Abstract
Recently, Hines (2014) wrote an evocative paper challenging findings from both histological and morphological studies of Einstein’s brain. In this discussion paper, I extend Hines’ theoretical point and further discuss how best to determine ‘abnormal’ morphology. To do so, I assess the sulcal patterning of Einstein’s fusiform gyrus (FG) for the first time. The sulcal patterning of the FG was unconsidered in prior studies because the morphological features of the mid-fusiform sulcus have only been clarified recently. On the one hand, the sulcal patterning of Einstein’s FG is abnormal relative to averages of ‘normal’ brains generated from two independent datasets (N = 39 and N = 15, respectively). On the other hand, within the 108 hemispheres used to make these average brains, it is not impossible to find FG sulcal patterns that resemble those of Einstein. Thus, concluding whether a morphological pattern is normal or abnormal heavily depends on the chosen analysis method (e.g. group average vs. individual). Such findings question the functional meaning of morphological ‘abnormalities’ when determined by comparing an individual to an average brain or average frequency characteristics. These observations are not only important for analyzing a rare brain such as that of Einstein, but also for comparing macroanatomical features between typical and atypical populations.
Keywords: History of science, Fusiform gyrus, Mid-fusiform sulcus, Morphology, Albert Einstein
1. Introduction
Very rarely do we have the opportunity to potentially link morphological features of the brain to the cognitive processes of thinkers who are outliers in their intellectual ability. This is not a new endeavor. Indeed, the late 1800s saw a rise in rather secretive societies where ‘eminent’ men would donate their brains upon death for members of the society to analyze. Perhaps the most infamous of which was Spitzka’s study of the American Anthropometric Society (AAS) (Spitzka, 1907), which included a haphazard treatment of Walt Whitman’s brain (Weiner, 2014). Surprisingly, Spitzka’s methodological process and motivation over 100 years ago is not that different from the ones used to assess the gross anatomical structure of Einstein’s brain in recent years (Falk, 2009; Falk, Lepore, & Noe, 2013; Witelson, Kigar, & Harvey, 1999). Specifically, the overall goal is to identify atypical or abnormal neuroanatomical features, which are then used to explain the superior intellectual ability and cognitive skills of the person of interest. This process is a highly contentious topic with the definition of ‘abnormal’ relative to ‘average’ often being the point of contention (Galaburda, 1999; Hines, 2014).
Recently, Hines (2014) referred to this general process as ‘neuromythology’ and particularly emphasized that morphological differences in Einstein’s brain are merely consequences of random variation in morphological patterns. This point is worth expanding on because it reveals a flagrant problem in ascribing meaning to anatomical deviations from an average brain. In this discussion paper, I use Einstein’s fusiform gyrus (FG) as a test case, taking into consideration the morphological patterns of the mid-fusiform sulcus, which have only recently been clarified (Weiner et al., 2014) and as such, have gone unconsidered in prior studies of Einstein’s brain (Falk, 2009; Falk et al., 2013; Witelson et al., 1999). By comparing Einstein’s sulcal patterning on the FG relative to two independent control datasets (N = 39 and N = 15, respectively), I show that the sulcal patterning of Einstein’s FG in both the right and left hemispheres is a clear outlier compared to the averages resulting from both groups. However, I also show that it is possible to identify individuals with FG sulcal patterns resembling those of Einstein. These results caution the general approach of comparing single subjects to a group average and further illustrate the possibility that morphological patterns of a single brain can appear to be an outlier in the context of an average brain, but still be within the bounds of normal deviation from one individual to the next.
1.1. Einstein’s fusiform gyrus is the seat of his intellectual prowess (or is it?)
The fusiform gyrus (FG) contains a shallow, longitudinal sulcus referred to as the mid-fusiform sulcus (MFS; Nasr et al., 2011; Weiner et al., 2014). The MFS bisects the FG into lateral and medial partitions and is both a functional and cytoarchitectonic landmark in the human brain (Weiner et al., 2014). The MFS is identifiable in every brain, but its morphological features can vary from one brain to the next. For example, the MFS is fractionated in some hemispheres, while in others it is not. Further, the MFS sometimes shares a sulcal bed with nearby sulci (Weiner et al., 2014) such as the occipitotemporal sulcus (‘oct’ in Fig. 1a) and collateral sulcus (‘col’ in Fig. 1a). The MFS has only been included in reference atlases recently (Petrides, 2012), which is why prior studies (Falk, 2009; Falk et al., 2013; Witelson et al., 1999) did not report its morphological features in Einstein’s brain.
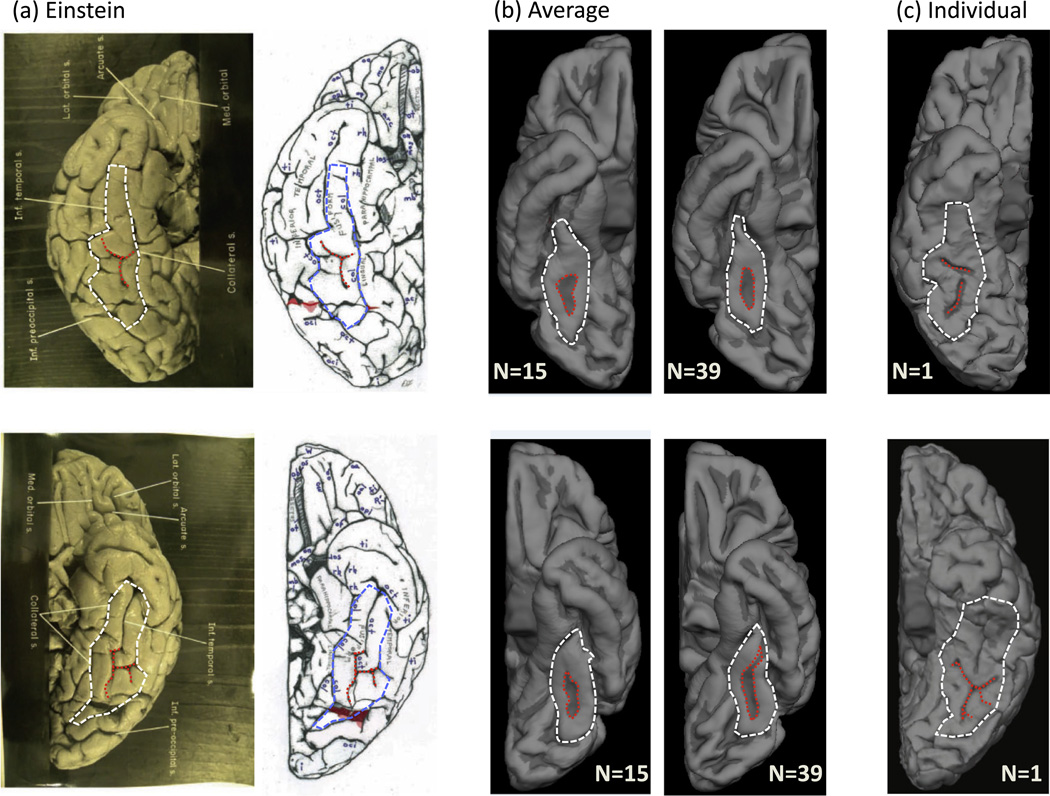
Fig. 1.
Einstein’s sulcal patterning on the fusiform gyrus (FG) is abnormal relative to a group average, but reflective of a subset of individuals. (a) Original (left) and schematic (right) images from Falk et al. (2013) for the right (top) and left (bottom) hemispheres of Einstein. Outlines of the FG (white/blue) and the mid-fusiform sulcus (MFS; red) have been added. (b) Average inflated cortical surfaces from two different independent sets of ‘typical’ brains. Left: N = 15; Right: N = 39 (FreeSurfer template). Red: MFS. White: FG. (c) Example pial surfaces from individual subjects for the right (top) and left (bottom) hemispheres. Red: MFS. White: FG. Einstein’s MFS in both hemispheres deviates from the group, but resembles sulcal patterns reflective of a minority of individuals. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In Einstein’s right and left hemispheres (Fig. 1a), the MFS contains both transverse and longitudinal components. In an independent average of 15 brains (Fig. 1b), the average MFS does not contain a transverse component in either hemisphere. This is consistent with an additional average brain (N = 39), which serves as the FreeSurfer template (freesurfer.net). Thus, compared to two independent averages of 54 healthy (e.g. ‘normal’) brains, the sulcal patterning of Einstein’s FG appears abnormal. Consequently, these results would be evidence for one to conclude that Einstein’s sulcal patterns are abnormal compared to the average morphological characteristics of the FG possessed by the general population. And by extension, one could go so far as to conclude that this MFS abnormality may be the anatomical seat of his intellectual prowess.
Nevertheless, when considering individual brains, it becomes clear that the MFS can develop branches that are not strictly longitudinal. Indeed, sulcal patterns within individuals can resemble those of Einstein (Fig. 1c). Obviously, these individuals contributed to the generation of the average brain. However, because there is less variability in the longitudinal component than the transverse component (Weiner et al., 2014), only the longitudinal component is captured in the average brain and the transverse component is not. Therefore, the accurate conclusion is not that Einstein’s sulcal patterning on the FG deviates from the average brain, but instead that Einstein’s sulcal patterning on the FG reflects a minority of healthy individuals and is within the range of normal variation.
1.2. In vivo measurements vs. postmortem atlases: Inactive conclusions evolve into active, testable hypotheses
Hines (2014) concludes his article by quoting Galaburda (1999), who asserted that the quest for finding gross anatomical markers of greatness will continue (see also Burrell, 2004). I agree that it is highly probable that such examinations will continue and as such, I suggest that future studies that wish to do so should pair these measurements with in vivo anatomical and potentially, functional measurements. It is feasible to use atlases containing images and frequency characteristics of postmortem brains to assess morphological characteristics of a postmortem brain – that is, to compare like vs. like. However, the limitations of using atlases of postmortem brains are that (a) the researcher is limited to the information provided by the author and (b) the assessment ends with a conclusion of ‘normal’ or ‘abnormal’ relative to that limited information. On the contrary, comparisons to in vivo measurements (a) allow an assessment relative to the group norm, as well as relative to each individual that contributes to that norm (Fig. 1) and (b) allow for further examination of testable hypotheses since the measurements are from living individuals. That is, it is possible to assess and measure the functional meaning of particular brain features present in some individuals and not others since these individuals are still alive. For example, do individuals with shared morphological features also share cognitive capacities? Is it functionally meaningful that both Einstein and a minority of individuals have a transverse branch of the MFS while a majority do not? Researchers would not even have to acquire their own anatomical and functional data and instead could use those provided by large databases. For example, the Human Connectome Project (humanconnectomeproject.org) contains anatomical and functional data from hundreds of participants and has the ability to directly assess (a) the probability that a morphological pattern deviates from a norm, (b) the probability that a morphological pattern of a given individual is possible considering those patterns of every other individual (in several hundred brains), and (c) if there is a potential functional effect of any measureable anatomical deviation. As data sharing and scientific transparency among institutions become more common place (scitran.stanford.edu), this proposed approach will become even more feasible in years to come.
Aside from whether or not any anatomical deviation is functionally meaningful, large datasets offer the benefit of new quantifications. While I have used the FG and the MFS as a test case, it is likely that this discrepancy between the group norm and individuals exists for several additional sulci – especially tertiary sulci that are often more variable from one hemisphere to the next. This stresses the importance of moving toward a quantifiable definition of ‘abnormal’ in the context of morphology. When it comes to the length or depth of sulci, or the surface area or volume of a gyrus or lobe, or even cortical thickness, quantifying deviation is rather easy it is just a number relative to the confidence interval of the norm. When it comes to morphology, however, this definition becomes more complex because it requires manual work to identify particular morphological patterns in individual hemispheres. Such quantifications are not only important for comparisons between typical brains and those of prominent historical figures, but also for comparisons between typical brains and those of patient populations to determine potential associations between abnormal cortical folding patterns and particular disorders. Finally, it is important to consider that there is not a one-to-one mapping between a morphological abnormality and ‘greatness’ (or on the other end of the spectrum, dysfunction). Instead, any abnormality likely reflects an aggregate effect and a complicated interaction of a variety of neurobiological features and may not be anything other than a coincidence without any functional meaning.
2. Conclusion
In this paper, I have assessed anatomical features of Einstein’s fusiform gyrus (FG) that were unconsidered by prior examinations. I show that on the one hand, the sulcal patterning of the FG in Einstein’s brain appears to be an outlier relative to the average morphological patterns present in two independent sets of brains. On the other hand, I show that there are individuals with FG sulcal patterns similar to those of Einstein. Therefore, Einstein is within the normal range of variation despite his deviation from the group norm. Since identifying abnormalities is a general goal of neurology, this discrepancy between group averages and individual subjects not only affects these rare opportunities to measure morphological features in eminent historical figures, but also raises the question of what the best approach is for determining morphological abnormalities in patient populations.
The methodological approach suggested in this paper aims to alter prior approaches from ‘neuromythology’ (Hines, 2014) to new ways of generating testable hypotheses. Though I have used the sulcal patterning of the FG and Einstein as a proof of concept, one thing is for sure: It is time for morphological analyses to evolve from when Spitzka examined the brains of eminent men over 100 years ago (Spitzka, 1907). In this age of big data and imaging of the living human brain, I believe even Einstein himself would agree that a change is needed and that the approach suggested in the present article is, if nothing else, a step in the right direction.
Acknowledgments
I thank Melina Uncapher, Jason Yeatman, and Kalanit Grill-Spector for helpful comments on prior versions of the manuscript. I thank Michael Barnett for help in analyzing data included in Fig. 1. This work is supported by 1R01EY02391501A1.
References
- Burrell B. Postcards from the brain museum. New York: Broadway Books; 2004. [Google Scholar]
- Falk D. New information about Albert Einstein’s brain. Frontiers in Evolutionary Neuroscience. 2009;1:3. doi: 10.3389/neuro.18.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk D, Lepore FE, Noe A. The cerebral cortex of Albert Einstein: A description and preliminary analysis of unpublished photographs. Brain. 2013;136:1304–1327. doi: 10.1093/brain/aws295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM. Albert Einstein’s brain. The Lancet. 1999;354:1821. doi: 10.1016/s0140-6736(05)70590-0. [author reply 1822]. [DOI] [PubMed] [Google Scholar]
- Hines T. Neuromythology of Einstein’s brain. Brain and Cognition. 2014;88:21–25. doi: 10.1016/j.bandc.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Nasr S, Liu N, Devaney KJ, Yue X, Rajimehr R, Ungerleider LG, et al. Scene-selective cortical regions in human and nonhuman primates. Journal of Neuroscience. 2011;31:13771–13785. doi: 10.1523/JNEUROSCI.2792-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. The human cerebral cortex: An MRI atlas of the sulci and gyri in stereotaxic space. Amsterdam: Elsevier/Academic Press; 2012. [Google Scholar]
- Spitzka EA. A study of the brains of six eminent scientists and scholars belonging to the American Anthropometric Society, together with a description of the Skull of Professor E.D. Cope. Transactions of the American Philosophical Society. 1907;21:175–308. [Google Scholar]
- Weiner KS. History: Two brains and a forgotten theory. Nature. 2014;509(7498):33. doi: 10.1038/509033e. [DOI] [PubMed] [Google Scholar]
- Weiner KS, Golarai G, Caspers J, Chuapoco MR, Mohlberg H, Zilles K, et al. The mid-fusiform sulcus: A landmark identifying both cytoarchitectonic and functional divisions of human ventral temporal cortex. Neuroimage. 2014;84:453–465. doi: 10.1016/j.neuroimage.2013.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL, Harvey T. The exceptional brain of Albert Einstein. The Lancet. 1999;353:2149–2153. doi: 10.1016/S0140-6736(98)10327-6. [DOI] [PubMed] [Google Scholar]