Abstract
Introduction
Persistent or recurrent hyperthyroidism after treatment with radioactive iodine (RAI) is common, and many patients require either additional doses or surgery before they are cured. The purpose of this study was to identify patterns and predictors of failure of RAI in patients with hyperthyroidism.
Methods
We conducted a retrospective review of patients treated with RAI from 2007–2010. Failure of RAI was defined as receipt of additional dose(s) and/or total thyroidectomy. Using a Cox proportional hazards model, we conducted univariate analysis to identify factors associated with failure of RAI. A final multivariate model was then constructed with significant (p < 0.05) variables from the univariate analysis.
Results
Of the 325 patients analyzed, 74 patients (22.8%) failed initial RAI treatment. 53 (71.6%) received additional RAI, 13 (17.6%) received additional RAI followed by surgery, and the remaining 8 (10.8%) were cured after thyroidectomy. The percentage of patients who failed decreased in a step-wise fashion as RAI dose increased. Similarly, the incidence of failure increased as the presenting T3 level increased. Sensitivity analysis revealed that RAI doses < 12.5 mCi were associated with failure while initial T3 and free T4 levels of at least 4.5 pg/mL and 2.3 ng/dL, respectively, were associated with failure. In the final multivariate analysis, higher T4 (HR 1.13, 95% CI 1.02–1.26, p=0.02) and methimazole treatment (HR 2.55, 95% CI 1.22–5.33, p=0.01) were associated with failure.
Conclusions
Laboratory values at presentation can predict which patients with hyperthyroidism are at risk for failing RAI treatment. Higher doses of RAI or surgical referral may prevent the need for repeat RAI in selected patients.
Keywords: radioactive iodine, relapse, hyperthyroidism, thyroidectomy
Introduction
Hyperthyroidism impacts almost all organ systems, leading to agitation, insomnia, tachycardia, dyspnea, anemia, hyperdefecation, myopathy, and increased bone turnover (1–3). The most common causes of hyperthyroidism are Graves’ disease, toxic solitary nodule, or toxic multinodular goiter (4).
Three treatment options exist for patients suffering from hyperthyroidism. Radioactive iodine (RAI) and surgery are considered definitive treatment options since the purpose is to either destroy or remove all hyperfunctioning thyroid tissue (4, 5). Antithyroid medications can be used for a defined period of time (12–18 months) in hopes of remission. However, these medications are generally not long-term treatment options due to their toxicities. Therefore, most patients suffering from hyperthyroidism must ultimately choose between RAI and surgery.
RAI continues to be the treatment of choice for most U.S. providers when given a hypothetical case of uncomplicated Graves’ disease (6, 7). Previously, guidelines favored radioactive iodine over surgery, but these most recent guidelines considered thyroidectomy as an equal treatment option to radioactive iodine (8, 9).
The reported recurrence rates after RAI treatment range from 10 to 40 percent of patients, with more severe cases of hyperthyroidism associated with higher rates of failure (10–12). Absolute contraindications to RAI are few and include pregnancy, lactation, and inability to comply with radiation safety guidelines after treatment (8, 13). Beyond these few contraindications, providers lack specific selection criteria for treating hyperthyroid patients with RAI, medication, or surgery (4). Although the use of surgery for the primary treatment of Graves’ disease is increasing, surgical referral commonly occurs after other modalities have failed. Since RAI can lead to significant fibrosis and scarring of the thyroid, thyroidectomy after RAI becomes more challenging. Therefore, it would be advantageous for providers to be able to predict up front which patients may be at high risk of RAI failure and instead undergo thyroidectomy as the initial treatment option (14, 15).
Amidst the uncertainty regarding the optimal therapy for hyperthyroidism, the purpose of this study is two-fold. First, we describe a large, tertiary referral center’s experience in treating hyperthyroidism with RAI and detail patterns of failure. Second, we identify factors associated with failure.
Methods
After obtaining institutional review board (IRB) approval, we conducted a retrospective review of patients treated with RAI from 2007–2010 at our institution. We selected adult (≥ 18 years old) patients receiving RAI for hyperthyroidism. Children, pregnant women, and patients treated for thyroid cancer were excluded. Also excluded were patients lacking follow-up (<1 month) data in the medical record after their treatment date. For patients meeting these selection criteria, further details of their treatment for hyperthyroidism were abstracted from the electronic medical records including medications, lab results, physical exam findings, demographics, co-morbidities, thyroid imaging, and doses of RAI. Patients were classified as having Graves’ disease if they either had positive TSH receptor stimulating antibodies or homogenous uptake on radioiodine uptake scan.
Failure of RAI was defined as requiring treatment with an additional dose(s) of RAI and/or total thyroidectomy for treatment of hyperthyroidism. Kaplan-Meier disease-free survival estimates were plotted to analyze the timing of failures. Using a Cox proportional hazards model, we conducted multivariate analysis to identify factors associated with failure of RAI. All statistical analyses were performed using STATA v. 12.1 (StataCorp, College Station, TX).
Results
Patient and disease characteristics
There were 325 patients included in this study. Nearly 73% were female, and 84% were Caucasian (Table 1). The average age was 43.4 ± 15.5 years (Table 1). This cohort was fairly healthy with few co-morbidities (Table 1).
Table 1.
Patient Characteristics (n = 325)
| Variable | # (%) |
|---|---|
| DEMOGRAPHICS | |
| Age | 43.4 ± 15.5 |
| Female gender | 237 (72.9) |
| Caucasian | 273 (84.0) |
| CO-MORBIDITIES | |
| Median # co-morbidities | 1 (0 – 10) |
| CAD | 6 (1.9) |
| Diabetes | 27 (8.3) |
| CHF | 12 (3.7) |
| BMI (kg/m2) | 27.1 ± 6.4 |
| Smoker | 82 (25.2) |
| ETIOLOGY | |
| Graves’ disease | 260 (80.3) |
| Toxic multinodular goiter | 51 (15.7) |
| Solitary toxic nodule | 14 (4.3) |
| HISTORY & PHYSICAL EXAM | |
| Heart rate | 82.9 ± 15.8 |
| Systolic blood pressure (mm Hg) | 122.1 ± 15.6 |
| Diastolic blood pressure (mm Hg) | 70.9 ± 10.0 |
| Goiter on exam | 173 (53.6) |
| Compressive symptoms | 34 (10.5) |
| Eye disease | 87 (26.9) |
| Family history+ | 66 (21.4) |
| IMAGING | |
| Largest dimension on U/S (cm) | 5.3 ± 1.0 |
| Uptake %* | 47.5 ± 18.9 |
| LABS | |
| Median TSH (uIU/mL) | 0.01 (IQR 0.02) |
| Median free T4 (ng/dL) | 2.6 (IQR 1.9) |
| Median T3 (pg/mL) | 7.8 (IQR 6.6) |
| Positive anti-TPO antibodies# | 63 (58.3) |
| Positive anti-Tg antibodies# | 23 (67.7) |
| Positive TRAB# | 91 (44.4) |
| Creatinine (mg/dL) | 0.7 ± 0.2 |
Data are represented as the mean ± the standard deviation for normally distributed continuous variables. Categorical variables are represented as the number with the corresponding percentage in parentheses. Continuous variables with a non-normal distribution are represented as the median with the inter-quartile range (IQR) in parentheses.
CAD, coronary artery disease, CHF, congestive heart failure, BMI, body mass index, kg, kilograms, m2, meters squared mm Hg, millimeters of mercury, U/S, ultrasound, cm, centimeters, IU, international units, mL, milliliters, ng, nanograms, dL, deciliters, pg, picograms, mg, milligrams.
Family history of any thyroid disease (not limited to hyperthyroidism)
Thyroid uptake percentage from a radioactive iodine uptake scan
Not all patients had these labs drawn
Of the total cohort, 260 patients (80.3%) were treated for Graves’ disease while 51 (15.7%) had toxic multinodular goiter and 14 (4.3%) had a solitary toxic nodule (Table 1). Eighty-seven patients (26.9%) presented with eye disease (Table 1).
As expected, this cohort presented with laboratory evidence of hyperthyroidism (Table 2). Accordingly, the average percentage uptake on radioiodine uptake was 47.5 ± 18.9% (Table 2). Detailed laboratory indices are given in Table 1.
Table 2.
Management of Hyperthyroidism
| Variable | # (%) |
|---|---|
| MEDICATIONS | |
| PTU | 70 (21.5) |
| Methimazole | 156 (48.2) |
| Steroids | 44 (13.5) |
| Beta-blockers | 46 (14.2) |
| >1 anti-thyroid medication* | 60 (18.5) |
| RAI | |
| Median dose (mCi) | 10.9 (IQR 7.1) |
| Median dose/% uptake | 0.23 (IQR 0.32) |
| Median time from onset of symptoms (months) | 8.2 (IQR 1.4) |
| FAILURES | |
| Total Failures | 74 (22.8) |
| Median initial dose (mCi) | 8.6 (IQR 4.4) |
| Median initial dose/% uptake | 0.14 (IQR 0.12) |
| Median time to failure (months) | 10.4 (IQR 9.3) |
| TREATMENT OF FAILURES | |
| Additional RAI | 53 (71.6) |
| Additional RAI + Surgery | 13 (17.6) |
| Surgery | 8 (10.8) |
Data are represented as the number with the percentage in parentheses for categorical variables and the median with the inter-quartile range (IQR) in parentheses for continuous variables since these data were distributed non-normally.
PTU, propylthiouracil, RAI, radioactive iodine, mCi, milli-Curies
Anti-thyroid medications include PTU, methimazole, steroids, and beta-blockers (when used for hyperthyroidism)
Initial Management of Hyperthyroidism and Failures
At presentation, nearly half of this cohort was treated with methimazole (48.2%), 21.5% were treated with PTU, 13.5% received steroids, and 14.2% were treated with beta-blockers (Table 2). Sixty patients (18.5%) were treated with more than one anti-thyroid medication (methimazole, PTU, beta blockers, and/or steroids when given specifically for hyperthyroidism).
The median dose of RAI administered to this cohort was 10.9 mCi with a range of 5 to 37.7 mCi (Table 2). Patients received RAI at a median of 8.2 months from presentation (Table 2).
A total of 74 patients (22.8%) failed RAI, and the median time to re-treatment was 10.4 months (IQR 9.3). When considering the subset of patients who failed, we found that their median dose of RAI was significantly lower than those who did not fail (8.6 ± 4.5 mCi vs. 11.8 ± 7.2 mCi, p < 0.01, Table 2). Sixty percent of patients receiving ≤ 8 mCi failed. Fifty-three of the 74 patients (71.6%) who failed RAI were treated with additional doses of RAI, 13 (17.6%) received additional doses of RAI followed by surgery, and the remaining 8 patients (10.8%) were treated with surgery for their recurrence (Table 2).
The Kaplan-Meier estimates for RAI failure were plotted to further examine the time course for failures. The median follow-up time for the entire cohort was 1.2 years (IQR 1.6 years). When examining the Kaplan-Meier estimates, it appeared that there were both early and late failures (Figure 1). We divided the entire follow-up time available into thirds, and 75.7% of failures occurred in the first 1.3 years after RAI. Only 9.5% of failures occurred in the middle period between 1.3 and 2.6 years. Nearly 15% of failures occurred in the later third, after 2.6 years (Figure 1). Since remission might be expected within six months, we also calculated the percentage that failed within six months of receiving RAI. Only 15 of the 74 failures (20.3%) occurred within six months.
Figure 1. Disease-free survival.
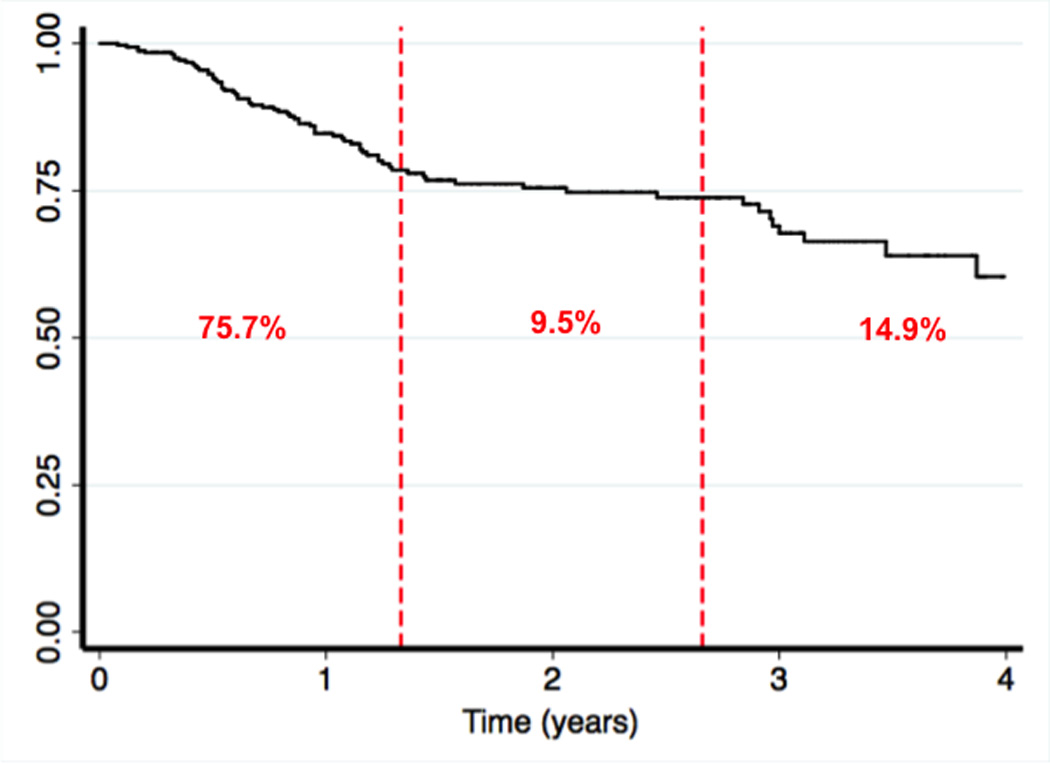
The Kaplan-meier disease-free survival estimates is displayed for the entire cohort. The dashed red lines divide the study time into three equal periods with the percentage of patients failing within each period shown in red.
Univariate analysis
To analyze the factors associated with failure of RAI, we conducted univariate analysis on all pre-treatment variables. Older age and female gender were associated with success of RAI treatment while non-Caucasian race was associated with failure (HR 1.95, 95% C.I. 1.16 – 3.26, p = 0.01, Table 3). Graves’ disease was associated with failure (HR 2.83, 95% C.I. 1.22 – 6.54, p = 0.02) while toxic multinodular goiter was associated with treatment success (Table 3). A higher heart rate at presentation and a greater uptake percentage on radioiodine uptake were associated with failure (Table 3). More severe thyroid function labs at presentation were also associated with RAI failure (Table 3). Further analysis revealed that there was a step-wise increase in the proportion of failures with increasing T3 levels (Supplementary Figure 1B). A similar pattern was noted for T4 levels (data not shown). Of note, the proportion of patients failing decreased with increasing doses of RAI (Supplementary Figure 1A). An RAI dose less than 13 mCi was significantly associated with failure (HR 2.22, 95% C.I. 1.29 – 3.84, p < 0.01) while higher doses were protective against failure (Table 3). Patients treated with methimazole or more than one anti-thyroid medication were also more likely to fail RAI (Table 3).
Table 3.
Univariate Analysis
| Variable | Hazard Ratio | 95% C.I. | p value |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Age at presentation | 0.98 | 0.97 – 0.99 | 0.04 |
| Female gender | 0.45 | 0.28 – 0.72 | <0.01 |
| Race (Non-Caucasian vs. Caucasian) | 1.95 | 1.16 – 3.26 | 0.01 |
| CO-MORBIDITIES | |||
| # co-morbidities | 0.81 | 0.70 – 0.93 | <0.01 |
| CAD | 1.22 | 0 – 1 | 1.00 |
| Diabetes | 0.28 | 0.07 – 1.14 | 0.08 |
| CHF | 0.69 | 0.17 – 2.81 | 0.60 |
| Smoker | 0.92 | 0.61 – 1.39 | 0.70 |
| BMI | 0.97 | 0.93 – 1.00 | 0.10 |
| Pregnant | 0.42 | 0.10 – 1.45 | 0.53 |
| ETIOLOGY | |||
| Graves’ disease | 2.83 | 1.22 – 6.54 | 0.02 |
| Toxic multinodular goiter | 0.39 | 0.15 – 0.95 | 0.04 |
| Solitary toxic nodule | 0.45 | 0.21 – 1.02 | 0.34 |
| HISTORY & PHYSICAL EXAM | |||
| Family History | 0.97 | 0.53 – 1.79 | 0.94 |
| Eye disease | 1.43 | 0.89 – 2.32 | 0.14 |
| Goiter on exam | 1.71 | 1.05 – 2.78 | 0.03 |
| Compressive symptoms | 1.19 | 0.59 – 2.40 | 0.62 |
| Heart rate | 1.03 | 1.01 – 1.04 | <0.01 |
| Systolic blood pressure | 1.01 | 0.99 – 1.03 | 0.11 |
| Diastolic blood pressure | 1.00 | 0.98 – 1.03 | 0.62 |
| IMAGING | |||
| Largest dimension on U/S | 1.30 | 0.81 – 2.11 | 0.28 |
| Uptake %* | 1.04 | 1.02 – 1.05 | <0.01 |
| LABS | |||
| TSH | 0.75 | 0.58 – 0.98 | 0.03 |
| 0.001 – 0.01 | 2.58 | 1.32 – 5.05 | <0.01 |
| 0.01 – 0.015 | 1.00 | 0.63 – 1.61 | 0.98 |
| 0.015 – 0.05 | 0.63 | 0.31 – 1.27 | 0.20 |
| 0.05 – 0.25 | 1.33 | 0.66 – 2.67 | 0.43 |
| >0.25 | 0.29 | 0.07 – 1.18 | 0.08 |
| T3 | 1.68 | 1.21 – 2.33 | <0.01 |
| 0 – 3 | 0.28 | 0.07 – 1.16 | 0.08 |
| 3 – 5.5 | 0.56 | 0.26 – 1.23 | 0.15 |
| 5.5 – 8 | 0.63 | 0.27 – 1.47 | 0.29 |
| >8 | 2.03 | 1.28 – 3.23 | <0.01 |
| Free T4 | 1.55 | 1.25 – 1.93 | <0.01 |
| 0 – 2 | 0.66 | 0.38 – 1.14 | 0.14 |
| 2 – 3.5 | 0.59 | 0.33 – 1.07 | 0.08 |
| 3.5 – 5 | 0.73 | 0.38 – 1.38 | 0.33 |
| >5 | 3.52 | 2.19 – 5.67 | <0.01 |
| T4:T3 ratio | 0.83 | 0.35 – 1.96 | 0.66 |
| Anti-TPO antibodies (present vs. Absent) | 0.92 | 0.39 – 2.17 | 0.86 |
| Anti-Tg antibodies (present vs. Absent) | 1.48 | 0.29 – 7.65 | 0.64 |
| TSH Receptor antibodies (present vs. absent) | 1.00 | 0.98 – 1.00 | 0.33 |
| Creatinine | 0.86 | 0.22 – 3.35 | 0.83 |
| MEDICATIONS | |||
| Steroids | 1.32 | 0.72 – 2.41 | 0.36 |
| PTU | 1.31 | 0.79 – 2.17 | 0.29 |
| Methimazole | 1.93 | 1.21 – 3.09 | <0.01 |
| Beta blockers | 1.29 | 0.75 – 2.20 | 0.36 |
| >1 anti-thyroid medication | 1.76 | 1.06 – 2.93 | 0.03 |
| RAI | |||
| Time from onset of symptoms | 0.98 | 0.89 – 1.07 | 0.61 |
| Dose | 0.49 | 0.31 – 0.76 | <0.01 |
| 4 – 13 | 2.22 | 1.29 – 3.84 | <0.01 |
| 13 – 22 | 0.64 | 0.35 – 1.14 | 0.13 |
| 22 – 31 | 0.26 | 0.06 – 1.06 | 0.06 |
| 31 – 40 | 6 X 10−16 | 0.00 – 4 X 10−17 | <0.01 |
CAD, coronary artery disease, CHF, congestive heart failure, BMI, body mass index, U/S, ultrasound, TSH, thyroid stimulating hormone, TPO, thyroid peroxidase, Tg, thyroglobulin, PTU, propylthiouracil, RAI, radioactive iodine
Thyroid uptake percentage from a radioactive iodine uptake scan
Multivariate analysis
Next we constructed a multivariate model using significant (p < 0.05) variables from our univariate analysis. When controlling for all other factors, a greater heart rate, percentage uptake on radioiodine uptake scan, TSH ≤ 0.01 uIU/mL, free T4 > 5 ng/dL were all significantly associated with failure. Treatment with methimazole was also an indicator of failure (HR 2.55, 95% C.I. 1.22 – 5.33, p = 0.01, Table 4). One of the strongest associations with failure of RAI was a dose of less than 13 mCi (HR 2.97, 95% C.I. 1.34 – 6.55, p < 0.01, Table 4). Age, gender, disease etiology, presence of goiter, or co-morbidities were not significantly associated with RAI failure (Table 4).
Table 4.
Final Multivariate Model
| Variable | Hazard Ratio | 95% C.I. | p value |
|---|---|---|---|
| Female gender | 0.87 | 0.44 – 1.71 | 0.69 |
| Age at presentation | 1.00 | 0.98 – 1.02 | 0.89 |
| Race (Non-Caucasian vs. Caucasain) | 1.54 | 0.74 – 3.21 | 0.25 |
| Graves’ disease | 3.00 | 0.33 – 27.54 | 0.33 |
| Toxic multinodular goiter | 0.51 | 0.06 – 4.38 | 0.54 |
| # co-morbidities | 0.91 | 0.77 – 1.06 | 0.23 |
| Diabetes | 0.43 | 0.10 – 1.90 | 0.27 |
| Heart rate | 1.02 | 1.00 – 1.03 | 0.04 |
| Goiter on exam | 0.94 | 0.54 – 1.63 | 0.82 |
| Uptake % | 1.03 | 1.01 – 1.05 | <0.01 |
| TSH ≤ 0.01 uIU/mL | 2.05 | 1.00 – 4.18 | 0.05 |
| T3 > 8 pg/mL | 0.91 | 0.53 – 1.58 | 0.74 |
| Free T4 > 5 ng/dL | 2.08 | 1.20 – 3.62 | 0.01 |
| RAI dose < 13 mCi | 2.97 | 1.34 – 6.55 | <0.01 |
| Methimazole | 2.55 | 1.22 – 5.33 | 0.01 |
| >1 anti-thyroid medication | 1.18 | 0.57 – 2.45 | 0.65 |
Significant items are indicated in bold type.
TSH, thyroid stimulating hormone, RAI, radioactive iodine, IU, international units, mL, milliliters, pg, picograms, ng, nanograms, dL, deciliters, mCi, milli-Curies
Since our entire cohort included all types of hyperthyroidism, we repeated this analysis on just the subset of patients with Graves’ disease (n = 260). Like the overall cohort, heart rate (HR 1.02, 95% C.I. 1.00 – 1.04, p = 0.02), free T4 > 5 ng/dL (HR 2.23, 95% C.I. 1.28 – 3.87, p < 0.01), percentage uptake (HR 1.02, 95% C.I. 1.00 – 1.04, p = 0.04), and methimazole use (HR 2.00, 95% C.I. 1.08 – 3.70, p = 0.03) were independently associated with failure in the Graves’ subset. Unlike the overall cohort, lower doses of RAI and lower TSH values at presentation did not independently predict failure in the multivariate model, although both were significant on univariate analysis.
Sensitivity analysis
To provide clinically meaningful values, we conducted sensitivity analysis to identify thresholds for the continuous clinical variables significant in our multivariate model. Initial free T4 and heart rate levels of at least 2.3 ng/dL and 78 beats per minute were associated with failure. RAI doses < 12.5 mCi were also associated with failure.
Discussion
In this examination of a single tertiary referral center’s experience with treatment of hyperthyroidism with RAI, nearly 23% of patients failed RAI treatment. The vast majority of these failures (75.7%) occurred within the first third of our follow-up time (1.3 years after treatment), but a significant portion of these failures (14.9%) happened beyond 2.6 years after treatment. A higher heart rate at presentation or more severe laboratory indices of hyperthyroidism (lower TSH and higher free T4) were associated with failure. Importantly, lower doses of RAI (< 13 mCi) were also associated with failure. These results can help clinicians in two ways: 1) identify patients at risk for failing RAI treatment and direct them to thyroidectomy, and 2) increase RAI doses for selected patients undergoing RAI.
In contrast to many studies on this topic, failure was defined here using a patient-centered designation—the need for repeat or alternate therapy for hyperthyroidism. Others have defined successful or failed treatment of hyperthyroidism based on thyroid function studies at defined time points (16–18). Here, we did not make any divisions between persistence and recurrence, and instead opted for a more all-encompassing examination of failure of RAI. However, only 15 patients failed within six months of treatment. Historically, patients were treated with the goal of achieving euthyroidism, but modern treatment strategy is to completely ablate the thyroid, rendering patients hypothyroid (4). In one study, Kendall-Taylor and colleagues found that only 5.6% of patients failed RAI at one year when the intent was complete ablation of the thyroid (19). In this retrospective series, we could not ascertain the intention of the treating provider(s). A variety of different providers (> 3), doses, and dosing strategies were used, but this enabled us to look at a range of doses, and enabled us to analyze the impact of dosage on failure rates. Importantly, we examined thyroid size both in terms of the clinician’s exam and largest dimension on ultrasound. Although goiter on exam was significant on univariate analysis (Table 3), it did not independently predict failure in the multivariate model (Table 4). Therefore, we feel a lower dose of RAI independently predicted failure regardless of thyroid size.
It is important to place the failure rate we found here in context with the failure rates for the other two treatment modalities, surgery and anti-thyroid medications. Anti-thyroid medications were associated with a much higher failure rate (52%) compared to RAI (15%) and surgery (10%) (20). With a shift in surgical thinking away from subtotal and toward total thyroidectomy, this 10% recurrence rate after surgery may reflect older literature (21–23). Hence, although the failure rate after RAI is not negligible, it is certainly better than continued anti-thyroid medications.
The failure rate for RAI reported here is slightly higher than the range reported in the literature of 14–20% (16, 18, 20, 24–26). This is likely a reflection of the variability in treatment strategies used by the physicians at our institution and the lack of a formal dosing algorithm. However, prospective studies report an even higher rate of failure than we did here in this retrospective series. For example, Metso and colleagues reported that 25% of patients relapsed after a single dose of RAI (27). In a randomized prospective study comparing RAI, surgery, and antithyroid medications for patients with Graves’ disease, Torring et al. report a 21% failure rate within the RAI group. When considering all patients who received more than one dose of RAI as a failure, then the rate of relapse in Torring’s trial exceeds 45% (10). Although the selection criteria and definition of failure may differ somewhat between these studies and our results, it appears that a single dose of RAI fails in at least 20–25% of all-comers, and patients should understand this when considering the various treatment options for hyperthyroidism. As mentioned above, the failure rate is much lower (5–6%) when the intention is complete ablation of the thyroid, or hypothyroidism. Therefore, if patients choose RAI with the expectation that it will work the first time, then the results reported here support the concept that higher doses should be used for this purpose (10, 19, 28). Alternatively, some patients might elect total thyroidectomy to eliminate the risk of recurrence altogether.
The relationship between dose and failure we found here is supported by the literature on this topic. Similar to these studies, we found that lower doses of RAI were associated with failure while higher doses were protective (16, 18, 24, 25). For example, Sztal-Mazer et al. found that patients treated with ≤ 15 mCi experienced a 26% failure rate (18). After the period of this study, our institution standardized its practice and now only gives doses greater than 8 mCi. It is hypothesized that higher doses carry additional risk for local side effects such as radiation thyroiditis and salivary gland dysfunction in addition to the development or exacerbation of opthalmopathy (20, 29, 30). The cancer incidence is higher among those previously treated with RAI for Graves’ disease, and there was a dose-response relationship (31, 32). RAI also increased the risk of thyroid cancer in patients with toxic multinodular goiter, but the underlying etiology may be the disease itself (31, 33). Unfortunately, we do not have accurate information regarding adverse outcomes from RAI in this study, but these risks must be balanced against the risk of recurrence.
Similar to our findings, others have also found that the percentage uptake (16) and higher pre-treatment free T4 (16, 24) were associated with failure. Finally, we also found that methimazole use predicted failure of RAI. Some evidence suggests that pre-treatment with antithyroid medications increases the risk of failure (34). In one study, this occurred with propylthiouracil, but not methimazole (35). We believe that methimazole is a marker for the severity of hyperthyroidism rather than intrinsically causing failure, but we continue to evaluate the use of preoperative medications in patients undergoing surgery.
Although this study is one of the largest to date examining failure of RAI, it is limited by its retrospective nature. These results may reflect the use and referral patterns unique to our center. In addition, we did not capture data related to the dosing strategy or rationale. These data reflect the practice of several different (> 3) nuclear medicine specialists. While many calculate an RAI dose based on estimated thyroid weights, these are simply estimations subject to considerable error and bias. Instead, we have included measured thyroid size by ultrasound in our univariate analysis, and these measures did not meet statistical criteria for inclusion in our final model. Furthermore, we have repeated our analysis by normalizing the doses to the percentage uptake (mCi/% uptake) and achieved very similar results (data not shown). Although our median follow-up time exceeds one year, we may not have captured failures treated outside our center. Those with little follow-up data (< 1 month) were excluded. Finally, while this cohort is purposefully heterogeneous to reflect all etiologies of hyperthyroidism, this was controlled for in our multivariate analysis.
In this large retrospective analysis of RAI treatment for hyperthyroidism with over four years of follow-up, nearly 23% of patients failed RAI, requiring a second dose or surgery in order to achieve cure. More severe laboratory indices (TSH, free T4) and a higher heart rate at presentation independently predict failure of RAI in the treatment of hyperthyroidism. Patients who present with these findings may prefer thyroidectomy as their initial definitive treatment option for hyperthyroidism in order to avoid relapse. Nonetheless, the decision regarding the optimal initial therapy remains complex and must consider many different factors including co-morbidities, living situation, smoking status, and the presence or absence of eye disease. Importantly, higher doses of RAI also protect against failure. This information can assist providers in counseling patients about their treatment options, selecting patients for surgery, or adjusting RAI doses to minimize the risk of failure.
Supplementary Material
Data are represented as the percentage of patients within the indicated RAI dose (A) or initial T3 level (B) who failed RAI.
Acknowledgements
This study was supported by NIH T32 CA009614-23.
Footnotes
Disclosure Summary: All authors have nothing to disclose.
References
- 1.Astrup A, Buemann B, Christensen NJ, Madsen J, Gluud C, Bennett P, Svenstrup B. The contribution of body composition, substrates, and hormones to the variability in energy expenditure and substrate utilization in premenopausal women. J Clin Endocrinol Metab. 1992;74(2):279–286. doi: 10.1210/jcem.74.2.1530952. [DOI] [PubMed] [Google Scholar]
- 2.Wilber JF. Thyrotropin releasing hormone: secretion and actions. Annu Rev Med. 1973;24:353–364. doi: 10.1146/annurev.me.24.020173.002033. [DOI] [PubMed] [Google Scholar]
- 3.Siegel E, Tobias CA. Actions of thyroid hormones on cultured human cells. Nature. 1966;212(5068):1318–1321. doi: 10.1038/2121318a0. [DOI] [PubMed] [Google Scholar]
- 4.Ross DS. Radioiodine therapy for hyperthyroidism. N Engl J Med. 2011;364(6):542–550. doi: 10.1056/NEJMct1007101. [DOI] [PubMed] [Google Scholar]
- 5.Schussler-Fiorenza CM, Bruns CM, Chen H. The surgical management of Graves' disease. J Surg Res. 2006;133(2):207–214. doi: 10.1016/j.jss.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Wartofsky L, Glinoer D, Solomon B, Nagataki S, Lagasse R, Nagayama Y, Izumi M. Differences and similarities in the diagnosis and treatment of Graves' disease in Europe, Japan, and the United States. Thyroid. 1991;1(2):129–135. doi: 10.1089/thy.1991.1.129. [DOI] [PubMed] [Google Scholar]
- 7.Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves' disease. J Clin Endocrinol Metab. 2012;97(12):4549–4558. doi: 10.1210/jc.2012-2802. [DOI] [PubMed] [Google Scholar]
- 8.Bahn Chair RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, Montori VM, Rivkees SA, Ross DS, Sosa JA, Stan MN. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21(6):593–646. doi: 10.1089/thy.2010.0417. [DOI] [PubMed] [Google Scholar]
- 9.Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, Segal RL. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8(6):457–469. [PubMed] [Google Scholar]
- 10.Torring O, Tallstedt L, Wallin G, Lundell G, Ljunggren JG, Taube A, Saaf M, Hamberger B. Graves' hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine--a prospective, randomized study. Thyroid Study Group. J Clin Endocrinol Metab. 1996;81(8):2986–2993. doi: 10.1210/jcem.81.8.8768863. [DOI] [PubMed] [Google Scholar]
- 11.Porterfield JR, Jr, Thompson GB, Farley DR, Grant CS, Richards ML. Evidence-based management of toxic multinodular goiter (Plummer's Disease) World J Surg. 2008;32(7):1278–1284. doi: 10.1007/s00268-008-9566-0. [DOI] [PubMed] [Google Scholar]
- 12.Hegedus L. Treatment of Graves' hyperthyroidism: evidence-based and emerging modalities. Endocrinol Metab Clin North Am. 2009;38(2):355–371. doi: 10.1016/j.ecl.2009.01.009. ix. [DOI] [PubMed] [Google Scholar]
- 13.Read CH, Jr, Tansey MJ, Menda Y. A 36-year retrospective analysis of the efficacy and safety of radioactive iodine in treating young Graves' patients. J Clin Endocrinol Metab. 2004;89(9):4229–4233. doi: 10.1210/jc.2003-031223. [DOI] [PubMed] [Google Scholar]
- 14.Wong KP, Lang BH. Graves' ophthalmopathy as an indication increased the risk of hypoparathyroidism after bilateral thyroidectomy. World journal of surgery. 2011;35(10):2212–2218. doi: 10.1007/s00268-011-1236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudberg C, Johansson H, Akerstrom G, Tuvemo T, Karlsson FA. Graves' disease in children and adolescents. Late results of surgical treatment. European journal of endocrinology / European Federation of Endocrine Societies. 1996;134(6):710–715. doi: 10.1530/eje.0.1340710. [DOI] [PubMed] [Google Scholar]
- 16.Alexander EK, Larsen PR. High dose of (131)I therapy for the treatment of hyperthyroidism caused by Graves' disease. J Clin Endocrinol Metab. 2002;87(3):1073–1077. doi: 10.1210/jcem.87.3.8333. [DOI] [PubMed] [Google Scholar]
- 17.Chen DY, Jing J, Schneider PF, Chen TH. Comparison of the long-term efficacy of low dose 131I versus antithyroid drugs in the treatment of hyperthyroidism. Nucl Med Commun. 2009;30(2):160–168. doi: 10.1097/MNM.0b013e3283134d4d. [DOI] [PubMed] [Google Scholar]
- 18.Sztal-Mazer S, Nakatani VY, Bortolini LG, Boguszewski CL, Graf H, de Carvalho GA. Evidence for higher success rates and successful treatment earlier in Graves' disease with higher radioactive iodine doses. Thyroid. 2012;22(10):991–995. doi: 10.1089/thy.2011.0362. [DOI] [PubMed] [Google Scholar]
- 19.Kendall-Taylor P, Keir MJ, Ross WM. Ablative radioiodine therapy for hyperthyroidism: long term follow up study. British medical journal. 1984;289(6441):361–363. doi: 10.1136/bmj.289.6441.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundaresh V, Brito JP, Wang Z, Prokop LJ, Stan MN, Murad MH, Bahn RS. Comparative effectiveness of therapies for Graves' hyperthyroidism: a systematic review and network meta-analysis. The Journal of clinical endocrinology and metabolism. 2013;98(9):3671–3677. doi: 10.1210/jc.2013-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Bargren A, Schaefer S, Chen H, Sippel RS. Total thyroidectomy: a safe and effective treatment for Graves' disease. The Journal of surgical research. 2011;168(1):1–4. doi: 10.1016/j.jss.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch KC, McHenry CR. Total thyroidectomy: is morbidity higher for Graves' disease than nontoxic goiter? The Journal of surgical research. 2011;170(1):96–99. doi: 10.1016/j.jss.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 23.Yip J, Lang BH, Lo CY. Changing trend in surgical indication and management for Graves' disease. American journal of surgery. 2012;203(2):162–167. doi: 10.1016/j.amjsurg.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Allahabadia A, Daykin J, Holder RL, Sheppard MC, Gough SC, Franklyn JA. Age and gender predict the outcome of treatment for Graves' hyperthyroidism. J Clin Endocrinol Metab. 2000;85(3):1038–1042. doi: 10.1210/jcem.85.3.6430. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Jimenez S, Pachon-Burgos A, Aguilar-Salinas CA, Andrade V, Reynoso R, Rios A, Reza-Albarran AA, Mehta R, Gonzalez-Trevino O, Gomez-Perez FJ, Perez-Enriquezi B, Rull JA. Radioiodine treatment in autoimmune hyperthyroidism: analysis of outcomes in relation to dosage. Arch Med Res. 2007;38(2):185–189. doi: 10.1016/j.arcmed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Grosso M, Traino A, Boni G, Banti E, Della Porta M, Manca G, Volterrani D, Chiacchio S, AlSharif A, Borso E, Raschilla R, Di Martino F, Mariani G. Comparison of different thyroid committed doses in radioiodine therapy for Graves' hyperthyroidism. Cancer Biother Radiopharm. 2005;20(2):218–223. doi: 10.1089/cbr.2005.20.218. [DOI] [PubMed] [Google Scholar]
- 27.Metso S, Jaatinen P, Huhtala H, Luukkaala T, Oksala H, Salmi J. Long-term follow-up study of radioiodine treatment of hyperthyroidism. Clin Endocrinol (Oxf) 2004;61(5):641–648. doi: 10.1111/j.1365-2265.2004.02152.x. [DOI] [PubMed] [Google Scholar]
- 28.Caruso DR, Mazzaferri EL. Intervention in Graves' disease. Choosing among imperfect but effective treatment options. Postgrad Med. 1992;92(8):117–124. doi: 10.1080/00325481.1992.11701555. 128-119, 133-114. [DOI] [PubMed] [Google Scholar]
- 29.de los Santos ET, Mazzaferri EL. Thyrotoxicosis. Results and risks of current therapy. Postgrad Med. 1990;87(5):277–278. 281-276, 291-274. [PubMed] [Google Scholar]
- 30.Stan MN, Bahn RS. Risk factors for development or deterioration of Graves' ophthalmopathy. Thyroid. 2010;20(7):777–783. doi: 10.1089/thy.2010.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ron E, Doody MM, Becker DV, Brill AB, Curtis RE, Goldman MB, Harris BS, 3rd, Hoffman DA, McConahey WM, Maxon HR, Preston-Martin S, Warshauer ME, Wong FL, Boice JD., Jr Cancer mortality following treatment for adult hyperthyroidism. Cooperative Thyrotoxicosis Therapy Follow-up Study Group. JAMA. 1998;280(4):347–355. doi: 10.1001/jama.280.4.347. [DOI] [PubMed] [Google Scholar]
- 32.Metso S, Auvinen A, Huhtala H, Salmi J, Oksala H, Jaatinen P. Increased cancer incidence after radioiodine treatment for hyperthyroidism. Cancer. 2007;109(10):1972–1979. doi: 10.1002/cncr.22635. [DOI] [PubMed] [Google Scholar]
- 33.Smith JJ, Chen X, Schneider DF, Nookala R, Broome JT, Sippel RS, Chen H, Solorzano CC. Toxic nodular goiter and cancer: a compelling case for thyroidectomy. Ann Surg Oncol. 2013;20(4):1336–1340. doi: 10.1245/s10434-012-2725-4. [DOI] [PubMed] [Google Scholar]
- 34.Walter MA, Briel M, Christ-Crain M, Bonnema SJ, Connell J, Cooper DS, Bucher HC, Muller-Brand J, Muller B. Effects of antithyroid drugs on radioiodine treatment: systematic review and meta-analysis of randomised controlled trials. BMJ. 2007;334(7592):514. doi: 10.1136/bmj.39114.670150.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imseis RE, Vanmiddlesworth L, Massie JD, Bush AJ, Vanmiddlesworth NR. Pretreatment with propylthiouracil but not methimazole reduces the therapeutic efficacy of iodine-131 in hyperthyroidism. J Clin Endocrinol Metab. 1998;83(2):685–687. doi: 10.1210/jcem.83.2.4538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data are represented as the percentage of patients within the indicated RAI dose (A) or initial T3 level (B) who failed RAI.


