Abstract
Background
Ultrasound (US) is a standard preoperative study in thyroid cancer. Accurate identification of lymph node (LN) disease in the central neck by US is debated, leading some surgeons to perform prophylactic central dissection. The aim of this study was to evaluate if US performed by a surgeon with specialization in thyroid sonography correctly determined clinical N0 status.
Methods
Retrospective identification of cN0 thyroid cancer patients from a prospectively maintained database was performed. Exclusion criteria included LN dissection with thyroidectomy or missing pre-operative US. Demographics and outcomes were reviewed. Patients were categorized by who performed the thyroid US (surgeon vs. non-surgeon). Additional radioactive iodine (RAI) treatments or subsequent positive pathology defined recurrence.
Results
From 2005 to 2012, 177 patients met criteria. 48 patients had surgeon US versus 129 patients with non-surgeon US. Groups were equivalent in age, gender, and tumor size. 46% had a pre-operative diagnosis of cancer, while 19% had benign and 35% had indeterminate diagnoses. Surgeon US documented LN status more frequently (69% vs. 20%, p<0.01). RAI treatment and dose were equivalent. RAI uptake was lower with surgeon US (0.06%±0.02 vs. 0.20%±0.03, p<0.01). Recurrence rates were higher in non-surgeon US (12% vs. 0%, p=0.01). Median time to recurrence was 11 months.
Conclusions
Surgeons with thyroid US expertise correctly identify patients as N0 which may eliminate the need for prophylactic LN dissection without increasing risk of early recurrence. Since not all thyroid cancers are diagnosed pre-operatively, US examination of the thyroid should include routine evaluation of the cervical LNs.
Keywords: Well differentiated thyroid cancer, surgeon performed ultrasound, ultrasonography, N0 disease, thyroid cancer staging, lymph node dissection, persistence, recurrence
Introduction
Cervical lymph node (LN) involvement in well-differentiated thyroid cancer (DTC) is common. For patients over age 45, it also impacts staging1, 2. Preoperative physical exam and ultrasound (US) are the mainstays for determining LN involvement prior surgery, although occasionally suspicious central LNs are encountered at time of operation prompting a therapeutic central lymph node dissection (LND)1, 3–8. Patients felt to be clinically node negative (cN0) based on preoperative US do not need a therapeutic LND, although the use of prophylactic central LND in cN0 patients is hotly debated9–11.
Currently, preoperative assessment of the cervical LN in thyroid cancer patients is performed via US due to increased sensitivity to detect metastatic involvement of LN when compared to manual palpation1, 3–8, 10. Traditionally, this assessment was performed by radiologists, however in the recent decade, US has become a common tool for the surgeon and endocrinologist alike3–5, 7, 12–22. Use of US during surgical training has become integrated into multiple different specialties; trauma, breast, abdominal, vascular, critical care, and head and neck surgery 23. As interpretation of US images can vary greatly, expertise in thyroid imaging as well as consistency of whom is performing the study results in optimal outcomes 11, 15, 16, 24, 25. Access to a specialized thyroid sonographer is not available at all institutions. In cases where the department of radiology does not have the resources to dedicate a single individual or team with expertise in thyroid imaging, the surgeon sonographer with specialization in the care of thyroid cancer can provide consistency in interpretation and expertise in thyroid imaging 3, 11, 12, 15, 18, 20, 26.
The aim of this study was to assess recurrence rates in cN0, DTC patients, and determine if surgeon performed US in contrast to non-surgeon performed US resulted in differences in early disease recurrence.
Methods
With IRB approval, a retrospective review of a prospectively collected thyroid database at a large tertiary referral center was performed. Patients with cN0, DTC with a minimum of 6 months of follow-up were included. The diagnosis of DTC was based on either fine needle aspiration (FNA) cytology or final surgical pathology. In some instances, the diagnosis of cancer was not known at time of US examination, or surgery. As institutional practice involves compartment based LND for clinically N1a or N1b disease, patients undergoing LND, either central or lateral, at the time of initial thyroidectomy were excluded. Prophylactic LND of the central or lateral compartment for well-differentiated thyroid cancer is not performed at our institution. Patients without documented preoperative US were excluded. Patients found to have micro papillary thyroid cancer (PTC, <1 cm) were only included if an additional worrisome feature was noted on final pathology (multi-focality, extra-thyroidal extension, lymphovascular invasion or positive margins).
Patients were categorized by who performed the US; the operative surgeon, or a non-surgeon. The surgeon performing thyroid ultrasound had successfully completed the American College of Surgeons Head and Neck US course, and currently serves as a course instructor. Surgeon performed US occurred during initial clinic visit, occasionally these were repeated in the operating room prior incision. The study institution does not have a dedicated individual or team of radiologists who specialize in thyroid cancer; thyroid US is done by a variety of different radiologists with expertise in US, but not necessarily thyroid cancer. To determine if the central and lateral compartments were assessed during US, the provider needed to specifically comment on LN with an associated descriptor as well as which compartments were evaluated. If no comment was specifically found regarding LN in both the central and lateral neck, the patient was classified as no LN evaluation.
Some patients had multiple tumor histologies on final pathology (i.e. PTC and Follicular, PTC and Hurthle Cell, etc.). For this reason, the frequency of each tumor type was totaled. Administration and dosing of radioactive iodine ablation (RAI) was determined by the endocrinologists within the study institution. Patients were monitored for recurrence by endocrinology with suppressed thyroglobulin levels and an US examination at 6 months, followed by a stimulated thyroglobulin level and US examination at one year1. Diagnostic whole body scan was generally performed if US or thyroglobulin results were concerning for residual or recurrent disease. Follow-up after one year relied on annual suppressed thyroglobulin level, and US evaluation of the neck. Disease recurrence was defined as the need for additional RAI treatment, as positive FNA or positive final pathology on operative re-exploration. Staged lymph node dissections, or staged completion thyroidectomies were not considered recurrences. Time to recurrence was calculated in months from time of initial operation, to time of subsequent intervention (RAI or surgical resection).
Statistical analysis was performed using IBM SPSS Statistics, version 20.0. Pearson’s Chi-Square, Fisher’s Exact, and unpaired t-tests were performed as appropriate. Kaplan Meier survival analysis was performed with outcome listed as time to recurrence or time to last disease free follow-up. Comparison of estimated disease free curves was performed using Mantel-Cox Log Rank. Data are expressed as mean ± standard error of the mean, or as number (percentage), unless otherwise specified. A p-value ≤ 0.05 was determined to be significant.
Results
Between 2005 and 2012, 322 patients with cN0, DTC were identified. Seventy three patients were excluded for less than 6 month follow up available within the electronic medical record. An additional 59 patients with micro PTC with low risk features on histology (uni-focal, intra-thyroidal, no lymphovascular invasion, and negative margins) were also excluded. Finally, 13 (4%) patients were noted to have no documented preoperative US, by either radiology report or by reference via clinician note and were excluded. The final study population was 177 patients.
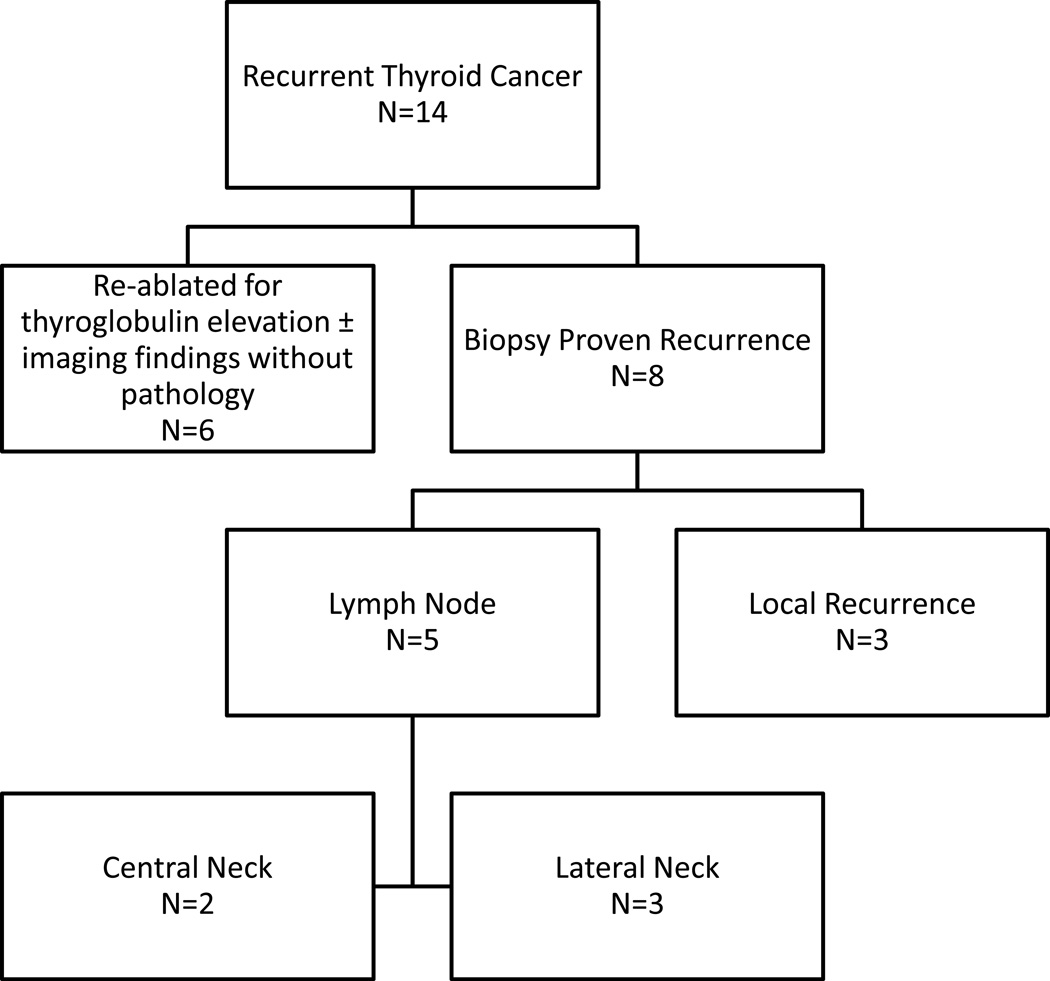
The study population had an average age of 49 ± 1.1 years, and 73% were female. Eighty-one patients (46%) had a diagnosis of thyroid cancer prior to surgery, while 63 patients (35%) had indeterminate biopsy results, and 34 patients (19%) were undergoing surgery for a presumed benign condition (Graves’, goiter, etc.). Surgeon performed US occurred in 48 cases (27%), while the remaining 129 patients (73%) had a non-surgeon performed US. Regardless of the sonographer (surgeon versus non-surgeon), only 59 patients (33%) had a full LN evaluation documented at time of US. However, the timing of the US may have occurred before the diagnosis of cancer was established. Overall, 14 recurrences (8%) were noted (Figure 1).
Figure 1.
Breakdown of patients considered to have persistent and/or recurrent disease based on treatment and/or location/type of disease.
Patients were grouped based on who performed their US evaluation, surgeon or non-surgeon. Patient age (p=0.77), and gender (p=0.57) were equivalent between groups (Table 1). The preoperative diagnosis based on FNA results and/or clinical diagnosis (ie. Graves’) were of similar distribution of benign, indeterminate and malignant between groups (p=0.26). Patients with a surgeon performed US were much more likely to have evaluation of their cervical LN than those patients undergoing ultrasound evaluation by a non-surgeon provider (69% vs. 20%, p<0.01).
Table 1.
Patient pre-operative demographic information.
| Non-Surgeon Sonographer |
Surgeon Sonographer |
P-Value | ||
|---|---|---|---|---|
| N | 129 | 48 | ||
| Age (Years) | 50 ± 1.4 | 49 ± 2.2 | 0.75 | |
| Female | 92 (71%) | 37 (77%) | 0.57 | |
| Pre-Op Diagnosis | 0.28 | |||
| Benign | 23 (18%) | 11 (23%) | ||
| Indeterminate | 43 (33%) | 20 (42%) | ||
| Malignant | 63 (49%) | 17 (35%) | ||
| Documented Assessment of Cervical Lymph Nodes with US | 26 (20%) | 33 (69%) | <0.01 | |
Data expressed as mean ± standard error of the mean, or number (percentage).
With the exception of the surgeon performed US group having a higher incidence of follicular thyroid carcinoma (19% vs. 4%, p<0.01), the groups had equal rates of PTC (p=0.21), hurthle cell carcinoma (p=1) and background thyroiditis (p=0.60, Table 2). The surgeon performed US group had a greater incidence of lymphovascular invasion noted on histology (13% vs. 3%, p=0.03), while the remaining histologic characteristics of the primary tumor were equivalent. On final pathology, tumor size (p=0.13) and total gland weight (p=0.93) did not differ.
Table 2.
Tumor type and pathologic characteristics.
| Non-Surgeon Sonographer |
Surgeon Sonographer |
P-Value | ||
|---|---|---|---|---|
| N | 129 | 48 | ||
| Tumor Type | ||||
| Papillary | 121 (94%) | 42 (88%) | 0.21 | |
| Follicular | 5 (4%) | 9 (19%) | <0.01 | |
| Hurthle Carcinoma | 5 (4%) | 1 (2%) | 1.0 | |
| Micro PTC | 26 (20%) | 8 (17%) | 0.39 | |
| Pathologic Characteristics | ||||
| Multi-Focal | 75 (58%) | 30 (63%) | 0.73 | |
| Extra-thyroidal Extension | 13 (10%) | 4 (8%) | 1.0 | |
| Positive Margin | 8 (6%) | 3 (7%) | 1.0 | |
| Lymphovascular Invasion | 4 (3%) | 6 (13%) | 0.03 | |
| Lymphocytic Thyroiditis | 44 (34%) | 19 (40%) | 0.60 | |
| Tumor Size (cm) | 1.8 ± 0.1 | 2.2 ± 0.2 | 0.13 | |
| Size of Micro PTC (cm) | 0.6 ± 0.05 | 0.6 ± 0.07 | 0.71 | |
| Gland Weight (gm) | 25 ± 3.9 | 26 ± 2.6 | 0.92 | |
Data expressed as number (percentage) or as mean ± standard error of the mean.
RAI was used with equal frequency (p=0.41), and equivalent doses (p=0.31, Table 3). Median follow-up was shorter in the surgeon performed US group (20 months vs. 34 months, p<0.01). However, median time to recurrence was 11 months, with first recurrence detected at 6 months, and last recurrence detected at 6 years. Only 2 recurrences were diagnosed beyond 15 months, and occurred between 4 and 6 years after initial surgery. Of the remaining patients, disease was detected within the first year from surgery in 7, and in 5 patients, shortly after the 1 year anniversary of their initial operation. No patient in the surgeon performed US group had evidence of disease recurrence at time of last follow-up, compared to 14 patients (12%) in the non-surgeon performed US group (p=0.01).
Table 3.
Post-operative management and disease specific outcomes.
| Non-Surgeon Sonographer |
Surgeon Sonographer |
P-Value | |
|---|---|---|---|
| RAI | 114 (88%) | 45 (94%) | 0.41 |
| RAI Dose (mCi) | 83 ± 5 | 93 ± 6 | 0.31 |
| Remnant Uptake | 0.2 ± 0.03 | 0.06 ± 0.02 | <0.01 |
| Follow Up (Months) | 34 (16–64) | 20 (10–34) | <0.01 |
| Disease Recurrence | 14 (12%) | 0 | 0.01 |
| Time to Recurrence (Months) | 11 (6.6) | 0 | <0.01 |
Data expressed as number (percentage), mean ± standard error of the mean, or median (interquartile range) as appropriate.
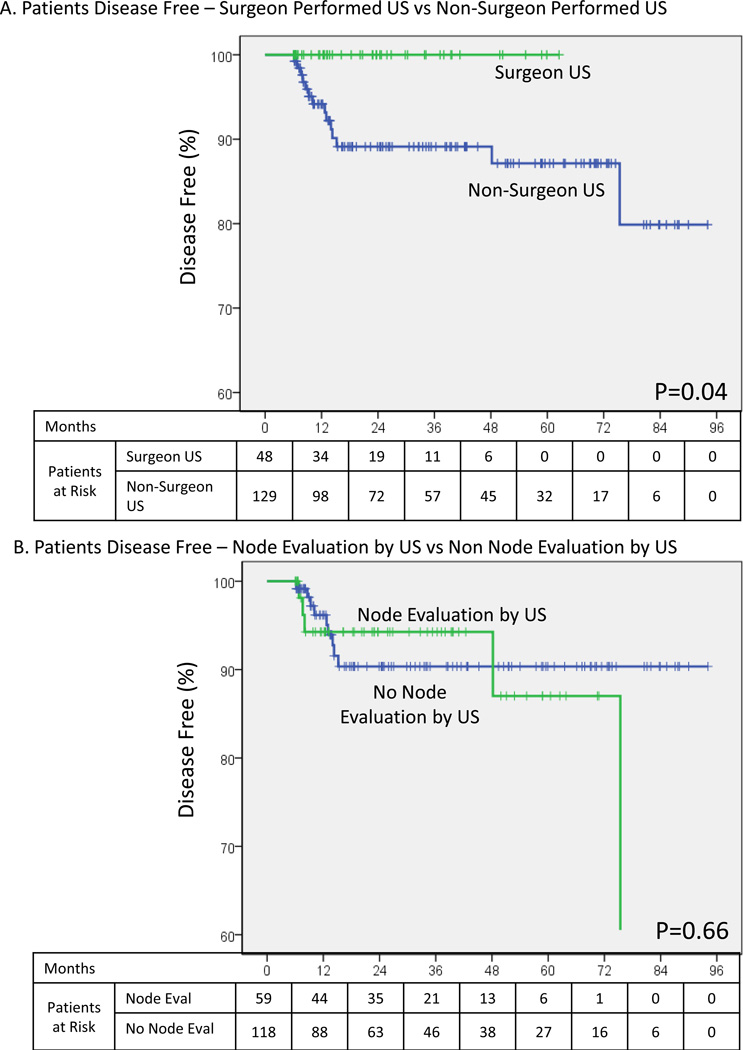
Grouping patients based on if the operative surgeon performed an US evaluation of the neck, a Kaplan Meier curve for disease free interval was constructed (Figure 2A). Patients having US exam performed only by a non-surgeon were disease free 94% at 1 year, 89% at 2 years, and 87% at 5 years. This was in marked contrast to the group with surgeon performed US who were disease free 100% at 1, 2 and 5 years (p=0.04). To ensure that the specialty of the individual performing the US evaluation was not a confounder for LN assessment, an additional analysis specific to documented LN assessment was also performed (Figure 2B). Estimated disease free status did not differ between these groups (p=0.66).
Figure 2.
Disease free status. A – Based on who performed the pre-operative ultrasound evaluation. B – Based on if pre-operative ultrasound evaluation included a lymph node assessment.
Discussion
In the hands of an experienced thyroid surgeon, trained in thyroid US, the classification of a patient as cN0 and forgoing prophylactic LND resulted in no recurrences to date, with actuarial follow-up out to 5 years. In contrast, patients undergoing a non-surgeon US experienced a recurrence rate of 12%, with 86% of recurrences occurring within the first 15 months of diagnosis. This early time to recurrence is suggestive of unrecognized disease present at time of initial diagnosis, or persistent disease. These data also support previous reports that a negative US of the central neck by experienced sonographers predicts long-term regional control, and that the microscopic disease found during prophylactic dissection may not impact short-term disease free survival 10, 11, 27, 28.
US is a highly operator dependent modality, and variability in image interpretation between sonographers is problematic15, 16, 24, 25, 29. Rosario evaluated US assessment of the cervical LN during surveillance in patients with known high risk PTC 29. Radiologists at a diagnostic imaging center, without specific specialization in thyroid imaging, missed half of the cervical metastasis caught two weeks later by a specialized thyroid sonographer. Previous work from this institution, as well as from other authors, have described the omission of LN commentary on thyroid US reports, even when the evaluation of the cervical LN were specifically requested 3, 26, 29, 30. For purposes of this study, patients with omitted LN commentary were classified as not having the assessment performed.
Surgeons have access to all pertinent clinical information at time of US, excellent understanding of the local anatomy, as well as feedback from final pathologic results to continue to learn the finer nuances of ultrasound findings within the neck 3, 4, 15, 18, 20. Radiology educational literature emphasizes the importance of repetition, in addition to familiarity with the key imaging characteristics, for greater accuracy of thyroid US interpretation24, 25. Thyroid surgeons, by using US weekly in both the clinic and operating room, can quickly develop the skills needed to proficiently and accurately perform thyroid US.
The timing of thyroid US during the course of patient work up may also influence image interpretation. During a thyroid nodule work up, an US along with thyroid function tests are initially ordered1. As the patient is deemed to need further evaluation and if necessary, referral for endocrinology or surgical consultation, the underlying index of suspicion for malignancy increases. At this time, a provider specialized in the care of thyroid cancer can scrutinize the US characteristics of the nodule, the remaining thyroid, as well as assess LN appearance. These variables can be placed within the context of the patient history, physical findings and biopsy results, to formulate an opinion regarding both the suspicion for malignancy and LN involvement 3, 7, 20, 31. The findings of improved short-term disease free survival with surgeon US within this study are supportive of this as well.
Only 46% of our study population had an established diagnosis of cancer before surgery, which likely influenced the extent of the pre-operative ultrasound evaluation. The remainder of patients had indeterminate or benign pathologies, requiring operative intervention. Given these non-malignant diagnoses, under current guidelines, LN assessment would not be indicated32. However, when patients undergo a diagnostic lobectomy for indeterminate cytology, and final pathology returns as malignant, LN assessment in a recently operated neck may be less reliable. Findings of suspicious cervical lymphadenopathy in the setting of suspicious or indeterminate cytology may prompt additional evaluation, and confirm the diagnosis of malignancy in time to alter the operative plan 3, 30, 33.
LN assessment is recommended to occur via physical exam at the initial stage of thyroid nodule work up, however studies have shown that US is superior to physical exam in detecting worrisome LN 1, 7, 8, 33, 34. For these reasons, the authors advocate the routine cervical LN assessment with clear documentation of findings during initial thyroid US 2–4, 20, 35. Evaluation of the LN at time of initial thyroid US would not add a substantial amount of time to the examination, and would streamline care by avoiding additional appointments for dedicated LN assessment.18 Results of this study have prompted ongoing quality improvement and continuing medical education within the study institution, as well as the surrounding medical community, emphasizing the importance of lymph node involvement at time of thyroid US.
Management of thyroid cancer requires a strong interdisciplinary team to facilitate the diagnosis, management, and long term follow-up. Dedicated endocrinologists, surgeons, radiologist and nuclear medicine physicians are critical to ensure a successful thyroid cancer program 6. However, not every institution has all of these resources at their disposal, and overlapping skill sets between the providers may be necessary 12, 20, 33. While these results are specific to surgeon performed US within the study institution, a dedicated thyroid sonographer of any specialty could achieve comparable outcomes.
As this study is retrospective in nature, it is has its inherent flaws. The study population consists of only patients with negative findings on US, who did not undergo LN excision. Given the initial patient selection based on an absence of LND at time of initial surgery, as well as the presence of cancer on final pathology, it is unknown how many patients had negative US imaging, but during thyroidectomy suspicious LN were encountered prompting subsequent LND. Therefore, sensitivity, specificity, positive or negative predictive value of US on the detection of LN metastases cannot be calculated. While clinically significant disease was not identified in follow up, this does not equate to the absence of microscopic disease. The length of follow-up included can attest to early recurrence or persistence, but long term (>5 year) outcomes cannot be assumed based on these data. Ongoing data collection for these cohorts of patients is being performed to see how long-term recurrence rates may differ between the cohorts. This will also determine the durability of the initial US evaluation.
While the study population does not differ in basic patient demographics, they are inherently different by the mere fact that a portion of the non-surgeon group includes patients erroneously categorized as cN0 who with follow up have evidence of persistent disease. This disease was likely present at time of initial preoperative consultation, but was missed. Patients undergoing surgeon US had this disease initially detected, and were able to undergo therapeutic LND. However, this very fact drives home the point that surgeon US can correctly stratify patients before operative intervention.
Conclusion
Here in, we demonstrate that a surgeon sonographer with expertise in thyroid cancer can provide an accurate assessment of the LN status in both the central and lateral neck, as demonstrated by the 100% disease free status at time of last follow-up. This implies that a thorough US examination of the cervical LN can detect clinically relevant disease in DTC. A negative, high quality US of the cervical LN may obviate the need for a prophylactic central LND. As not all patients have an established diagnosis of cancer at time of thyroid US, additional information provided by a LN evaluation can lead to the correct diagnosis. Assessment of the cervical LN should be a standard part of any thyroid US. It is critical that an experienced sonographer provide this assessment to enable the proper extent of surgery and reduce early recurrence.
Synopsis.
Thorough, ultrasound examination of the cervical lymph node stations performed by a surgeon with expertise in thyroid sonography can correctly identify patients as clinically N0 in well differentiated thyroid cancer, without increased risk of early recurrence or missed disease.
Footnotes
Poster Presentation at American Thyroid Association, San Juan, Puerto Rico, October 2013. For consideration in Annals of Surgical Oncology
References
- 1.Cooper DS, et al. American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2009 Nov;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.American Thyroid Association Surgery Working G; American Association of Endocrine S; American Academy of O-H et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2009 Nov;19(11):1153–1158. doi: 10.1089/thy.2009.0159. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaglia PJ. Surgeon-performed ultrasound in patients referred for thyroid disease improves patient care by minimizing performance of unnecessary procedures and optimizing surgical treatment. World journal of surgery. 2010 Jun;34(6):1164–1170. doi: 10.1007/s00268-010-0402-y. [DOI] [PubMed] [Google Scholar]
- 4.Milas M, Stephen A, Berber E, Wagner K, Miskulin J, Siperstein A. Ultrasonography for the endocrine surgeon: a valuable clinical tool that enhances diagnostic and therapeutic outcomes. Surgery. 2005 Dec;138(6):1193–1200. doi: 10.1016/j.surg.2005.08.032. discussion 1200-1191. [DOI] [PubMed] [Google Scholar]
- 5.Miller BS, Gauger PG, Broome JT, Burney RE, Doherty GM. An international perspective on ultrasound training and use for thyroid and parathyroid disease. World journal of surgery. 2010 Jun;34(6):1157–1163. doi: 10.1007/s00268-010-0481-9. [DOI] [PubMed] [Google Scholar]
- 6.Schneider DF, Chen H. New developments in the diagnosis and treatment of thyroid cancer. CA: a cancer journal for clinicians. 2013 Jun 24; doi: 10.3322/caac.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solorzano CC, Carneiro DM, Ramirez M, Lee TM, Irvin GL., 3rd Surgeon-performed ultrasound in the management of thyroid malignancy. The American surgeon. 2004 Jul;70(7):576–580. discussion 580-572. [PubMed] [Google Scholar]
- 8.Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003 Dec;134(6):946–954. doi: 10.1016/s0039-6060(03)00424-0. discussion 954-945. [DOI] [PubMed] [Google Scholar]
- 9.Carling T, Carty SE, Ciarleglio MM, et al. American Thyroid Association Design and Feasibility of a Prospective Randomized Controlled Trial of Prophylactic Central Lymph Node Dissection for Papillary Thyroid Carcinoma. Thyroid : official journal of the American Thyroid Association. 2012;22(3):237–244. doi: 10.1089/thy.2011.0317. [DOI] [PubMed] [Google Scholar]
- 10.Randolph GW, Duh Q-Y, Heller KS, et al. The Prognostic Significance of Nodal Metastases from Papillary Thyroid Carcinoma Can Be Stratified Based on the Size and Number of Metastatic Lymph Nodes, as Well as the Presence of Extranodal Extension. Thyroid : official journal of the American Thyroid Association. 2012;22(11):1144–1152. doi: 10.1089/thy.2012.0043. [DOI] [PubMed] [Google Scholar]
- 11.Moreno MA, Edeiken-Monroe BS, Siegel ER, Sherman SI, Clayman GL. In papillary thyroid cancer, preoperative central neck ultrasound detects only macroscopic surgical disease, but negative findings predict excellent long-term regional control and survival. Thyroid : official journal of the American Thyroid Association. 2012 Apr;22(4):347–355. doi: 10.1089/thy.2011.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-azawi D, Mann GB, Judson RT, Miller JA. Endocrine surgeon-performed US guided thyroid FNAC is accurate and efficient. World journal of surgery. 2012 Aug;36(8):1947–1952. doi: 10.1007/s00268-012-1592-2. [DOI] [PubMed] [Google Scholar]
- 13.Choi JS, Chung WY, Kwak JY, Moon HJ, Kim MJ, Kim EK. Staging of papillary thyroid carcinoma with ultrasonography: performance in a large series. Annals of surgical oncology. 2011 Dec;18(13):3572–3578. doi: 10.1245/s10434-011-1783-3. [DOI] [PubMed] [Google Scholar]
- 14.Goldfarb M, Gondek SS, Sanchez Y, Lew JI. Clinic-Based Ultrasound Can Predict Malignancy in Pediatric Thyroid Nodules. Thyroid : official journal of the American Thyroid Association. 2012;22(8):827–831. doi: 10.1089/thy.2011.0494. [DOI] [PubMed] [Google Scholar]
- 15.Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. The Laryngoscope. 2011 Mar;121(3):487–491. doi: 10.1002/lary.21227. [DOI] [PubMed] [Google Scholar]
- 16.Kabaker AS, Tublin ME, Nikiforov YE, et al. Suspicious Ultrasound Characteristics PredictBRAFV600E-Positive Papillary Thyroid Carcinoma. Thyroid : official journal of the American Thyroid Association. 2012;22(6):585–589. doi: 10.1089/thy.2011.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kangelaris GT, Kim TB, Orloff LA. Role of ultrasound in thyroid disorders. Otolaryngologic clinics of North America. 2010 Dec;43(6):1209–1227. doi: 10.1016/j.otc.2010.08.006. vi. [DOI] [PubMed] [Google Scholar]
- 18.Lee CY, Snyder SK, Lairmore TC, Dupont SC, Jupiter DC. Utility of surgeon-performed ultrasound assessment of the lateral neck for metastatic papillary thyroid cancer. Journal of oncology. 2012;2012:973124. doi: 10.1155/2012/973124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The American Association of Clinical Endocrinologists and the American Association of Endocrine Surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2005 Jan-Feb;11(1):49–54. doi: 10.4158/EP.11.1.49. [DOI] [PubMed] [Google Scholar]
- 20.Mendez W, Rodgers SE, Lew JI, Montano R, Solorzano CC. Role of surgeon-performed ultrasound in predicting malignancy in patients with indeterminate thyroid nodules. Annals of surgical oncology. 2008 Sep;15(9):2487–2492. doi: 10.1245/s10434-008-0052-6. [DOI] [PubMed] [Google Scholar]
- 21.Akinci B, Demir T, Yener S, et al. Beneficial effect of endocrinologist-performed ultrasonography on preoperative parathyroid adenoma localization. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2009 Jan-Feb;15(1):17–23. doi: 10.4158/EP.15.1.17. [DOI] [PubMed] [Google Scholar]
- 22.Zangeneh F, Powell CC, Gharib H. A survey on the use of thyroid ultrasonography in clinical endocrinology training programs. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2003 Mar-Apr;9(2):162–163. doi: 10.4158/EP.9.2.162. [DOI] [PubMed] [Google Scholar]
- 23.Rozycki GS. Surgeon-performed ultrasound: its use in clinical practice. Annals of surgery. 1998 Jul;228(1):16–28. doi: 10.1097/00000658-199807000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HG, Kwak JY, Kim EK, Choi SH, Moon HJ. Man to man training: can it help improve the diagnostic performances and interobserver variabilities of thyroid ultrasonography in residents? European journal of radiology. 2012 Mar;81(3):e352–e356. doi: 10.1016/j.ejrad.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Park CS, Jung SL, et al. Observer variability and the performance between faculties and residents: US criteria for benign and malignant thyroid nodules. Korean journal of radiology : official journal of the Korean Radiological Society. 2010 Mar-Apr;11(2):149–155. doi: 10.3348/kjr.2010.11.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poehls JL, Chen H, Sippel RS. Preoperative ultrasonography findings predict the need for repeated surgery in papillary thyroid cancer. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2012 May-Jun;18(3):403–409. doi: 10.4158/EP11221.OR. [DOI] [PubMed] [Google Scholar]
- 27.Shen WT, Ogawa L, Ruan D, Suh I, Duh QY, Clark OH. Central neck lymph node dissection for papillary thyroid cancer: the reliability of surgeon judgment in predicting which patients will benefit. Surgery. 2010 Aug;148(2):398–403. doi: 10.1016/j.surg.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Raffaelli M, De Crea C, Sessa L, et al. Prospective evaluation of total thyroidectomy versus ipsilateral versus bilateral central neck dissection in patients with clinically node-negative papillary thyroid carcinoma. Surgery. 2012 Dec;152(6):957–964. doi: 10.1016/j.surg.2012.08.053. [DOI] [PubMed] [Google Scholar]
- 29.Rosario PW. Ultrasonography for the follow-up of patients with papillary thyroid carcinoma: how important is the operator? Thyroid : official journal of the American Thyroid Association. 2010 Jul;20(7):833–834. doi: 10.1089/thy.2010.0025. [DOI] [PubMed] [Google Scholar]
- 30.Roy R, Kouniavsky G, Venkat R, et al. The role of preoperative neck ultrasounds to assess lymph nodes in patients with suspicious or indeterminate thyroid nodules. Journal of surgical oncology. 2012 May;105(6):601–605. doi: 10.1002/jso.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sippel RS, Elaraj DM, Khanafshar E, Kebebew E, Duh QY, Clark OH. Does the presence of additional thyroid nodules on ultrasound alter the risk of malignancy in patients with a follicular neoplasm of the thyroid? Surgery. 2007 Dec;142(6):851–857. doi: 10.1016/j.surg.2007.08.011. discussion 857 e851-852. [DOI] [PubMed] [Google Scholar]
- 32.AIUM Practice Guideline for the Performance of Ultrasound Examinations of the Head and Neck. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2014 Feb;33(2):366–382. doi: 10.7863/ultra.33.2.366. [DOI] [PubMed] [Google Scholar]
- 33.Lew JI, Solorzano CC. Use of ultrasound in the management of thyroid cancer. The oncologist. 2010;15(3):253–258. doi: 10.1634/theoncologist.2009-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacomini CP, Jeffrey RB, Shin LK. Ultrasonographic evaluation of malignant and normal cervical lymph nodes. Seminars in ultrasound, CT, and MR. 2013 Jun;34(3):236–247. doi: 10.1053/j.sult.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Stack BC, Jr, Ferris RL, Goldenberg D, et al. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2012 May;22(5):501–508. doi: 10.1089/thy.2011.0312. [DOI] [PubMed] [Google Scholar]