Abstract
Introduction
To determine if higher achieved mean arterial blood pressure (MAP) during treatment with therapeutic hypothermia (TH) is associated with neurologically intact survival following cardiac arrest.
Methods
Retrospective analysis of a prospectively collected cohort of 188 consecutive patients treated with TH in the cardiovascular intensive care unit of an academic tertiary care hospital.
Results
Neurologically intact survival was observed in 73/188 (38.8%) patients at hospital discharge and in 48/162 (29.6%) patients at a median follow up interval of 3 months. Patients in shock at the time of admission had lower baseline MAP at the initiation of TH (81 versus 87 mmHg; p=0.002), but had similar achieved MAP during TH (80.3 versus 83.7 mmHg; p=0.11). Shock on admission was associated with poor survival (18% versus 52%; p<0.001). Vasopressor use among all patients was common (84.6%) and was not associated with increased mortality. A multivariable analysis including age, initial rhythm, time to return of spontaneous circulation, baseline MAP and achieved MAP did not demonstrate a relationship between MAP achieved during TH and poor neurologic outcome at hospital discharge (OR 1.28, 95% CI 0.40–4.06; p=0.87) or at outpatient follow up (OR 1.09, 95% CI 0.32–3.75; p=0.976).
Conclusion
We did not observe a relationship between higher achieved MAP during TH and neurologically intact survival. However, shock at the time of admission was clearly associated with poor outcomes in our study population. These data do not support the use of vasopressors to artificially increase MAP in the absence of shock. There is a need for prospective, randomized trials to further define the optimum blood pressure target during treatment with TH.
1. Introduction
Cardiac arrest is a major public health issue, with an annual incidence approaching 400,000 in the United States[1]. Mild therapeutic hypothermia (TH) is now an established and recommended therapy to improve survival and neurologic outcome following cardiac arrest[2–4]. Despite advances in post-resuscitation care, long-term outcomes following cardiac arrest remain dismal. Approximately 60% of patients suffering cardiac arrest will die in the field, and of those who survive to hospitalization, approximately 75% will not survive to discharge[5]. Given the high mortality seen in patients who are successfully resuscitated and survive to hospitalization, attention has turned to optimizing post-resuscitation management.
In-hospital mortality following cardiac arrest is often a result of the “post-cardiac arrest syndrome,” a complex condition characterized by multi-organ ischemic reperfusion injury, particularly in the brain and myocardium[6]. Profound reversible myocardial dysfunction and a robust pro-inflammatory response are pathophysiologic derangements seen in this syndrome that can result in hemodynamic instability[7–9]. Circulatory shock - a physiologic state of inadequate tissue perfusion and oxygenation related to systemic hypotension - is common in the post-resuscitation period and known to be associated with increased mortality[7,10,11].
Clinical guidelines for the hemodynamic management of patients undergoing TH are not clearly specified, largely due to the lack of robust clinical data. This has resulted in a wide range of hemodynamic targets in the published literature and subsequent inconsistency in clinical practice. Several TH studies do not define hemodynamic goals such as a target mean arterial blood pressure (MAP)[3,12–15]. In studies with a defined target MAP, the range is remarkably variable, ranging from >60 mmHg[16] to 90–100 mmHg[4].
Prior animal and human studies show that cerebral blood flow and autoregulation are abnormal following cardiac arrest[27,28]. Therefore, it has been suggested that MAP should be maintained at a higher level to secure cerebral blood flow[17]. In light of recent studies suggesting that the use of vasopressors following cardiac arrest may improve the likelihood of return of spontaneous circulation (ROSC) and short-term survival at the expense of worse long-term clinical outcomes, MAP goals following successful resuscitation need to be carefully considered[18–21].
Given the high incidence of shock in the post-resuscitation time period, the harm associated with both systemic arterial hypotension and vasopressor use, and the paucity of clinical data that supports an ideal MAP target during TH, there is an urgent need for high quality clinical data to better define hemodynamic goals in this high risk patient population. We hypothesized that higher achieved MAP (specifically, a MAP ≥80 mmHg) during treatment with TH may be associated with improved neurologic outcomes.
2. Methods
2.1. Study Design
The study population included 188 consecutive patients following cardiac arrest that had successful ROSC and were treated with TH at Vanderbilt University Medical Center between May 2007 and March 2012. All patients were cooled externally using an active surface-cooling device to maintain a core body temperature of 32–34 degrees Celsius for a total of 24 hours following ROSC, after which they were rewarmed actively at a rate of 0.25 degrees Celsius per hour. The standardized TH protocol at our institution recommends a MAP target of 80–90 mmHg and norepinephrine as the initial vasopressor of choice to treat hypotension.
Following approval from the institutional review board, data were collected prospectively on these patients, including initial rhythm, time to ROSC, receipt of bystander cardiopulmonary resuscitation, Cerebral Performance Category (CPC) score at hospital discharge, CPC score at outpatient follow up and time to follow up. In addition, we retrospectively collected all noninvasive (NIMAP) and invasive/arterial (AMAP) mean arterial blood pressure measurements as well as all vasopressor use and doses administered during treatment with TH. We defined shock on admission as a systolic blood pressure <90 mmHg or the need for any vasopressor or mechanical circulatory support at the time of admission. Medications considered vasopressors included norepinephrine, epinephrine, vasopressin, phenylephrine, dopamine, dobutamine and milrinone.
The cardiovascular portion of the Sequential Organ Failure Assessment (SOFA) score was used to reflect the achieved MAP for each patient. The SOFA score is a validated scoring system to quantify the number and severity of organ dysfunction over time in six organ systems (respiratory, hematologic, hepatic, cardiovascular, renal and neurologic)[22]. The SOFA score has been validated for both individual and aggregate organ dysfunction and provides clinicians with information on both the degree and progression of dysfunction. It has been studied and validated in critically ill patients including cardiovascular patients with acute myocardial infarction[23]. The cardiovascular portion of the SOFA score (CV SOFA) accounts for both MAP and vasopressor requirement. Points are assigned for decreasing MAP and increasing vasopressor use, and therefore, the CV SOFA score is inversely associated with hemodynamic stability.
For the purpose of this study, the baseline MAP was defined as the mean of the first 5 consecutive MAPs recorded during TH. Achieved MAP was defined as the mean of all consecutive MAPs recorded during the longest period of time with the lowest CV SOFA score during TH over a minimum of 5 consecutive blood pressure measurements. Using this method, we were able to define the achieved MAP as the mean value of recordings during the period of time when the patient was the most hemodynamically stable.
Nurses in the cardiovascular intensive care unit of our institution routinely chart both NIMAP and AMAP measurements at regular time intervals, recording pressures hourly as a minimum requirement although more frequently as the clinical condition of the patient dictates. Because MAPs obtained by noninvasive means can be recorded quickly and do not require a procedure (i.e. arterial line placement), we anticipated that the NIMAP values would be a more complete dataset compared to AMAP, and therefore, pre-specified to use NIMAP in the primary analysis. AMAP values were subsequently used for the secondary analysis. To our knowledge, no data exist comparing the accuracy of NIMAP and AMAP values in this patient population.
The primary outcome for this study was CPC score at the time of follow up. The CPC score was developed as a measure of central nervous system function after cardiac arrest and is recommended and commonly used for this purpose[3,4,24]. A CPC score between 3 and 5 defines a poor neurologic outcome in study patients and clinically represents a range of neurologic dysfunction from severe neurologic disability to brain death[24]. Consistent with these recommendations, a CPC score between 3 and 5 was used to define a poor neurologic outcome in this study. Secondary outcomes for this study included CPC score at hospital discharge, survival to hospital discharge and survival to follow up. We also evaluated outcomes based on vasopressor use and the presence of shock on admission.
2.2. Inclusion/Exclusion Criteria
All patients treated with TH at our institution who had at least 5 NIMAP measurements during hypothermia were included.
2.3. Statistical Analysis
Descriptive statistics were calculated as the median with interquartile range (IQR) for continuous variables. For categorical variables, frequencies and percentages were presented. Clinical characteristics between patients with and without vasopressor use, and between patients with and without shock on admission, were compared using the Wilcoxon rank sum test or Pearson Chi-square test where appropriate. A logistic regression model was developed to assess the association between achieved MAP and the neurologic outcome adjusting for baseline MAP, age, initial rhythm and time to ROSC. Achieved MAP, baseline MAP, age and time to ROSC were modeled as continuous variables with appropriate transformation using restricted cubic splines to account for non-linear relationships. All tests were two-tailed. All statistical analyses were performed using open source R statistical software[25] and rms package[26].
3. Results
Of 188 consecutive patients considered for inclusion, 174 had at least 5 consecutive NIMAPs recorded during TH and therefore, were included in the primary analysis. A total of 148 patients had at least 5 consecutive AMAPs recorded during TH and were included in the secondary analysis. Table 1 displays baseline characteristics of the cohort. The overall in-hospital mortality rate was 57.4% (108/188), and the cumulative mortality rate at follow up was 70.4% (114/162). Survival to hospital discharge with a good neurologic outcome (CPC 1–2) was observed in 73/188 patients (38.8%), and good neurologic outcome at follow up was observed in 48/162 patients (29.6%). The median time to follow up was 3 months (IQR 1–6 months).
Table 1. Baseline Characteristics.
Data are presented as median (25th percentile–75th percentile) for continuous variables and number (percentage) of patients for categorical variables. N represents the number of non-missing values. Shock on admission was defined as systolic blood pressure <90mmHg or the need for vasopressors or mechanical circulatory support (intra-aortic balloon pump, extracorporeal membrane oxygenation or ventricular assist device) at the time of admission. Index CV SOFA score indicates the score during the achieved MAP.
| Characteristic | N | Overall |
|---|---|---|
| Age (years) | 188 | 59 (50–68) |
| Male (%) | 188 | 118 (63%) |
| Out-of-hospital arrest (%) | 188 | 150 (80%) |
| Initial rhythm VT/VF (%) | 182 | 110 (60%) |
| Witnessed arrest (%) | 188 | 157 (84%) |
| Received bystander CPR (%) | 188 | 98 (52%) |
| Time to ROSC (minutes) | 173 | 18 (12–30) |
| Presence of shock on admission (%) | 188 | 54 (29%) |
| Placement of mechanical circulatory support device (%) | 188 | 32 (17%) |
| Baseline NIMAP (mmHg) | 174 | 84 (76–92) |
| Baseline AMAP (mmHg) | 148 | 86 (81–95) |
| Baseline CV SOFA Score | 174 | 0.09 (0.00–2.60) |
| Achieved NIMAP (mmHg) | 174 | 83.2 (76.4–89.6) |
| Achieved AMAP (mmHg) | 148 | 86 (80–92) |
| Index CV SOFA Score | 174 | 1.73 (0.53–2.71) |
| Initial body temperature (degrees Celsius) | 164 | 36.4 (35.5–36.8) |
| Time from arrest to initiation of TH (minutes) | 177 | 195 (90–320) |
| Time to reach target temperature (minutes) | 171 | 420 (248–550) |
| Time spent at target temperature (hours) | 176 | 19.0 (16.0–23.2) |
| ST-segment Elevation Myocardial Infarction (%) | 187 | 40 (21%) |
| Coronary angiography performed (%) | 188 | 115 (61%) |
| Percutaneous coronary intervention performed (%) | 188 | 62 (33%) |
| Peak Troponin I concentration (mcg/l) | 172 | 4.38 (0.84–20.70) |
| Time from arrest to follow up (months) | 53 | 3.0 (1.0–6.0) |
AMAP = Invasive (Arterial) Mean Arterial Blood Pressure; CPR = Cardiopulmonary Resuscitation; CV SOFA = Cardiovascular Sequential Organ Failure Assessment; NIMAP = Noninvasive Mean Arterial Blood Pressure; ROSC = Return of Spontaneous Circulation; TH = Therapeutic Hypothermia; VT = Ventricular Tachycardia; VF = Ventricular Fibrillation
The median number of NIMAP values during treatment with TH analyzed per patient was 23 (IQR 17–30), and the median number of AMAP values per patient was 26 (IQR 21–33). The relationship between baseline and achieved levels of NIMAP and AMAP are displayed in Figure 1. Vasopressors were administered to 159/188 (84.6%) patients at any point during treatment with TH. Table 2 displays the relationship between vasopressor use, achieved MAP and survival. The baseline CV SOFA score was significantly higher in patients who received vasopressors (1.60 versus 0.00; p < 0.001) as was the index CV SOFA score, which indicates the calculated score at achieved MAP (2.05 versus 0.04; p<0.001). Achieved NIMAP and AMAP values were significantly lower in patients who received vasopressors. However, there was not a statistically significant difference in the number of patients who achieved a MAP ≥80 mmHg during TH between those who did and those who did not receive vasopressors.
Figure 1. Baseline and Achieved Mean Arterial Blood Pressure During Hypothermia.
Patients achieving a good neurologic outcome (CPC 1–2) at hospital discharge are identified with a black triangle. Patients with a poor neurologic outcome (CPC 3–5) are identified with a red star.
AMAP = Invasive (Arterial) Mean Arterial Blood Pressure; NIMAP = Noninvasive Mean Arterial Blood Pressure
Table 2. Vasopressor Use, Mean Arterial Blood Pressure and Mortality.
Data are presented as median (25th percentile–75th percentile) for continuous variables and number (percentage) of patients for categorical variables. N represents the number of non-missing values. Index CV SOFA score indicates the score during the achieved MAP.
| N | No Vasopressor (n = 29) |
Vasopressor (n = 159) |
p-value | |
|---|---|---|---|---|
| Baseline CV SOFA Score | 174 | 0.00 (0.00–0.20) | 1.60 (0.00–2.60) | <0.001a |
| Index CV SOFA Score | 174 | 0.04 (0.00–0.12) | 2.05 (0.94–2.87) | <0.001a |
| Achieved NIMAP (mmHg) | 174 | 86.7 (79.7–92.5) | 82.7 (75.8–88.8) | 0.021a |
| Achieved AMAP (mmHg) | 148 | 93 (84–98) | 85 (80–91) | 0.019a |
| Achieved NIMAP ≥80 (%) | 174 | 18 (72%) | 88 (59%) | 0.22b |
| Achieved AMAP ≥80 (%) | 148 | 18 (82%) | 95 (75%) | 0.51b |
| Survival to hospital discharge (%) | 188 | 16 (55%) | 64 (40%) | 0.14b |
| Survival to outpatient follow up (%) | 162 | 11 (41%) | 37 (27%) | 0.17b |
Wilcoxon rank sum test
Pearson Chi-square test
AMAP = Invasive (Arterial) Mean Arterial Blood Pressure; CV SOFA = Cardiovascular Sequential Organ Failure Assessment; NIMAP = Noninvasive Mean Arterial Blood Pressure
Table 3 displays the relationship between shock at the time of admission, MAP values during TH and mortality. Patients who met the definition of shock at the time of admission had significantly lower baseline NIMAP (p=0.002) and AMAP (p<0.001) at initiation of TH and were less likely to survive to hospital discharge (18% versus 52%; p<0.001) or follow up (12% versus 38%; p<0.001). Patients with and without shock on admission had similar achieved NIMAP values during treatment with TH (p=0.11).
Table 3. Shock at Admission, Mean Arterial Blood Pressure and Mortality.
Data are presented as median (25th percentile–75th percentile) for continuous variables and number (percentage) of patients for categorical variables. N represents the number of non-missing values. Shock on admission was defined as systolic blood pressure <90mmHg or the need for vasopressors or mechanical support at the time of admission.
| N | Shock at admission (n = 54) |
No shock at admission (n = 134) |
p-value | |
|---|---|---|---|---|
| Baseline NIMAP (mmHg) | 174 | 81 (72–87) | 87 (77–94) | 0.002a |
| Baseline AMAP (mmHg) | 148 | 82 (74–86) | 88 (82–97) | <0.001a |
| Achieved NIMAP (mmHg) | 174 | 80.3 (73.8–88.5) | 83.7 (77.2–90.1) | 0.11a |
| Achieved AMAP (mmHg) | 148 | 82 (73–87) | 88 (82–94) | <0.001a |
| Survival to hospital discharge (%) | 188 | 10 (18%) | 70 (52%) | <0.001b |
| Survival to outpatient follow up (%) | 162 | 6 (12%) | 42 (38%) | <0.001b |
Wilcoxon rank sum test
Pearson Chi-square test
AMAP = Invasive (Arterial) Mean Arterial Blood Pressure; NIMAP = Noninvasive Mean Arterial Blood Pressure
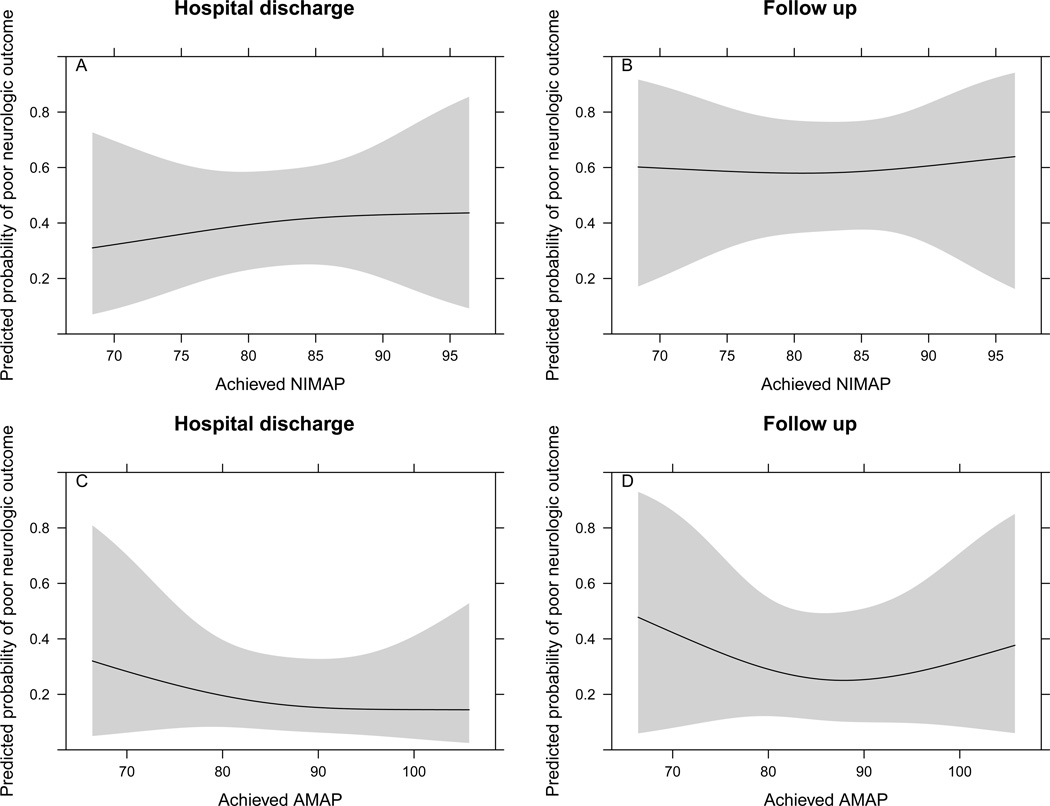
Using data reduction methods and to account for variables known to be associated with poor outcomes following cardiac arrest, a multivariable logistic regression model was developed to examine the association between achieved NIMAP and neurologically intact survival at hospital discharge and follow up. After controlling for age, initial rhythm, estimated time to ROSC and baseline NIMAP, achieved NIMAP during TH was not significantly associated with neurologic outcomes at hospital discharge (p=0.87) or at a median follow up interval of 3 months (p=0.976)(Table 4, Figure 2A–B). Results were similar when the analysis was repeated using AMAP values (p=0.717 and p=0.714, respectively)(Table 4, Figure 2C–D).
Table 4. Achieved Mean Arterial Blood Pressure as a Predictor of Poor Outcome.
Relationship between achieved MAP and poor neurologic outcome adjusting for age, initial rhythm, time to ROSC and baseline MAP. Poor neurologic outcome was defined as CPC 3–5.
| Odds of poor outcome at hospital discharge using NIMAP | |||
|---|---|---|---|
| Characteristic | Odds ratio | 95% confidence interval | p-value |
| Age | 2.03 | 1.00–4.11 | 0.051 |
| Initial rhythm (PEA/Asystole) | 8.48 | 3.02–23.83 | <0.001 |
| Time to ROSC | 9.09 | 3.63–22.77 | <0.001 |
| Baseline NIMAP | 0.62 | 0.20–1.91 | 0.619 |
| Achieved NIMAP | 1.28 | 0.40–4.06 | 0.87 |
| Odds of poor outcome at outpatient follow up using NIMAP | |||
| Characteristic | Odds ratio | 95% confidence interval | p-value |
| Age | 2.48 | 1.08–5.69 | 0.055 |
| Initial rhythm (PEA/Asystole) | 3.97 | 1.43–11.03 | 0.008 |
| Time to ROSC | 5.84 | 2.45–13.91 | 0.003 |
| Baseline NIMAP | 0.95 | 0.29–3.04 | 0.481 |
| Achieved NIMAP | 1.09 | 0.32–3.75 | 0.976 |
| Odds of poor outcome at hospital discharge using AMAP | |||
| Characteristic | Odds ratio | 95% confidence interval | p-value |
| Age | 2.55 | 1.11–5.86 | 0.027 |
| Initial rhythm (PEA/Asystole) | 8.02 | 2.68–23.95 | <0.001 |
| Time to ROSC | 15.5 | 3.30–72.78 | 0.002 |
| Baseline AMAP | 1.07 | 0.49–2.33 | 0.643 |
| Achieved AMAP | 0.73 | 0.32–1.67 | 0.717 |
| Odds of poor outcome at outpatient follow up using AMAP | |||
| Characteristic | Odds ratio | 95% confidence interval | p-value |
| Age | 3.46 | 1.31–9.12 | 0.025 |
| Initial rhythm (PEA/Asystole) | 4.08 | 1.31–12.71 | 0.015 |
| Time to ROSC | 11.32 | 2.02–63.39 | 0.017 |
| Baseline AMAP | 1.05 | 0.42–2.59 | 0.989 |
| Achieved AMAP | 0.88 | 0.33–2.35 | 0.714 |
AMAP = Invasive (Arterial) Mean Arterial Blood Pressure; NIMAP = Noninvasive Mean Arterial Blood Pressure; PEA = Pulseless Electrical Activity; ROSC = Return of Spontaneous Circulation
Figure 2. Achieved Noninvasive and Invasive Mean Arterial Blood Pressure During Hypothermia and Outcome.
Association between achieved mean arterial blood pressure (NIMAP - A, B; AMAP - C, D) and log odds for a poor neurologic outcome (CPC 3–5) at hospital discharge and follow up.
NIMAP model is adjusted to: baseline MAP 84.2 mmHg, age 59 years, initial rhythm ventricular tachycardia or ventricular fibrillation, and time to ROSC 18 minutes. AMAP model is adjusted to: baseline MAP 86 mmHg, age 59 years, initial rhythm ventricular tachycardia or ventricular fibrillation, and time to ROSC 18 minutes. AMAP = Invasive (Arterial) Mean Arterial Blood Pressure; NIMAP = Noninvasive Mean Arterial Blood Pressure
4. Discussion
The principal findings of this investigation examining mean arterial blood pressure goals in our study population of patients treated with TH following cardiac arrest are: 1) shock at the time of admission was associated with poor outcomes and 2) there was no association between achieved MAP during treatment with TH and outcomes at hospital discharge or at a median follow up interval of 3 months. These data are consistent with previous studies showing that hemodynamic instability is poorly tolerated following cardiac arrest[10]. Furthermore, the data refute our hypothesis that achieving a higher MAP during treatment with TH is associated with improved neurologic outcomes.
While multiple studies demonstrate the benefit of TH, uniformity does not exist in the blood pressure targets utilized in those studies. In fact, several TH studies do not define hemodynamic goals such as threshold MAP[3,12–15]. Even in those studies with a defined target MAP, the range is quite variable, including: >60 mmHg[16], >65–70 mmHg[29–31], 65–100 mmHg[32], 80–100 mmHg[33,34] and 90–100 mmHg[4].
The most recent American Heart Association (AHA) guidelines for post-resuscitation care recommend the use of fluid administration as well as vasoactive, inotropic and inodilator agents titrated as needed to optimize blood pressure, cardiac output and systemic perfusion. Although human studies have not established targets for blood pressure, the AHA guidelines state that a MAP ≥65 mmHg is generally considered to be a reasonable goal[2]. The International Liaison Committee on Resuscitation recommends that targeting a MAP of 65–100 mmHg is a reasonable goal, although the organization acknowledges that little evidence is available to support this recommendation[6]. The variability in guideline recommendations above coupled with the deficiency of evidence in this area may result in inconsistent clinical practice within this critically ill patient population.
The TH protocol utilized at our institution was developed based on the best available evidence, review of the most up-to-date practice guidelines and local expert consensus opinion. As such, our institutional protocol recommends a goal MAP of 80–90 mmHg during treatment with TH. In this retrospective analysis, we observed that shock at the time of admission, defined as a systolic blood pressure <90 mmHg or the need for vasopressors or mechanical support, was associated with poor outcomes. Based on this observation, we speculated that the rapid identification and aggressive treatment of shock may represent an opportunity to improve outcomes in this population and warranted further investigation. However, when analyzing MAP as a continuous variable, we did not identify a threshold MAP achieved during treatment with TH that was associated with improved neurologically intact survival. Our findings support the current AHA guidelines targeting a normal MAP (i.e. ≥65 mmHg). These data contradict the hypothesis that higher achieved MAP during TH may improve outcomes, and do not support the practice of using vasopressors to artificially increase MAP in the absence of shock.
To our knowledge, there are no large prospective randomized trials that are designed to determine the optimum MAP during treatment with TH. Gaieski et al. conducted a trial examining the feasibility of establishing an integrated post-cardiac arrest resuscitation algorithm combining TH and early goal-directed hemodynamic optimization within 6 hours of admission[33]. Such a treatment algorithm appears to be feasible and represents a promising conceptual strategy; however, the trial was not powered to assess hard outcomes.
More recently, Beylin and colleagues reported findings from their study that specifically examined the relationship between MAP targets and use of vasoactive agents on survival and neurologic outcomes in post-arrest TH patients. They showed that survivors had higher MAPs at 1 hour and 6 hour time points compared to non-survivors. In addition, for patients requiring vasoactive agents, survivors also had higher MAPs at these specified time points compared to non-survivors[34]. While their study shares some similarity to ours in regards to subject number, single center site and retrospective design, we present several additional strengths of our investigation. These include: collection of both noninvasive and invasive blood pressure data; analysis of MAP as a continuous variable with multiple measurements taken over time (median NIMAP per patient = 23, median AMAP per patient = 26); use of a CV SOFA score as a means to better identify the period of achieved hemodynamic stability; and evaluation of CPC score at a median of 3 month follow up. We argue that assessing MAP at 6 hour time intervals has the potential to miss important dynamic features of continuous blood pressure management, especially within the initial hours of presentation where the patient may be the most critically ill. We agree with the authors that additional prospective trials with larger cohorts using continuous data may help determine if maintaining a MAP between 80–100 mmHg, as they chose to use, translates to improved clinical end points.
Furthermore, the potential adverse effects associated with vasopressor use cannot be minimized. Several recent studies have examined the relationship between vasopressor use and outcomes following cardiac arrest. While most studies agree that the use of sympathomimetic drugs such as epinephrine during resuscitation improves the likelihood of successful ROSC and short-term survival, recent data suggest that any short-term benefit may come at the expense of worse long-term outcomes[18–21]. These studies raise the concern that in the absence of shock, the use of vasopressors to maintain a MAP greater than normal (i.e. drug-induced hypertension) during TH may be harmful. Although our study was not designed to examine the effect of vasopressor use on the primary endpoint, we did not find a statistically significant difference in mortality based on vasopressor use alone. One potential explanation for this finding may be that several hemodynamically stable patients received vasopressors according to our protocol in order to maintain a MAP between 80–90 mmHg.
4.1. Study Limitations
This study has a number of limitations. This is a retrospective observational study that only allows us to suggest association rather than causation. While we attempted to account for the most important potential confounders in the multivariable analysis, it is possible that other unidentified variables may have influenced the results. Although the SOFA score is validated in a number of clinical scenarios including acute myocardial infarction, it has never been validated in patients treated with TH. The CV SOFA score does not consider some vasopressors including neosynephrine, vasopressin or milrinone, one of which was used in 52/188 (27.6%) patients in this study. Patients with less than 5 MAP values recorded in the electronic medical record were excluded from the analysis. It is possible that these patients represent the most hemodynamically unstable patients and therefore died (or had care withdrawn) early in their hospital course, resulting in survivorship bias. It is not clear how this small number of patients (n=14) may have affected the results. Finally, we did not have a method to address hypertensive patients who may have required antihypertensive medications during TH.
5. Conclusions
Despite treatment with TH and advances in post-resuscitation care, survival following cardiac arrest remains poor. It is clear that the presence of shock at the time of admission is associated with worse outcomes. In this retrospective cohort study, we did not find an association between higher achieved MAP during treatment with TH and neurologic outcomes at hospital discharge or at a median follow up interval of 3 months. These data support current guidelines to maintain a normal MAP during treatment with TH, but do not support the practice of using vasopressors to artificially elevate MAP in the absence of shock. The early identification and aggressive treatment of shock may be important opportunities for improved outcomes in this population, but the ideal threshold to which MAP should be targeted during TH remains in question. There is a need for prospective, randomized trials to better delineate hemodynamic goals during treatment with TH.
Acknowledgements
None.
Funding Sources
MNY is supported by the National Institute of Health Training in Cardiovascular Research grant, 5T32 HL7411-34. Research reported in this publication has also been supported by the National Center for Advancing Translational Sciences of the National Institute of Health under Award Number UL1 TR000445, as well as the Vanderbilt University Medical Center Division of Cardiovascular Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Vanden Hoek Kronick SL. Part 9: Post-Cardiac Arrest Care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18_suppl_3):S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 3.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 4.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 5.Kern KB. Optimal Treatment of Patients Surviving Out-of-Hospital Cardiac Arrest. JACC Cardiovasc Interv. 2012;5(6):597–605. doi: 10.1016/j.jcin.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RSB, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. Post-Cardiac Arrest Syndrome: Epidemiology, Pathophysiology, Treatment, and Prognostication A Consensus Statement From the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Stroke Council. Circulation. 2008;118(23):2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 7.Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40(12):2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Bailén M, Hoyos EAde, Ruiz-Navarro S, Díaz-Castellanos MÁ, Rucabado-Aguilar L, Gómez-Jiménez FJ, Martínez-Escobar S, Moreno RM, Fierro-Rosón J. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66(2):175–181. doi: 10.1016/j.resuscitation.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, Dhainaut JF, Cavaillon JM. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106(5):562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 10.Kilgannon JH, Roberts BW, Reihl LR, Chansky ME, Jones AE, Dellinger RP, Parrillo JE, Trzeciak S. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation. 2008;79(3):410–416. doi: 10.1016/j.resuscitation.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emergency Cardiac Care Committee and Subcommittees, American Heart Association. Guidelines for cardiopulmonary resuscitation and emergency cardiac care, III: adult advanced cardiac life support. JAMA. 1992;268(16):2199–2241. [PubMed] [Google Scholar]
- 12.Kupchik NL. Development and implementation of a therapeutic hypothermia protocol. Crit Care Med. 2009;37(7 Suppl):S279–S284. doi: 10.1097/CCM.0b013e3181aa61c5. [DOI] [PubMed] [Google Scholar]
- 13.Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol. 2009;133(2):223–228. doi: 10.1016/j.ijcard.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Busch M, Soreide E, Lossius HM, Lexow K, Dickstein K. Rapid implementation of therapeutic hypothermia in comatose out-of-hospital cardiac arrest survivors. Acta Anaesthesiol Scand. 2006;50(10):1277–1283. doi: 10.1111/j.1399-6576.2006.01147.x. [DOI] [PubMed] [Google Scholar]
- 15.Haugk M, Testori C, Sterz F, Uranitsch M, Holzer M, Behringer W, Herkner H. Time to Target Temperature Study Group. Relationship between time to target temperature and outcome in patients treated with therapeutic hypothermia after cardiac arrest. Crit Care. 2011;15(2):R101. doi: 10.1186/cc10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mooney MR, Unger BT, Boland LL, Burke MN, Kebed KY, Graham KJ, Henry TD, Katsiyiannis WT, Satterlee PA, Sendelbach S, Hodges JS, Parham WM. Therapeutic Hypothermia After Out-of-Hospital Cardiac Arrest: Evaluation of a Regional System to Increase Access to Cooling. Circulation. 2011;124(2):206–214. doi: 10.1161/CIRCULATIONAHA.110.986257. [DOI] [PubMed] [Google Scholar]
- 17.Sundgreen C, Larsen FS, Herzog TM, Knudsen GM, Boesgaard S, Aldershvile J. Autoregulation of Cerebral Blood Flow in Patients Resuscitated From Cardiac Arrest. Stroke. 2001;32(1):128–132. doi: 10.1161/01.str.32.1.128. [DOI] [PubMed] [Google Scholar]
- 18.Arrich J, Sterz F, Herkner H, Testori C, Behringer W. Total epinephrine dose during asystole and pulseless electrical activity cardiac arrests is associated with unfavourable functional outcome and increased in-hospital mortality. Resuscitation. 2012;83(3):333–337. doi: 10.1016/j.resuscitation.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL. Effect of adrenaline on survival in out-of-hospital cardiac arrest: A randomised double-blind placebo-controlled trial. Resuscitation. 2011;82(9):1138–1143. doi: 10.1016/j.resuscitation.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA. 2012;307(11):1161–1168. doi: 10.1001/jama.2012.294. [DOI] [PubMed] [Google Scholar]
- 21.Larabee TM, Liu KY, Campbell JA, Little CM. Vasopressors in cardiac arrest: A systematic review. Resuscitation. 2012;83(8):932–939. doi: 10.1016/j.resuscitation.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 23.Huang SS, Chen YH, Lu TM, Chen LC, Chen JW, Lin SJ. Application of the Sequential Organ Failure Assessment score for predicting mortality in patients with acute myocardial infarction. Resuscitation. 2012;83(5):591–595. doi: 10.1016/j.resuscitation.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Becker LB, Aufderheide TP, Geocadin RG, Callaway CW, Lazar RM, Donnino MW, Nadkarni VM, Abella BS, Adrie C, Berg RA, Merchant RM, O'Connor RE, Meltzer DO, Holm MB, Longstreth WT, Halperin HR American Heart Association Emergency Cardiovascular Care Committee, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124(19):2158–2177. doi: 10.1161/CIR.0b013e3182340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2012. R: A language and environment for statistical computing. ISBN 3-900051-07-0, URL http://www.R-project.org/ [Google Scholar]
- 26.Harrell FE. rms: Regression Modeling Strategies. R package version 3.5-0. 2012 http://CRAN.R-project.org/package=rms. [Google Scholar]
- 27.Fischer EG, Ames A, Lorenzo AV. Cerebral blood flow immediately following brief circulatory stasis. Stroke. 1979;10(4):423–427. doi: 10.1161/01.str.10.4.423. [DOI] [PubMed] [Google Scholar]
- 28.Cohan SL, Mun SK, Petite J, Correia J, Tavelra Da Silva AT, Waldhorn RE. Cerebral blood flow in humans following resuscitation from cardiac arrest. Stroke. 1989;20(6):761–765. doi: 10.1161/01.str.20.6.761. [DOI] [PubMed] [Google Scholar]
- 29.Sunde K, Pytte M, Jacobsen D, Mangschau A, Jensen LP, Smedsrud C, Draegni T, Steen PA. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Castren M, Silfvast T, Rubertsson S, Niskanen M, Valsson F, Wanscher M, Sunde K Task Force on Scandinavian Therapeutic Hypothermia Guidelines, Clinical Practice Committee Scandinavian Society of Anaesthesiology and Intensive care Medicine. Scandinavian Clinical practice guidelines for therapeutic hypothermia and post-resuscitation care after cardiac arrest. Acta Anaesthesiol Scand. 2009;53(3):280–288. doi: 10.1111/j.1399-6576.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 31.Zobel C, Adler C, Kranz A, Seck C, Pfister R, Hellmich M, Kochanek M, Reuter H. Mild therapeutic hypothermia in cardiogenic shock syndrome*. Crit Care Med. 2012;40(6):1715–1723. doi: 10.1097/CCM.0b013e318246b820. [DOI] [PubMed] [Google Scholar]
- 32.Bro-Jeppesen J, Kjaergaard J, Horsted TI, Wanscher MC, Nielsen SL, Rasmussen LS, Hassager C. The impact of therapeutic hypothermia on neurological function and quality of life after cardiac arrest. Resuscitation. 2009;80(2):171–176. doi: 10.1016/j.resuscitation.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Gaieski DF, Band RA, Abella BS, Neumar RW, Fuchs BD, Kolansky DM, Merchant RM, Carr BG, Becker LB, Maguire C, Klair A, Hylton J, Goyal M. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–424. doi: 10.1016/j.resuscitation.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Beylin ME, Perman SM, Abella BS, Leary M, Shofer FS, Grossestreuer AV, Gaieski DF. Higher mean arterial pressure with or without vasoactive agents is associated with increased survival and better neurological outcomes in comatose survivors of cardiac arrest. Intensive Care Med. 2013;39(11):1981–1988. doi: 10.1007/s00134-013-3075-9. [DOI] [PubMed] [Google Scholar]