20 Gy and cultured for the indicated times. Autophagy was assessed by Western blot. Data shown are representative of 2 independent experiments. f B16F10 cells were transfected with LC3-GFP and irradiated with 20 Gy. Punctae formation was assessed 24 h later. Typical examples of three performed experiments are shown
Autophagy is a cellular stress response that has been well documented in irradiated cells [36]. We monitored autophagy induction by tracing the cytosolic form of the microtubule-associated protein 1A/1B-light chain 3 (LC3) (LC3-I), and its form conjugated to phosphatidylethanolamine (LC3-II), a marker of autophagy. Irradiation of B16F10 as well as LLC cells resulted in elevated LC3-II 24 h after the treatment (Fig. 3d). Further investigation into the kinetics of autophagy revealed that formation of LC3-II was observed as early as 3 h after irradiation (Fig. 3e). Autophagy induction by ionizing radiation was further confirmed with confocal microscopy showing LC3 punctae formation in response to ionizing radiation (Fig. 3f).
To evaluate the role of radiation-induced autophagy in MPR up-regulation, 3MA, a specific autophagy inhibitor, was used. 3MA abrogated MPR up-regulation in B16F10, LLC, and 4T1 cells caused by radiation (Fig. 4a). In order to confirm this finding, MPR up-regulation was evaluated in B16F10 cell lines stably transfected with shRNA specific for atg5 and beclin1—two major components of the autophagy pathway (Fig. 4b). As expected, down-regulation of Atg5 abrogated formation of LC3-II in response to both TAX and radiation (Fig. 4c). Also, down-regulation of Atg5 abrogated the radiation-inducible redistribution of MPR to the cell surface (Fig. 4d vs. Fig. 3b). When atg5 shRNA B16F10 cells and beclin1 shRNA B16F10 cells were treated with ionizing radiation or Taxol (as a positive control), MPR up-regulation was blunted (Fig. 4e). These data indicate that MPR up-regulation during radiation is controlled by autophagy.
Fig. 4.
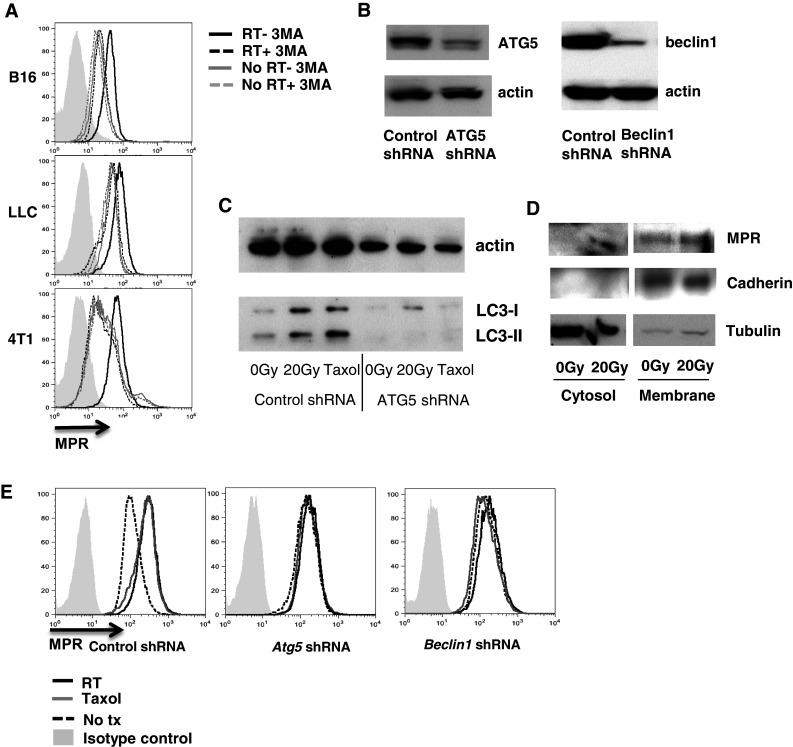
MPR up-regulation is dependent on autophagy. a MPR up-regulation was abrogated by 3MA. The indicated tumor cells were irradiated with 20 Gy and cultured for 24 h in the presence or absence of 3MA. Expression of MPR was analyzed by flow cytometry. Data shown are representative of 2 independent experiments. b Atg5 or beclin1 shRNA down-regulated protein expression in stably transfected B16F10 cells. Proteins were detected by Western blot. c LC3II lipidation induced by 20 Gy irradiation or 12.5nM Taxol was down-regulated in cells transfected with atg5 shRNA. Cells were evaluated 24 h after the treatment by Western blot. Two experiments with the same results were performed. d Membrane and cytosolic fractions of irradiated B16F10 atg5 shRNA analyzed 24 h after irradiation by Western blotting. Two experiments with the same results were performed. e Radiation or TAX-inducible MPR up-regulation was abrogated in B16F10 cells transfected with atg5 or beclin1 shRNA. Cells were treated with 20 Gy radiation. and MPR surface expression was analyzed 24 h later. Data shown are representative of 3 independent experiments
Mechanism of MPR up-regulation caused by radiation- and chemotherapy-induced autophagy
To address how autophagy causes redistribution of MPR to the cell surface, we tested the hypothesis that MPR trafficking within the cell can be affected by mobilization of MPR to autophagosomes. B16F10 tumor cells were transfected with LC3-GFP to detect the formation of autophagosomes. After treatment of the cells with radiation or TAX, LC3 punctae became easily visualized by confocal microscopy. When untreated cells were stained with MPR antibody, no colocalization of MPR and LC3 was seen. In contrast, after radiation or TAX treatment, colocalization of MPR with autophagosomes was readily detectable (Fig. 5a). This suggests a direct involvement of autophagosomes in MPR redistribution. Fusion of autophagosomes with lysosomes is the main feature of autophagy. We asked whether this was a requirement for up-regulation of MPR expression. As we demonstrated earlier, TAX up-regulated MPR on the tumor cell surface within 24 h after starting treatment [27, 28]. At that time point, evidence of autophagy and colocalization of MPR with autophagosomes were evident (Fig. 5a). However, no colocalization of LC3 with LAMP2, a marker of lysosomes, was seen (Fig. 5b).
Fig. 5.
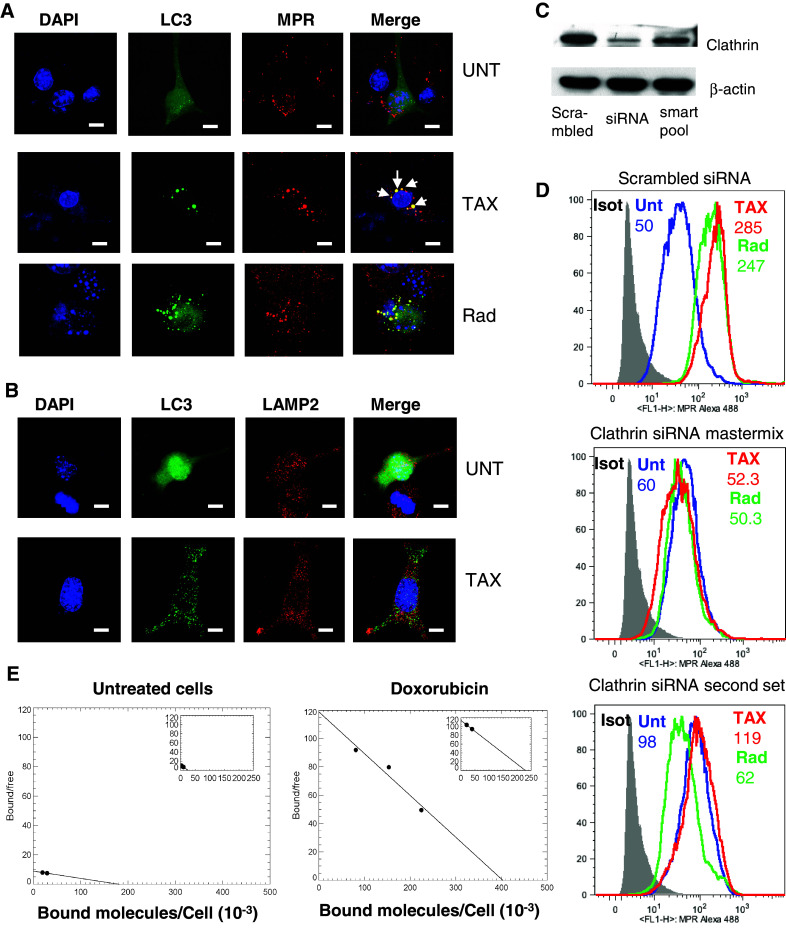
MPR redistribution following radiation or chemotherapy is directly associated with autophagosome and is dependent on clathrin. a Colocalization of LC3-positive autophagosomes (green LC3 punctae) and MPR (red) after treatment of LC3-GFP-transfected B16F10 cells with TAX or radiation. (Bars = 25 µm). A representative of four experiments with the same results is shown. b Colocalization of autophagosomes (green LC3 punctae) with lysosomes (red LAMP2) after treatment of LC3-GFP-transfected B16F10 cells with TAX. Three experiments with the same results are shown. c Down-regulation of clathrin by siRNA. Two different sets of siRNA were used. Clathrin heavy chain expression was evaluated by Western blot. d MPR up-regulation was abrogated by clathrin down-regulation. B16F10 cells were transfected with scrambled siRNA or two different sets of clathrin siRNA, and 2 days later, cells were treated with TAX or 20 Gy irradiation. After 24-h culture, cells were analyzed for MPR expression. Data shown are representative of 2 independent experiments. e U266 cells were treated overnight with 25nM doxorubicin. Next day, the cells were washed and a Scatchard assay was performed to estimate the fraction of ligand-bound receptors versus empty receptors using human [125I] IGF-II as described in methods. Six experiments were performed
Inside the cell, MPR is transported via clathrin-coated vesicles. We asked whether radiation or chemotherapy-inducible up-regulation of MPR on the tumor cell surface requires clathrin. Clathrin was down-regulated on B16F10 cells using different sets of specific siRNA Mastermix combining 5 different siRNA sets, and a second set with a single independent siRNA was used to knock down Clathrin on B16F10 cells (Fig. 5c). Cells were treated with radiation or TAX. Down-regulation of clathrin in B16F10 cells abrogated up-regulation of MPR on the cell surface caused by radiation or TAX (Fig. 5d). Since MPR is mobilized to autophagosomes but does not undergo degradation (no changes in the total protein level) and does not colocalize with lysosomes, we hypothesized that the receptor recycles back to the surface empty after losing its cargo. To test this hypothesis in principle, we measured binding of the major MPR ligand—[125I] IGF-II on the surface of tumor cells. Since only human [125I] IGF-II was available, we had to use human cell line. We have previously established redistribution of MPR to the cell surface in U266 myeloma in response to chemotherapy (doxorubicin) [27]. Therefore, we used this cell line for the experiments. In untreated cells, specific binding of labeled ligand was not detected at the highest available concentration (2758 Ci/mmol) (Fig. 5e). In contrast, if tumor cells were treated with doxorubicin, the binding of labeled IGFII became clearly detectable with Kd 20.19 pM (Fig. 5e).
Discussion
This study describes the potentiating effect of XRT on immunotherapy of tumors is dependent on up-regulation of MPR mediated by autophagy. While ionizing radiation has long been recognized as immunosuppressive [5, 7], in multiple preclinical studies and clinical phase I and II trials, XRT has effectively boosted immune responses [12–19]. In a recent case report, a complete response to a combination of ipilimumab with stereotactic ablative radiotherapy was observed in a patient with metastatic melanoma [37]. The mechanism of immune activation by radiation therapy includes the concept of immunogenic cell death in addition to engagement of cognate antigen [24, 25, 38]. A current model of immunologic cell death involves high-mobility group protein B1 (HMGB1) and ATP as DAMP [24, 26, 39], which also functions as a DC maturation signal [40], and enhances antigen presentation through relocalization of calreticulin [41]. However, there have been some contradictory findings. Indeed, the report by Teits-Tennenbaum et al. suggests that radiation therapy may enhance antitumor immune responses even in the absence of apoptosis [42]. Furthermore, irradiation of tumors resulted in superior presentation of tumor antigens to T cells without associated DC maturation [42]. While induction of NKG2D by ionizing radiation and subsequent tumor sensitization to NK cell cytotoxicity may partly explain such findings [43, 44], the hallmark of antimelanoma effector immune response is tumor-specific CTL activity. We have previously described novel mechanism that may explain the synergistic effect of conventional chemotherapy and immune therapy. Conventional chemotherapy induced autophagy and concomitant up-regulation of MPR, which resulted in tumor cell sensitization to CTLs [27]. Given that autophagy has been frequently associated with ionizing radiation in tumors, we explored the role of MPR and autophagy in the combined effects of XRT and immunotherapy.
Up-regulation of MPR was a common response to radiation in vitro in different tumors. More detailed studies were performed in vivo with B16F10 melanoma. We observed rapid but transient up-regulation of MPR expression after a single 15–20 Gy dose of XRT, which is a clinically relevant dose in melanoma patients who are being treated with stereotactic body radiation therapy or stereotactic radiosurgery [45]. There is evidence for an improved effect with 24 Gy in 3 fractions versus 20 Gy in a single fraction in breast carcinoma cells [14]. Further, previous studies showing relative radioresistance of regulatory T cells [10] and their dose-dependent increase in response to 0–20 Gy of XRT in C57BL/6 mice [11] suggest that a single high dose of XRT may have profound immunosuppressive effect. Regardless, we focused on a single radiation fraction to facilitate a better understanding of the temporal relationship between MPR up-regulation and therapeutic responses to immunotherapy. We used CTLA4 blockade which has been shown to be effective in murine preclinical studies as well as in humans in phase III trials in melanoma [3]. Administration of CTLA4 within the time frame of XRT-inducible MPR up-regulation potentiated the antitumor effect of XRT. CTLA4 alone had no impact on local tumor control consistent with prior publications [46]. This synergistic effect was dependent on MPR, since inhibition of MPR expression on tumor cells with shRNA completely abrogated the therapeutic synergy. MPR suppression did not result in altered tumor sensitivity to radiation. Our experiments also ruled out the possibility that MPR down-regulation in tumor cells somehow impacted the quality of T cell responses to the combined treatment. Thus, XRT sensitized tumor cells to immune therapy.
We also addressed the mechanism of MPR up-regulation in response to ionizing radiation. Our results showed that MPR synthesis or degradation was unaffected by radiation, and instead, MPR was found to be redistributed from the cytosol to the cell surface. This redistribution was dependent on autophagy as was evident from experiments with the autophagy inhibitor 3MA and atg5 or beclin1 shRNA. Recently, autophagy has been shown to be important for the immunogenicity of chemotherapy-induced cell death associated with ATP release [47, 48]. Our study demonstrated that in addition to possible immune activation, autophagy may play a major role in sensitization of tumor cells to immune therapy.
How exactly does autophagy cause MPR up-regulation? Our data showing colocalization of MPR with LC3 punctae representing autophagosomes suggest a direct role of autophagosomes in MPR redistribution. Our results demonstrated that radiation- or chemotherapy-induced MPR redistribution was dependent on clathrin, which represent a major mechanism of MPR transfer between the cell surface and cytosol [49], and was implicated in the formation of autophagosome precursors and ATG16L1-positive phagophore precursors [50, 51].
Our study presents a novel mechanism by which XRT may enhance antitumor effect of immunotherapy independent of any modulation of the host immune system. Radiation and chemotherapy induce cellular stress in tumor cells, which elicits a common protective response—autophagy. At the same time, autophagy redirects MPR with its ligands to the autophagosomes, as clathrin-coated vesicles or resulting from the fusion of autophagosomes with endosomes (where MPR are usually located). In both cases, low pH in autophagosomes resulted in the release of the MPR cargo. Empty MPRs are trafficking back to the surface. Since recycling of MPRs is regulated by binding to their ligands, this results in accumulation of empty receptors on the cell surface. This process not only affects normal recycling of MPR, but also makes these receptors available for binding to different ligands. GrzB released by activated CTLs is one such ligand [31]. As we demonstrated earlier in the model of chemotherapy-induced autophagy, this leads to the internalization of GrzB protease and apoptotic tumor cell death in an antigen-independent manner [27, 28]. This mechanism may play an important role in the context of relatively radioresistant tumors such as melanoma or renal cell carcinoma when suboptimal XRT alone may not cause enough cell death or when cancer immune therapy leads to generation of only suboptimal CTL responses. Whether significant clinical benefit in local control may be seen within the window of MPR up-regulation requires validation in clinical trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Jeffrey Weber for critical reading of the manuscript. This work was supported in part by pilot funds from Donald A. Adam Comprehensive Melanoma Research Center at H. Lee Moffitt Cancer Center and by NIH grant CA168536 to Dmitry I. Gabrilovich. This work was supported in part by microscopy core of H. Lee Moffitt Cancer Center.
Conflict of interest
Authors declare no conflicts of interests.
Abbreviations
- 3MA
3-Methyladenine
- ATG5
Autophagy protein 5
- ATG16L1
Autophagy-related protein 16-1
- CTL
Cytotoxic T lymphocyte
- CTLA4
Cytotoxic T lymphocyte antigen 4
- DAMP
Damage-associated molecular pattern
- DC
Dendritic cell
- GrzB
Granzyme B
- Gy
Gray
- IGFII
Insulin-like growth factor II
- iNOS
Inducible nitric oxide synthase
- LAMP2
Lysosome-associated membrane protein 2
- LLC
Lewis lung cancer
- LC3
Microtubule-associated protein 1A/1B-light chain 3
- MPR
Mannose-6-phopsphate receptor
- PD-L1
Programmed death-ligand 1
- shRNA
Short hairpin RNA
- siRNA
Small interfering RNA
- TAX
Taxol
- TGN
Trans-golgi network
- TLR
Toll-like receptor
- XRT
Radiation therapy
Footnotes
Sungjune Kim and Rupal Ramakrishnan have equally contributed to the work.
References
- 1.Aass N, De Mulder PH, Mickisch GH, Mulders P, van Oosterom AT, van Poppel H, Fossa SD, de Prijck L, Sylvester RJ. Randomized phase II/III trial of interferon alfa-2a with and without 13-cis-retinoic acid in patients with progressive metastatic renal cell Carcinoma: the European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group (EORTC 30951) J Clin Oncol. 2005;23(18):4172–4178. doi: 10.1200/JCO.2005.07.114. [DOI] [PubMed] [Google Scholar]
- 2.Sparano JA, Fisher RI, Sunderland M, Margolin K, Ernest ML, Sznol M, Atkins MB, Dutcher JP, Micetich KC, Weiss GR, et al. Randomized phase III trial of treatment with high-dose interleukin-2 either alone or in combination with interferon alfa-2a in patients with advanced melanoma. J Clin Oncol. 1993;11(10):1969–1977. doi: 10.1200/JCO.1993.11.10.1969. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borella L, Webster RG. The immunosuppressive effects of long-term combination chemotherapy in children with acute leukemia in remission. Cancer Res. 1971;31(4):420–426. [PubMed] [Google Scholar]
- 5.Gough MJ, Crittenden MR. Combination approaches to immunotherapy: the radiotherapy example. Immunotherapy. 2009;1(6):1025–1037. doi: 10.2217/imt.09.64. [DOI] [PubMed] [Google Scholar]
- 6.Harris J, Sengar D, Stewart T, Hyslop D. The effect of immunosuppressive chemotherapy on immune function in patients with malignant disease. Cancer. 1976;37(2 Suppl):1058–1069. doi: 10.1002/1097-0142(197602)37:2+<1058::AID-CNCR2820370813>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Hill-Kayser CE, Plastaras JP, Tochner Z, Glatstein E. TBI during BM and SCT: review of the past, discussion of the present and consideration of future directions. Bone Marrow Transpl. 2011;46(4):475–484. doi: 10.1038/bmt.2010.280. [DOI] [PubMed] [Google Scholar]
- 8.Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol. 2012;39(3):323–339. doi: 10.1053/j.seminoncol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Punt CJ, Suciu S, Gore MA, Koller J, Kruit WH, Thomas J, Patel P, Lienard D, Eggermont AM, Keilholz U. Chemoimmunotherapy with dacarbazine, cisplatin, interferon-alpha2b and interleukin-2 versus two cycles of dacarbazine followed by chemoimmunotherapy in patients with metastatic melanoma: a randomised phase II study of the European Organization for Research and Treatment of Cancer Melanoma Group. Eur J Cancer. 2006;42(17):2991–2995. doi: 10.1016/j.ejca.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, McBride WH, Schaue D. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81(4):1128–1135. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu Y, Jin S, Zhang A, Zhang B, Shi X, Wang J, Zhao Y. Gamma-ray resistance of regulatory CD4 + CD25 + Foxp3 + T cells in mice. Radiat Res. 2010;173(2):148–157. doi: 10.1667/RR0978.1. [DOI] [PubMed] [Google Scholar]
- 12.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728–734. [PubMed] [Google Scholar]
- 13.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelstein SE, Iclozan C, Bui MM, Cotter MJ, Ramakrishnan R, Ahmed J, Noyes DR, Cheong D, Gonzalez RJ, Heysek RV, Berman C, Lenox BC, Janssen W, Zager JS, Sondak VK, Letson GD, Antonia SJ, Gabrilovich DI. Combination of external beam radiotherapy (EBRT) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. Int J Radiat Oncol Biol Phys. 2012;82(2):924–932. doi: 10.1016/j.ijrobp.2010.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikitina EY, Gabrilovich DI. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001;94(6):825–833. doi: 10.1002/1097-0215(20011215)94:6<825::AID-IJC1545>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Teitz-Tennenbaum S, Li Q, Davis MA, Wilder-Romans K, Hoff J, Li M, Chang AE. Radiotherapy combined with intratumoral dendritic cell vaccination enhances the therapeutic efficacy of adoptive T-cell transfer. J Immunother. 2009;32(6):602–612. doi: 10.1097/CJI.0b013e3181a95165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, Ito F, Davis MA, McGinn CJ, Chang AE. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63(23):8466–8475. [PubMed] [Google Scholar]
- 19.Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, Duret H, Yagita H, Johnstone RW, Smyth MJ, Haynes NM. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72(13):3163–3174. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 20.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Nat Cancer Inst. 1979;63(5):1229–1235. [PubMed] [Google Scholar]
- 21.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392(6671):86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 22.Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186(8):1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schakel K, Garbi N, Jager D, Weitz J, Schmitz-Winnenthal H, Hammerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9(5):353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durante M, Reppingen N, Held KD. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol Med. 2013;19(9):565–582. doi: 10.1016/j.molmed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 27.Ramakrishnan R, Huang C, Cho HI, Lloyd M, Johnson J, Ren X, Altiok S, Sullivan D, Weber J, Celis E, Gabrilovich DI. Autophagy induced by conventional chemotherapy mediates tumor cell sensitivity to immunotherapy. Cancer Res. 2012;72(21):5483–5493. doi: 10.1158/0008-5472.CAN-12-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120(4):1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52(3):329–341. doi: 10.1016/S0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 30.Dressel R, Raja SM, Honing S, Seidler T, Froelich CJ, von Figura K, Gunther E. Granzyme-mediated cytotoxicity does not involve the mannose 6-phosphate receptors on target cells. J Biol Chem. 2004;279(19):20200–20210. doi: 10.1074/jbc.M313108200. [DOI] [PubMed] [Google Scholar]
- 31.Trapani JA, Sutton VR, Thia KY, Li YQ, Froelich CJ, Jans DA, Sandrin MS, Browne KA. A clathrin/dynamin- and mannose-6-phosphate receptor-independent pathway for granzyme B-induced cell death. J Cell Biol. 2003;160(2):223–233. doi: 10.1083/jcb.200210150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motyka B, Korbutt G, Pinkoski MJ, Heibein JA, Caputo A, Hobman M, Barry M, Shostak I, Sawchuk T, Holmes CF, Gauldie J, Bleackley RC. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell. 2000;103(3):491–500. doi: 10.1016/S0092-8674(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 33.Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 2008;68(5):1485–1494. doi: 10.1158/0008-5472.CAN-07-0562. [DOI] [PubMed] [Google Scholar]
- 34.Lomonaco SL, Finniss S, Xiang C, Decarvalho A, Umansky F, Kalkanis SN, Mikkelsen T, Brodie C. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int J Cancer. 2009;125(3):717–722. doi: 10.1002/ijc.24402. [DOI] [PubMed] [Google Scholar]
- 35.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188(2):277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies? Autophagy. 2009;5(4):442–450. doi: 10.4161/auto.5.4.7667. [DOI] [PubMed] [Google Scholar]
- 37.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, Swetter SM, Knox SJ. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5(6):404–407. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 39.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 40.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 41.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, Annaert W, Golab J, de Witte P, Vandenabeele P, Agostinis P. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31(5):1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teitz-Tennenbaum S, Li Q, Okuyama R, Davis MA, Sun R, Whitfield J, Knibbs RN, Stoolman LM, Chang AE. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J Immunother. 2008;31(4):345–358. doi: 10.1097/CJI.0b013e318163628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gannage M, Buzyn A, Bogiatzi SI, Lambert M, Soumelis V, Dal Cortivo L, Cavazzana-Calvo M, Brousse N, Caillat-Zucman S. Induction of NKG2D ligands by gamma radiation and tumor necrosis factor-alpha may participate in the tissue damage during acute graft-versus-host disease. Transplantation. 2008;85(6):911–915. doi: 10.1097/TP.0b013e31816691ef. [DOI] [PubMed] [Google Scholar]
- 44.Kim JY, Son YO, Park SW, Bae JH, Chung JS, Kim HH, Chung BS, Kim SH, Kang CD. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med. 2006;38(5):474–484. doi: 10.1038/emm.2006.56. [DOI] [PubMed] [Google Scholar]
- 45.Stinauer MA, Kavanagh BD, Schefter TE, Gonzalez R, Flaig T, Lewis K, Robinson W, Chidel M, Glode M, Raben D. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34. doi: 10.1186/1748-717X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martins I, Michaud M, Sukkurwala AQ, Adjemian S, Ma Y, Shen S, Kepp O, Menger L, Vacchelli E, Galluzzi L, Zitvogel L, Kroemer G. Premortem autophagy determines the immunogenicity of chemotherapy-induced cancer cell death. Autophagy. 2012;8(3):413–415. doi: 10.4161/auto.19009. [DOI] [PubMed] [Google Scholar]
- 48.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di Virgilio F, Zitvogel L, Kroemer G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334(6062):1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 49.Lin SX, Mallet WG, Huang AY, Maxfield FR. Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-Golgi network and a subpopulation of late endosomes. Mol Biol Cell. 2004;15(2):721–733. doi: 10.1091/mbc.E03-07-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12(8):747–757. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravikumar B, Moreau K, Rubinsztein DC. Plasma membrane helps autophagosomes grow. Autophagy. 2010;6(8):1184–1186. doi: 10.4161/auto.6.8.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H, Xie Y, Li W, Chibbar R, Xiong S, Xiang J. CD4(+) T cell-released exosomes inhibit CD8(+) cytotoxic T-lymphocyte responses and antitumor immunity. Cell Mol Immunol. 2011;8(1):23–30. doi: 10.1038/cmi.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.