Abstract
Creatine is an antioxidant, neuromodulator and key regulator of energy metabolism shown to improve depressive symptoms in humans and animals, especially in females. To better understand the pharmacological effects of creatine, we examined its influence on depression-related hippocampal gene expression and behaviors in the presence and absence of sex steroids. Sham-operated and gonadectomized male and female rats were fed chow alone or chow blended with either 2% or 4% w/w creatine monohydrate for five weeks before forced swim, open field, and wire suspension tests, or seven weeks total. Before supplementation, males were chronically implanted with an empty or a testosterone-filled (T) capsule (10-mm surface release), and females were administered progesterone (P, 250 μg), estradiol benzoate (EB, 2.5 μg), EB+P, or sesame oil vehicle weekly. Relative to non-supplemented shams, all hippocampal plasticity-related mRNAs measured, including brain-derived neurotrophic factor (BDNF), tyrosine kinase B, doublecortin, calretinin, and calbindin, were downregulated in sham males given 4% creatine, and BDNF, doublecortin, and calbindin mRNAs were downregulated in sham females given 4% creatine. In contrast, combined 4% creatine + T in castrates prevented downregulation of BDNF, doublecortin, and calretinin mRNAs. Similarly, combined 4% creatine + EB+P in ovariectomized females attenuated downregulation of BDNF and calbindin mRNA levels. Moderate antidepressant and anxiolytic-like behaviors were observed in EB+P-treated ovariectomized females fed creatine, with similar trends in T-treated castrates fed creatine. Altogether, these data show that chronic, high-dose creatine has opposing effects on neuroplasticity-related genes and depressive behavior in intact and gonadectomized male and female rats. The dose and schedule of creatine used negatively impacted hippocampal neuronal integrity in otherwise healthy brains, possibly through negative compensatory changes in energy metabolism, whereas combined creatine and sex steroids acted in a neuroprotective manner in gonadectomized rats, potentially by reducing metabolic complications associated with castration or ovariectomy.
Keywords: Creatine, Antidepressant, Depression, Sex hormone, Forced swim test, Neuroplasticity, qRT-PCR, Sex difference
1. Introduction
Creatine, N-(aminoiminomethyl)-N-methylglycine, is a naturally occurring nutrient found in high protein foods such as beef, fish and milk, and it can also be synthesized by the body from the amino acids L-arginine, glycine, and L-methionine (Wyss and Kaddurah-Daouk, 2000). Creatine has traditionally been understood as a vital regulator of adenosine triphosphate (ATP) levels in muscle and brain tissue (Wallimann et al., 1992), and the peripheral effects of creatine supplements have been studied most extensively in athletes and military personnel. More recent studies have demonstrated creatine’s neuromodulatory, antioxidant, anti-apoptotic, and anti-inflammatory properties in the central nervous system, motivating researchers to assess the therapeutic utility of creatine monohydrate for treating brain-related disorders (Allen, 2012; Andres et al., 2008; Gaulano et al., 2010).
Of clinical relevance, previous evidence has shown that impaired creatine metabolism has deleterious effects on brain integrity (in’t Zandt et al., 2004; Streijger et al., 2005) and cognitive development (Braissant and Henry, 2008; Jost et al., 2002; Schulze et al., 2003; Streijger et al., 2004, 2005). Conversely, supplementation with creatine has been shown to protect the brain from the negative effects of mild stress (McMorris et al., 2006, 2007a; Watanabe et al., 2002), age-related memory decline (Bender et al., 2008; McMorris et al., 2007b), brain and spinal cord injury (Rabchevsky et al., 2003; Sakellaris et al., 2006; Scheff & Dhillon et al., 2004; Sullivan et al., 2000), and muscle and neurodegenerative disorders such as Huntington’s disease (Rosas et al., 2014). The necessity of endogenous creatine for healthy brain function combined with evidence that dietary creatine improves brain-related conditions underscores its potential for treating mental illness (Andres et al., 2008; Gualano et al., 2010).
An increasing number of human and animal studies indicate that creatine is particularly promising for the treatment of depressive disorders (Allen, 2012). Rodent studies have found that creatine supplementation reduces immobility in the forced swim and tail suspension tests, which are animal models that robustly predict antidepressant treatment response in humans (Allen et al., 2010; 2012; Cryan et al., 2005; Cunha et al., 2012, 2013a b). Of particular interest, our laboratory has found sex differences in the effects of creatine on behavior in rats. Specifically, daily creatine supplementation (+4% w/w, five weeks, per os) decreased immobility behavior in female rats, an effect that is indicative of antidepressant-like properties, whereas the same dose and duration produced either no effect or increased immobility behaviors in male rats (Allen et al., 2010; 2012). Two clinical trials reported that adjunctive creatine accelerated treatment response in depressed female adolescents (Kondo et al., 2011) and women (Lyoo et al., 2012) resistant to conventional treatment, underscoring its translational potential and clinical utility.
The present research aimed to build upon and extend our previous rodent findings by examining two plausible neurobiological mechanisms that could explain the sex-specific antidepressant-like effects of dietary creatine in rats and possibly humans, namely the activational influence of sex steroids and the involvement of neurotrophin-related neuronal activity. Briefly, sex hormones exert important influences on physiology and behavior that extend well beyond reproduction, including the regulation of cognition, mood, mitochondrial function, neurogenesis and synaptic plasticity (for review, see Baudry et al., 2012; Walf and Frye, 2006). The effects of sex hormones on creatine metabolism are sex-specific. For instance, creatine kinase activity, which regulates the use and consumption of energy, increases and decreases in sync with the rat estrous cycle (Sömjen et al., 1991). Moreover, brain-type creatine kinase has been shown to be upregulated sex-specifically by estrogens in female rats and by testosterone in male rats (Malnick et al., 1983; Sömjen et al., 1989; 1997; 2011). Our previous forced swim data indicated that creatine supplementation produced antidepressant-like effects in females particularly during the proestrus and estrus phases of the estrous cycle, when levels of ovarian hormones are high (Allen et al., 2012). We hypothesized that cycling levels of estrogens augment the antidepressant-like effect of creatine in female rats by stimulating energy production and consumption. In males, it is possible that the stable, as opposed to cyclical, nature of testosterone precludes significant increases in creatine metabolism during testing.
Another consideration is that creatine may improve depressive symptoms by enhancing the growth or survival of neurons (Zainuddin and Thuret, 2012). Increasing evidence supports a relationship between creatine and brain-derived neurotrophic factor (BDNF), an essential mediator of synaptic plasticity, neural survival and energy metabolism associated with depression (Duman and Monteggia, 2006). For instance, in mice, oral supplementation with 1% creatine upregulated BDNF expression levels in whole brain hemisphere samples by 1.27 times compared to controls (Bender et al., 2008). In humans, three studies found that a single nucleotide polymorphism of the BDNF gene (val66met) that interferes with BDNF function is associated with lower levels of total creatine in hippocampal and cortical tissue in healthy and mood disordered patients (Frey et al, 2007; Gallinat et al, 2010; Stern et al, 2008;). Given this evidence, we examined the effects of creatine on mRNA levels of hippocampal BDNF, and its receptor Tyrosine Kinase B (TrkB), in the presence and absence of sex hormones to determine whether creatine alters neuroplasticity-related factors akin to conventional antidepressants.
Additionally, to develop a more complete profile of creatine’s effects on factors related to neural growth and survival, we also examined hippocampal mRNA levels of doublecortin (DCX), calretinin (CALR), and calbindin (CALB). DCX is classified as a microtubule-associated protein (MAP) and is involved in hippocampal cell division, differentiation and migration (des Portes et al., 1998). CALB and CALR are calcium-binding proteins that frequently colocalize with GABAergic interneurons and maintain intracellular calcium balance that is integral for neurotransmission (Todkar et al., 2012). These three markers have distinct developmental expression patterns within the subgranular zone of the dentate gyrus in adult hippocampus (see Brandt et al., 2003). DCX is expressed in newly born progenitor cells, whereas CALR and CALB are expressed in immature and mature granule cells, respectively.
We conducted two experiments in male (Experiment 1) and female (Experiment 2) rats using three doses of creatine monohydrate (+0%, +2%, or +4%, per os) to determine whether the previously observed effects of creatine on depression-related behavior are attributable to the actions of gonadal steroids during behavioral testing. This work paired classic hormone manipulation paradigms, namely bilateral castration or ovariectomy ± sex hormone replacement, with standard behavioral assays to evaluate potential hormone-creatine interactions on affective behavior. A secondary aim was to conduct a focused analysis on depression-related hippocampal gene expression to determine whether creatine alters mRNA levels of neurotrophic- and neurogenesis-related markers in adult hippocampal tissue. Due to the large number of experimental groups, we planned a priori to narrow the scope of this initial molecular investigation to compare the effects no-dose (+0%) and high-dose (+4%) creatine in pre-selected hormone groups, as 4% creatine is the dose previously shown to robustly alter affective behavior (Allen et al., 2010; 2012). We hypothesized that creatine would increase expression of plasticity-related markers in the same manner shown by chronic studies of conventional antidepressant medication, and that any positive mRNA alterations would correspond with antidepressant-like effects. Together, this work aimed to increase understanding of the behavioral, pharmacological, and neurobiological mechanisms of creatine and its potential to improve depressive symptoms.
2. Material and Methods
2.1. Experiment 1: Males
2.1.1. Animals and Housing
Seventy-two male Sprague-Dawley rats, approximately six weeks old, were obtained from Charles River Laboratories (Raleigh, NC). Rats were housed individually in hanging stainless-steel cages in a climate-controlled vivarium (22 ± 1°C) on a 12:12 h reverse light-dark cycle (lights on at 1900h). Rats were given seven days to acclimate to handling and housing conditions prior to the beginning of each experiment.
All procedures were approved by the Tufts University Institutional Animal Care and Use Committee (IACUC).
2.1.2. General Experimental Procedure
The duration of the experiment was a total of eleven weeks: one week habituation period, two weeks for surgery and recovery, five weeks of daily creatine supplementation, and two weeks for behavioral testing before euthanasia (Figure 1). Following habituation, rats were randomly assigned to undergo bilateral castration (GDX) or sham-surgery and to receive testosterone (T) maintenance or no T (described below). Upon recovery, equal numbers of rats from each hormone group (i.e., sham, GDX, GDX+T) were randomly assigned to receive 0%, 2%, or 4% creatine supplementation (Allen et al., 2010, 2012). Following five weeks of daily creatine treatment, rats performed in the forced swim, open field, and wire suspension tests, within a two-week period, prior to euthanasia. Rats were tested in two separate but consecutive squads under identical conditions and counterbalanced by diet and hormone conditions. This was necessary to study an adequate number of rats to achieve statistical power for behavioral measures.
Figure 1.
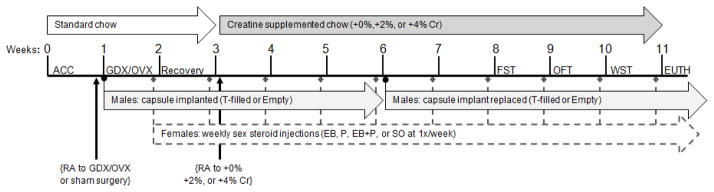
Timeline of study procedures in male (Exp. 1) and female (Exp. 2) rats. The order of surgery, sex steroid administration, dietary supplementation, and behavioral assessment procedures. Abbrev.: ACC, acclimation period; Cr, creatine; EB, estradiol benzoate; EUTH, euthanasia; FST, forced swim test; GDX, gonadectomy/bilateral castration; OFT, open field test; OVX, bilateral ovariectomy; P, progesterone; RA, random assignment; SO, sesame oil; T, testosterone; WST, wire suspension test.
2.1.3. Bilateral Castration and Testosterone Replacement
Bilateral castrations were performed to remove the primary endogenous source of androgens using IACUC-approved surgical procedures. All male rats were anesthetized with a single dose of Ketamine (80 mg/kg) and Xylazine (6 mg/kg) intraperitoneally and were randomly assigned to undergo bilateral castration (GDX) or sham surgery approximately one week following habituation (Frye and Wawrzycki, 2003).
Testosterone treatment was randomly assigned to one half of the GDX males (n = 24) to restore normal physiological levels using a single controlled-released Silastic capsule (10-mm surface release) filled with 100% crystalline testosterone (Sigma Chemical). Silastic implants provide sustained release of testosterone in a manner that mimics the naturally occurring, stable endogenous levels of testosterone observed in intact (and sham-operated) male rats (Smith et al., 1977; Svensson et al., 2003). Silastic implants are frequently utilized to administer hormones chronically in numerous rodent models of cognition, anxiety, and depression. All capsules were constructed with extreme care to avoid contamination according to the procedures described by Smith et al. (1977). The capsule was inserted subcutaneously in the region of the scrotum via the small skin incision made during gonadectomy surgery. The capsule was replaced once approximately four weeks later (two weeks prior to behavioral testing), in a brief procedure under isoflurane anesthesia, to ensure efficacy and stability of testosterone release (refer to Damassa et al., 1977; Smith et al., 1977; Svensson et al., 2003). The sham-operated (n = 24) and untreated GDX (n = 24) received an empty capsule made from the exact same materials under the same surgical conditions. Post-operative analgesia (Carprofen, 5 mg/kg) was administered subcutaneously immediately after surgery and given once daily for three days and then as necessary.
2.1.4. Diets
Beginning one week after surgery, an equal number of rats from each hormone group (sham, GDX, GDX+T) were randomly assigned to receive standard powdered rat chow alone or chow mixed with 2% or 4% w/w creatine monohydrate for five weeks prior to behavioral testing. The creatine-supplemented mixtures were made in-house using creatine monohydrate > 98% (Sigma Chemical; 0 kcal/g) and ground Purina chow #5001 (3.4 kcal/g). Food was presented in Wahmann LC306 (Timonium, MD) stainless-steel food cups, which were covered with lids and clipped to the floor of the cage to reduce spillage. Glass water bottles with drip-proof stoppers were fitted at the front of each cage. Body weight, and chow and water intakes were measured every other day at the same time of day. Chow was measured to the nearest 0.1 g and reported in calories (to the nearest 0.1 kcals). Water intake and body weights were recorded to the nearest 1.0 g. The amount of dietary creatine consumed was calculated and reported as the number of grams of creatine ingested per kilogram of rat (g/kg).
2.1.5. Forced Swim Test
Rats were tested in a modified version of the Porsolt forced swim test (Detke et al., 1997; Porsolt et al., 2001; Frye and Walf, 2002), an index of antidepressant-like behavior in rodents. Briefly, rats were placed in a clear Plexiglas cylinder (25 cm in diameter by 65 cm in height) filled to 48 cm with 25° C water (± 0.5° C) for a total of 10 minutes. To characterize the time course of treatment effects, the time-sampling technique developed by Detke et al. (1995) was used to score the entire ten minutes, as well as the first five minutes of the trial to enable comparisons to previous research (Allen et al., 2010, 2012). A rater blind to the experimental conditions recorded one of four predominant behaviors of the rat at the end of each five-second period of time, including counts of swimming, climbing, immobility, and diving (as described previously, Allen et al., 2010, 2012). Additionally, latency to the onset of immobility was calculated based on the first time a rat displayed an immobile posture for at least two consecutive five-second intervals (at least ten seconds).
2.1.6. Open Field Test
The open field test was used to measure anxiety-like behavior as well as locomotor activity and exploratory behaviors. One week after the forced swim test, rats were placed in a square apparatus (50 cm length X 50 cm width) for five minutes. The center of the field was illuminated by a 60 watt bulb, which was the only direct source of light in the room. Scored behaviors included the number of rears, stretch attends, grooming, and the number of times the animal returned to its starting corner. Also recorded were average speed, average distance traveled, and time spent in all corners, borders, and center of the field.
2.1.7. Wire Suspension Test
Psychomotor testing was conducted within two weeks after the forced swim test. The wire suspension test was used to exclude the possibility that differences in physical ability or motor function occurred as a result of dietary, surgical, or hormonal manipulations. The wire suspension task measured the ability of the animal to grasp a taut horizontal wire (2 mm. in diameter, 62 cm. above the table top) with its forepaws and to remain suspended. Latency to drop from the wire was measured in seconds, with a maximum testing time of 60 sec.
2.1.8. Tissue Preparation and Neurochemical Analyses
All rats were euthanized by decapitation under isoflurane anesthesia to collect trunk blood and brain tissue one day after the wire suspension test (seven weeks post-creatine). Rats from all diet and hormone groups completed the last behavioral test in the same period of time and were euthanized in a counterbalanced order over the course of four days.
Trunk blood was collected first, then the brain was quickly removed and the hippocampus and prefrontal cortex were rapidly dissected free-hand on an ice block in a sterile environment. Trunk blood samples were centrifuged for twenty minutes at 3000 g, and the plasma supernatant was collected and frozen (-80° C) until analysis of creatine content. Hippocampal and prefrontal cortex tissue from one hemisphere were collected for analysis of creatine and protein concentrations, and hippocampal tissue from the other hemisphere was collected for analysis of depression-related mRNA expression for a subset of samples selected a priori for genetic analysis. Note that the genes of interest were assessed in hippocampal tissue only, as it is a major site of adult neurogenesis. To control for possible lateralization effects, collection of left and right hemisphere samples were counterbalanced for all groups. All samples were frozen on dry ice and stored in a freezer (-80° C) until processing.
2.1.8.1. Creatine and Protein Assays
Analysis of all blood plasma, prefrontal cortex, and hippocampal samples for creatine levels were performed using a commercially available calorimetric assay kit (BioVision, Inc.), which uses an enzyme-linked technique that relies on oxidation of sarcosine to produce a change in optical absorbance. Creatine concentrations in blood plasma were compared to a standard creatine curve and calculated as nmol/ml. Creatine levels measured in the prefrontal cortex and hippocampus were also quantified from a standard creatine curve, normalized to the amount of protein in each tissue sample using a standard protein assay (below), and reported as a ratio of creatine:protein (nmol/mg).
Determination of protein levels in brain tissue samples was accomplished using the Bradford assay (Bradford, 1976). This common calorimetric technique uses Coomassie Brilliant Blue Dye (Bradford Reagent, Sigma-Aldrich). The amount of protein in the sample wells was quantified by comparing the absorbance values to a concurrent standard curve for protein generated with known concentrations of bovine serum albumin.
2.1.8.2. RNA Isolation and Quantitative Reverse Transcription-PCR (qRT-PCR) Analysis
Determination of relative expression levels of depression-related mRNAs in brain tissue was performed using quantitative reverse transcription-PCR (qRT-PCR) for all rats in all conditions (except where noted below). Total RNA was extracted using Tri-Reagent (Molecular Research Center, Inc.), treated with DNAse (2U TURBO DNAse, Ambion), and tested for genomic DNA contamination in PCRs. The quantity and integrity of mRNA was validated using a NanoDrop spectrophotometer (Thermo Scientific). cDNA was synthesized using 1.5 μg of total RNA, 200 units of Superscript II reverse transcriptase (Invitrogen) and 1 μl of random hexamers (Invitrogen) in a 20 μl reaction. qRT-PCR amplification was performed using a MX-3000P Stratagene cycler with SYBR green PCR master mix (Applied Biosystems) and a two-step protocol: (1) 95°C for 10 min followed by (2) 45 cycles with 95°C for 30 sec, 55°C or 59° for 30 sec, and 72°C for 30 sec. The annealing temperature in the second step varied depending on the optimal melting temperature of the primer sequence, which is detailed below.
Primer pairs specific to β-actin, BDNF, TrkB, DCX, CALR, and CALB were adapted from previously published research studies and validated using the NCBI primer BLAST software. β-actin was used as the normalizer. The specificity of product amplification for each intron-spanning primer pair was confirmed by 2% agarose gel electrophoresis and dissociation curve analysis, and the results revealed no evidence of primer dimerization. In addition, serial dilution curves were created for all primers used, and the efficiency of amplification was found to exceed 90% for all pairs. All target genes were found to have similar efficiencies to the housekeeping gene, β-actin. The amplicon length was kept as close as possible to 100–150 bp. The optimal annealing temperature was determined to be 55°C for the BDNF, TrkB, and CALB primer sets and 59° for the CALR and DCX primer sets. The efficiency and dissociation curves of β-actin were validated at both annealing temperatures. All samples and non-template controls were run in duplicate using the appropriate thermal profiles. The relative levels of expression of each mRNA sample were calculated using the comparative Ct method (Schmittgen and Livak, 2008).
The specific primer sequences used were as follows: β-actin forward: GGCTGTATTCCCCTCCATCG, β-Actin reverse: CCAGTTGGTAACAATGCCATGT (Cordeira et al., 2010), BDNF forward: TTAGCGAGTGGGTCACAGCG, BDNF reverse: ATTGGGTAGTTCGGCATTGC (Huang et al., 2010), TrkB forward: CCTCCACGGATGTTGCTGAC, TrkB reverse: GCAACATCACCAGCAGGCA (Cordeira et al., 2010), DCX forward: CTCCTATCTCTACACCCACAAGCC, DCX reverse: GAATCGCCAAGTGAATCAGAGTC (Kádár et al., 2011), CALR forward: CCACCTACAGGAAGAGCGTCAT, CALR reverse: GGCTCACTGCAAAGCACAATC (Dijk et al., 2004), and CALB forward: AGGGATGTGCTTCTGCTTGT, CALB reverse: CATCTGGCTACCTTCCCTTG (Xi et al., 2009).
2.1.9. Statistical Analysis
Data were analyzed using SPSS v.18.0 for MAC OSX. Behavioral and neurochemical data were analyzed using two-way ANOVA with diet and hormone as the between-subjects factors. Forced swim behavior was analyzed based on performance during the first five minutes of the test in addition to the full ten-minute test to fully characterize behavioral changes over time, and to facilitate comparisons to forced swim behavior reported in previous studies of creatine supplementation (Allen et al., 2010, 2012). Body weight, calories and creatine intake were analyzed using three-factor mixed ANOVAs, with two between-subjects factors (diet and hormone condition) and one within-subjects factor (weeks). Additionally, weekly averages for body weight, calorie and creatine consumption were analyzed using one-way ANOVAs when significant differences were detected to determine how each group differed each week.
Rats from all hormone and diet groups were included in all neurochemical and behavioral analyses, with the following exception. As mentioned above, a decision was made a priori to only examine samples of male rats given 0% or 4% creatine supplementation using qRT-PCR due to the large number of samples and inability to assay all at once. Thus, 2% creatine hippocampal samples were not collected for analysis using qRT-PCR. This determination was based on results from previous work that showed that 4% creatine produces more robust behavioral effects than 2% creatine, and therefore, potentially the most robust molecular effects (Allen et al., 2010; 2012). All male hormone groups were included in molecular analyses.
Planned post hoc comparisons were made using Tukey’s HSD (or the Tukey-Kramer method in cases where there were unequal sample sizes). Alpha was defined as p < 0.05
2.2. Experiment 2: Females
2.2.1. Methods
All methods for Experiment 2 were identical to Experiment 1 with two exceptions. First, 156 female Sprague-Dawley rats were used. Second, all females underwent ovariectomy or sham-surgery before random assignment to receive ovarian hormone replacement or sesame oil vehicle each week. Rats were tested in three separate but consecutive squads under identical conditions and counterbalanced by diet by hormone conditions due to the number of rats required to achieve statistical significance.
2.2.2. Ovariectomy and Ovarian Hormone Replacement
Ovariectomy was performed to remove the primary source of estrogens and progesterone. All female rats were anesthetized using a single dose of Ketamine (80 mg/kg) and Xylazine (6 mg/kg) administered intraperitoneally and were randomly assigned to undergo ovariectomy or sham surgery approximately one week following habituation to the laboratory environment (Frye, Bock and Kanarek, 1992; Petralia, DeBold, and Frye, 2007).
Within one week following ovariectomy (n = 120) or sham surgery (n = 36), the ovariectomized (OVX) rats were randomly assigned to one of four hormone replacement groups to receive: estradiol benzoate (EB, 2.5 μg), progesterone (P, 250.0 μg), EB+P, or sesame oil vehicle alone (all from Sigma Chemical). All sham-operated females received sesame oil vehicle only. EB was dissolved in sesame oil at a concentration of 50 μg/ml, and P was dissolved in sesame oil at a concentration of 5.0 mg/ml. All injections were administered subcutaneously at a volume of 0.05 ml per individual.
Sesame oil, EB, P, or EB+P was administered once per week, on the same day and time each week, for the duration of the experiment to mimic normal physiological levels. Subcutaneous injections were timed so that females received P and EB maintenance at 4 hours and 48 hours, respectively, prior to each behavioral test and time of euthanasia. This regimen was used because it mimics the gradual increase in estradiol levels and the pulsatile effect of progesterone during the estrous cycle (Petralia et al., 2007; Walf and Frye, 2006).
2.2.3. Statistical Analysis
Statistical analyses for Experiment 2 were identical to Experiment 1 with the exception that a decision was made a priori to focus the genetic analysis to include only sham, OVX, and OVX+EB+P female samples fed either 0% or 4% creatine. The reason is due to the large number of samples (diet x hormone) and inability to assay all samples at once using qRT-PCR. Thus, hippocampal samples from 2% creatine-fed rats were not collected for molecular analyses.
3. Results
3.1. Experiment 1: Effects of creatine on depression-related gene expression and behavior in sham-operated and castrated male rats, with and without testosterone replacement
3.1.1. Male Diets and Body Weights
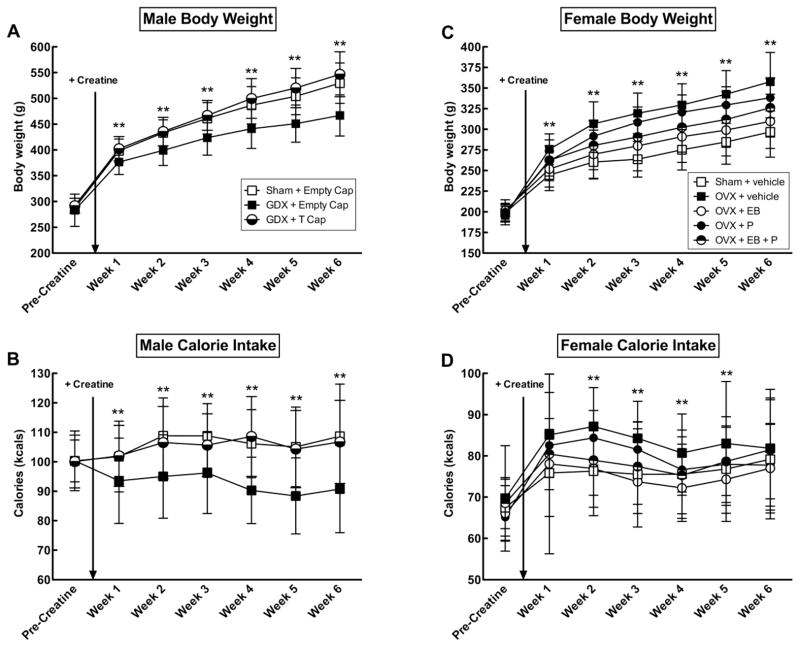
Body weights and caloric intake between hormone groups did not differ prior to surgery or creatine supplementation. Bilateral castration significantly reduced the body weights of GDX males (F(2,60) = 17.90, p < 0.001), whereas the body weights of GDX+T did not differ from shams for the duration of the study (all p > 0.05) (Figure 2a).
Figure 2.
Mean (± SEM) body weight and calorie intake in male (A–B; Exp. 1) and female (C–D; Exp. 2) rats across hormone conditions. The star symbol (*) indicates a significant effect (p < 0.05) of castration (GDX) or ovariectomy (OVX). GDX males implanted with an empty capsule weighed less and ate less than sham-operated rats and GDX rats implanted with a testosterone (T)-filled capsule, regardless of creatine. OVX+Vehicle (Veh) females weighed more and ate more than all other groups except OVX + P, regardless of creatine. Estradiol benzoate (EB) had an anorectic effect in EB- and P-treated OVX rats. EB alone normalized body weights and food intakes to sham levels, whereas P antagonized the effect of EB. OVX+EB+P rats weighed less than untreated OVX and OVX+P rats. All hormone groups consumed equal amounts of creatine on a gram per kilogram basis.
Similarly, bilateral castration significantly decreased caloric intake in GDX males (F(2, 61) = 8.94, p < 0.001), whereas calories consumed by GDX+T did not differ from shams for the duration of the study (all p > 0.05)(Figure 2b). GDX did not produce differences in the amount of creatine consumed on a gram per kilogram basis for the duration of the study.
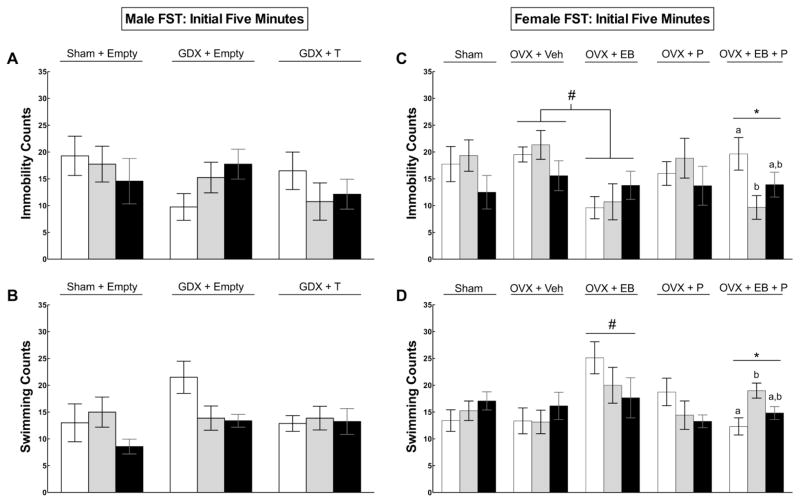
3.1.2. Male Forced Swim Test (FST)
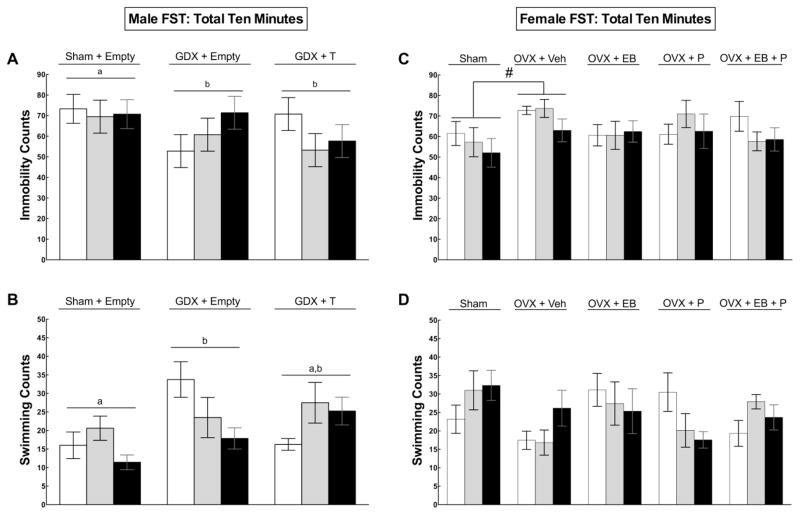
There were no significant main effects of hormone or creatine treatment during the initial five minutes of the FST (Figure 3a–b). During the full ten-minute FST, bilateral castration altered total immobility (F(2,60) = 3.17, p = 0.049) and swimming counts (F(2,60) = 3.24, p = 0.046) (Figure 3a). Post hoc analyses indicated that sham males displayed immobility more frequently than both GDX (p = 0.042) and GDX+T (p = 0.047). GDX rats swam more than shams (p = 0.017) but displayed a similar amount of swimming as GDX+T (p > 0.05). There was a correlation between lower body weight in GDX males without T and high levels of activity in the FST, which was not observed in shams or GDX+T.
Figure 3.
Mean (± SEM) immobility (A, C) and swimming (B, D) behaviors of male (left, Exp. 1) and female (right, Exp. 2) rats performing in the first five minutes of forced swim testing (FST). In males, there were no differences detected in sham or castrated (GDX) males given empty or testosterone (T)-filled capsules during the first five minutes (p > 0.05). In females, the star symbol (*) indicates a significant effect (p < 0.05) of creatine within the OVX+EB+P treated group, such that 2% and 4% creatine supplementation reduced immobility scores compared to non-supplemented shams. The pound symbol (#) indicates that OVX+EB rats displayed significantly less immobility than OVX+Vehicle (Veh) rats (3C) and OVX+EB rats swam more than all other groups (3D) (p’s < 0.05).
Also during the full ten-minute FST, there was a marginal creatine by hormone interaction effect for total immobility (F(2,60) = 2.13, p = 0.088) (Figure 4a). An exploratory post hoc analysis within the group of GDX+T rats showed that GDX+T rats supplemented with 2% and 4% creatine displayed less immobility than GDX+T fed 0% creatine, however this trend did not reach significance (F(2,20) = 2.78, p = 0.086). A second exploratory analysis within the group of GDX rats without T showed that GDX rats fed 4% creatine displayed more immobility than GDX rats fed 0% creatine (Figure 4b), but this trend also did not achieve significance (F(2,21) = 2.08, p = 0.150).
Figure 4.
Mean (± SEM) immobility (A,C) and swimming (B,D) behaviors of male (left, Exp. 1) and female (right, Exp. 2) rats performing in the full ten-minute forced swim test (FST). Means with different letters are statistically different (p < 0.05). In males, castrates (GDX) without testosterone (T) displayed less immobility and more swimming, but this hyperactive effect was correlated with lower body weight. A non-significant trend shows an antidepressant-like effect of creatine in GDX+T and a pro-depressant-like effect of creatine in GDX implanted with an empty capsule (4A). In females, the pound symbol (#) indicates that ovariectomized (OVX) rats without estradiol benzoate (EB), progesterone (P), or E+P displayed immobility more frequently relative to sham females (p < 0.05), but no other behavioral differences were detected in ten minutes.
3.1.3. Male Open Field Test
Hormone status altered the amount of time spent in the corners (F(2,61) = 4.10, p = 0.021) and the borders (F(2,61) = 3.75, p = 0.029) of the open field, but there were no differences in time spent in the center of the field (p > 0.05) (data not shown). Post hoc analyses indicated that GDX spent more time in the corners (228.80 sec ± 5.74 sec) (p = 0.027) and less time in the borders (77.66 s ± 5.50 sec) (p = 0.044) compared to shams (195.09 ± 8.44 sec and 100.07 ± 7.78 sec, respectively). GDX+T spent a similar amount of time in the corners and borders (201.23 ± 3.99 sec and 96.28 ± 5.53 sec, respectively) as shams.
Open field activity did not vary significantly as a function of creatine, and there were no creatine by hormone effects for any of the open field behaviors.
3.1.4. Male Wire Suspension Test
Latency to release grasp from the wire did not differ as a function castration or testosterone replacement (average hang time ± SEM: shams, 9.49 ± 1.60; GDX, 13.24 ± 2.44; GDX+T, 8.01 ± 0.86) nor were any differences in suspension time detected as a function of creatine (average hang time ± SEM: 0% creatine, 9.97 ± 1.62 s; 2% creatine, 10.74 ± 1.78 s; 4% creatine: 10.08 ± 2.06 s). No interaction effects were detected for hormone by diet (p > 0.05) (data not shown).
3.1.5. Male Creatine Concentrations
Plasma levels of creatine increased in rats supplemented with creatine in a dose-dependent manner (F(2,59) = 158.50, p < 0.001), such that males fed 4% creatine (1456.55 ± 65.63 nmol) had higher levels of plasma creatine compared to rats supplemented with 2% creatine (824.95 ± 39.63 nmol) (p < 0.001), and 2% creatine had higher levels than 0% creatine (276.30 ± 20.78 nmol)(p < 0.001).
Hippocampal creatine concentrations increased in supplemented rats (F(2,59) = 3.74, p = 0.03), whereby males fed 4% creatine (.336 ± 0.012 nmol/mg) showed greater concentrations compared to males fed 0% creatine (0.294 ± 0.011 nmol/mg) (p = 0.006), but males fed 2% creatine (0.310 ± 0.009 nmol/mg) did not differ from 0% creatine controls (p > 0.05). Prefrontal cortex levels of creatine did not differ as a function of diet in males (p > 0.05) (0%: 0.233 ± 0.014; 2%: 0.231 ± 0.022; 4%: 0.209 ± 0.015 nmol/mg).
3.1.6. Male mRNA Expression
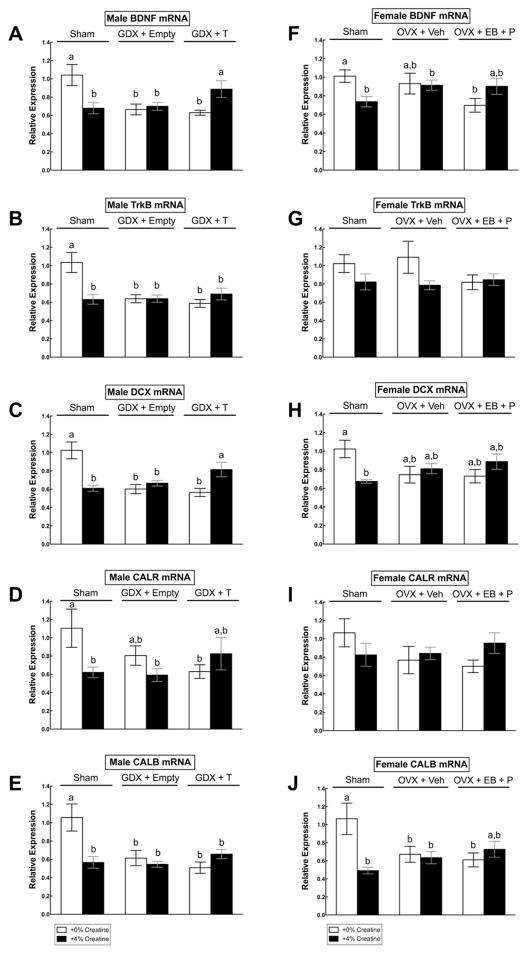
There were multiple creatine by hormone interaction effects on mRNA levels for all genes of interest: BDNF (F(2,40) = 9.50, p = < 0.001), TrkB (F(2,40) = 9.19, p < 0.001), DCX (F(2,40) = 16.20, p= < 0.001), CALR (F(2,40) = 3.58, p = 0.037), and CALB (F(2,40) = 8.29, p = 0.001) (Figure 5a–e). Post hoc analyses indicated that 4% creatine supplementation downregulated all mRNA expression levels in sham males relative to non-supplemented sham males (all p’s < 0.05). GDX rats without T also showed significant downregulation of all genes of interest regardless of creatine diet (all p’s < 0.05), whereas GDX+T given 4% creatine exhibited normal expression levels of BDNF, DCX and CALR mRNA as compared to non-supplemented shams (p > 0.05) (Figure 5a,c). Creatine did not prevent the downregulation of TrkB or CALB mRNA levels in GDX+T males (Figure 5b,e).
Figure 5.
Mean (± SEM) mRNA expression levels in hippocampal tissue in male (A–E, Exp. 1) and female (F–J; Exp. 2) rats across hormone and creatine groups. Means with different letters are statistically different (p < 0.05). In sham males, 4% creatine reduced all mRNA levels of interest in comparison to 0% creatine-fed shams, whereas in castrated (GDX) males, combined 4% creatine and testosterone (T) normalized BDNF, DCX, and CALR mRNA levels as compared to non-supplemented shams (5A–E). In sham females, 4% creatine reduced BDNF, DCX, and CALB mRNA levels in comparison to 0% creatine-fed shams, whereas in ovariectomized (OVX) females, 4% creatine partially normalized BDNF and CALB mRNA levels as compared to non-supplemented shams, but there was more variability (5F–J).
3.2. Experiment 2: Effects of creatine on depression-related gene expression and behavior in sham-operated and ovariectomized female rats, with and without ovarian hormone replacement
3.2.1. Female Diets and Body Weights
Average body weights and caloric intakes between hormone groups did not differ prior to surgery or creatine supplementation. Following bilateral OVX, body weights varied significantly between hormone treatment groups (F(4,139) = 18.95, p < 0.001)(Figure 2c). Post hoc analyses indicated that untreated OVX and OVX+P females weighed significantly more than sham-operated and OVX+EB rats (p < 0.05 for all weeks). OVX+EB rats weighed similarly to sham-operated females, whereas OVX+EB+P rats weighed more than shams and OVX + EB but less than OVX and OVX+P females.
Similarly, caloric intake varied significantly between the hormone groups after OVX (F(4,138) = 4.22, p = 0.003) (Figure 2d). Post hoc analyses indicated that untreated OVX rats consumed more calories than sham-operated females during Weeks 2 to 6 (all p’s < 0.05). OVX+EB females consumed similar levels of calories as sham-operated controls throughout the study (all p > 0.05). OVX+P and OVX+EB+P rats consumed more calories than shams during Week 2 (p’s < 0.001) and Week 3 (p’s < 0.01) only, but otherwise did not differ from any of the groups. OVX did not produce differences in the amount of creatine consumed on a gram per kilogram basis for the duration of the study.
3.2.2. Female Forced Swim Test (FST)
During the initial five minutes of FST, there was a main effect of hormone treatment, whereby OVX+EB rats displayed less immobility than untreated OVX rats (p = 0.023) (Figure 3c) and swam significantly more than all other diet by hormone groups (all p’s < 0.05) (Figure 3d). Also during the first five minutes of FST, there was a significant creatine by hormone interaction effect, such that creatine significantly reduced total immobility (F(2,33) = 3.88, p = 0.031) and increased swimming counts (F(2,33) = 5.73, p = 0.007) among the group of OVX+EB+P females fed creatine (Figure 3c–d). Females given 2% creatine displayed significantly less immobility compared to females given 0% creatine (p = 0.024) but did not differ from females given 4% creatine (p > 0.05). Additionally, females given 2% creatine swam more than females given 0% creatine (p = 0.006) but did not differ compared to females given 4% creatine (p > 0.05). Sham females showed a similar antidepressant-like trend as creatine-fed rats, but behavioral changes did not reach statistical significance.
During the full ten-minute FST, OVX females showed significantly greater immobility (F(1,69) = 7.71, p = 0.007) and less swimming activity (F(1,69) = 6.30, p = 0.014) compared to shams, but no other behavioral differences were found (Figure 4c–d).
3.2.3. Female Open Field Test
OVX significantly altered distance traveled (F(4,139) = 5.58, p < 0.001), average speed (F(4,139) = 5.53 p < 0.001), and time spent immobile (F(4,139) = 5.51, p < 0.001). Post hoc analyses indicated that untreated OVX rats traveled less (p = 0.021), moved more slowly (p = 0.021), and spent more time immobile (p < 0.001) compared to OVX+EB rats, but no other differences were detected between the groups (data not shown).
A significant creatine by hormone interaction effect was detected for latency to enter the center (F(8,130) = 2.71, p = 0.008) and the number of entries into the center of the field (F(8,139) = 2.60, p = 0.011) (data not shown). Specifically, within the OVX+EB group, OVX+EB females given 2% creatine entered the center more quickly than OVX+EB females given 0% creatine (p = 0.001) or 4% creatine (p < 0.001). In addition, OVX+EB females given 2% creatine entered the center more frequently than their counterparts given 0% creatine (p = 0.019) and 4% creatine (p = 0.005). Within the OVX+EB+P group, females given 4% creatine entered the center of the field faster than females given 2% creatine (p = 0.038), but neither creatine group differed from females given 0% creatine (p > 0.05).
3.2.4. Female Wire Suspension Test
Latency to drop from the wire did not differ as a function of OVX (average hang time ± SEM: sham-operated, 11.92 ± 1.75; OVX + sesame oil, 9.79 ± 1.05; OVX+EB, 10.87 ± 1.33; OVX+P, 11.68 ± 2.06; OVX+EB+P, 9.35 ± 0.79) nor were any differences in suspension time detected as a function of creatine supplementation (average hang time ± SEM: 0% creatine, 10.59 ± 0.086 s; 2% creatine, 10.78 ± 1.14 s; 4% creatine: 10.50 ± 1.23 s). No creatine by hormone interaction effects were found (p > 0.05) (data not shown).
3.2.5. Female Creatine Concentrations
Plasma levels of creatine increased in rats supplemented with creatine in a dose-dependent manner (F(2,129) = 335.85, p < 0.001), such that females fed 4% creatine (1250.23 ± 39.81 nmol/ml) had higher levels of plasma creatine compared to rats supplemented with 2% creatine (708.87 ± 19.18 nmol/ml) (p < 0.001), and rats given 2% creatine had higher levels than non-supplemented controls (215.13 ± 14.94 nmol/ml) (p < 0.001).
Hippocampal (F(2,129) = 4.32, p = 0.015) and prefrontal cortex (F(2,129) = 8.512, p < 0.001) levels of creatine also increased following dietary supplementation in a dose-dependent manner. Females supplemented with 4% creatine (0.346 ± 0.013 nmol/mg) had higher levels of hippocampal creatine compared to females fed 0% creatine (0.303 ± 0.006 nmol/mg) (p = 0.003), whereas females given 2% creatine (0.328 ± 0.008 nmol/mg) were similar to both the 0%- and 4%-supplemented groups (both p’s > 0.05). Similarly, females given 4% creatine (0.274 ± 0.007 nmol/mg) had higher levels of prefrontal creatine compared to 0% creatine controls (0.237 ± 0.006 nmol/mg) (p < 0.001), whereas females fed 2% creatine (0.256 ± 0.007 nmol/mg) showed similar levels of prefrontal creatine as the 0% and 4% creatine-treated groups (p > 0.05).
3.2.6. Female Hippocampal mRNA Expression
There were significant creatine by hormone interaction effects for the relative expression of BDNF (F(2,41) = 4.56, p = 0.016), DCX (F(2,41) = 6.31, p = 0.004) and CALB mRNAs in hippocampal tissue (F(2,41) = 7.65, p = 0.002) (Figure 5f,h,j). Post hoc analyses indicated that, in comparison to non-supplemented shams, BDNF, DCX, and CALB mRNA levels were downregulated in 4% creatine supplemented shams (all p’s < 0.05). Vehicle-treated OVX females showed significantly reduced CALB mRNA regardless of creatine diet (p < 0.01), with non-significant trends for downregulated BDNF, DCX and CALR mRNA levels. Levels of BDNF and CALB mRNA in OVX+EB+P females fed 4% creatine were partially normalized to non-supplemented sham levels. However, this group also did not differ from OVX+EB+P females given 0% creatine.
4. Discussion
Long-term daily creatine supplementation produced differential effects on hippocampal gene expression and affective behavior in sham-operated rats compared to gonadectomized rats treated with sex steroids. Given that adult hippocampal neurogenesis is known to play an integral role in antidepressant efficacy and some forms of hippocampal-dependent learning (Duman & Monteggia, 2006), these data provide further support that creatine influences neural processes underlying mood and cognition. This interpretation is strengthened by the demonstration that creatine is highly bioavailable in hippocampal tissue in a dose-dependent manner in males and females, and prefrontal cortex in females only. However, the manner in which creatine affects neural processes is more complex than was anticipated. While this is the first report to find a normalizing or protective effect of combined creatine and sex steroid treatment in gonadectomized rats (which corresponded to behavioral changes), it is also the first to find potentially negative effects of excess creatine on hippocampal neural integrity in otherwise healthy intact rats.
As a preface to this discussion, the reliability of these findings is strengthened by the large number of male and female samples that were assayed separately in different experiments using identical procedures. However, the interpretation of the relationship between molecular and behavioral changes induced by creatine and sex hormone treatment is limited by our priori study design, which did not allow for the genetic analysis of all experimental groups. This is particularly relevant to the 2% creatine group, which we did not anticipate having more robust effects than the 4% creatine group based on previous studies (Allen et al., 2010, 2012). This unexpected dose dependency may be explained by differences in study procedures (i.e. surgical experience, age of rats, batch of creatine monohydrate, composition of chow, multiple squads of rats) compared to previous studies. This underscores the need to study the effects of creatine on neurochemistry and behavior in males and females using multiple doses and time points. Nevertheless, the finding that chronic creatine supplementation had opposing effects on hippocampal plasticity-related genes in intact and gonadectomized male and female rats adds to the small but growing body of research linking creatine to brain function, mood and cognition.
In males, hippocampal BDNF, TrkB, DCX, CALR, and CALB mRNA levels in shams supplemented with 4% creatine were unexpectedly downregulated by ~30–40% compared to non-supplemented shams. Similarly, all genes of interest were downregulated in non- supplemented GDX males with and without T. The direction of this change is typically associated with a negative effect on neuroplasticity or a depression-like effect (Duman and Monteggia, 2006), as it suggests attenuation of factors involved in neural survival and differentiation. In contrast, BDNF, DCX, and CALR mRNA levels in GDX+T rats supplemented with 4% creatine were normalized to the levels of non-supplemented shams, indicating that combined treatment (creatine + T) may have protected the birth rate, growth and survival of adult-born hippocampal neurons in males. These molecular effects corresponded with behavioral alterations, given that GDX+T males supplemented with creatine showed marginally significant antidepressant-like behavioral trends in the forced swim test (i.e., reduced immobility) whereas creatine supplementation had no effect on shams, consistent with previous research (Allen et al., 2012). It is important to note that, while the physiological dose of T administered to GDX rats was sufficient for normalizing body weight and caloric intakes, it was not adequate enough to prevent downregulation of synaptic plasticity-related genes or to alter behavior in males without creatine supplementation. This is consistent with previous work, which has shown that high doses of T (>1.0 mg/kg) are required to reliably stimulate hippocampal plasticity in castrated male rats in chronic study designs (>15–30 d) (Spritzer and Galea, 2007, 2011). Thus, we conclude that creatine supplementation augmented the effects of a sub-optimal dose of T on hippocampal gene expression and antidepressant behavior in GDX males.
In females, hippocampal BDNF, DCX, and CALB mRNAs in shams supplemented with 4% creatine were also unexpectedly downregulated by ~20–50% compared to sham females fed non-supplemented chow. As with males, the direction of this change is associated with a negative effect on neurogenesis-related gene expression. In contrast, BDNF and CALB mRNA levels in OVX+EB+P rats supplemented with creatine did not differ from non-supplemented shams, indicating that combined treatment (EB+P + creatine) may have protected the growth and survival of mature neurons in females. Behavioral observations are consistent with these molecular effects, given that OVX+EB+P rats supplemented with creatine showed greater antidepressant-like behavior in the forced swim test (first 5 minutes only) as well as an anxiolytic-like effect in the open field test (i.e., latency to enter center of file, number of center entries). Inconsistent with previous studies, OVX did not reliably reduce hippocampal plasticity-related gene expression. There was more variability in mRNA levels within the vehicle-treated OVX group in comparison to the OVX+EB+P group, which limits the interpretation of the clinical significance of combined treatment effects in females. Nevertheless, OVX rats showed increased immobility in the forced swim test, which corresponds with increased depression-like behavior as predicted (Walf & Frye, 2006).
It is also important to note that there was also a marked antidepressant-like effect of EB alone in OVX+EB rats (i.e., reduced immobility, increased swimming activity), which was expected but could confound the interpretation of the positive creatine effect observed in the OVX+EB+P females. Fortunately, the inclusion of P-treated groups allowed us to separate out the effects of creatine and EB, as previous work has established that P has inhibitory effects on EB when administered in close proximity (Aguirre et al., 2010; Baudry et al., 2012). Consistent with this literature, antagonistic effects of P on the effects of EB were observed in OVX+EB+P rats across various measures, including forced swimming behaviors, body weights, and mRNA changes. Specifically, immobility counts of OVX+EB+P females given 0% creatine were higher than OVX+EB rats given 0% creatine but similar to non-supplemented sham and OVX+P groups, supporting our conclusion that P blocked the antidepressant-like EB effect on immobility counts in the absence of creatine supplementation. Additionally, P attenuated the anorectic effects of EB in OVX rats, such that OVX+EB+P females gained more weight than OVX + EB and sham females. Lastly, EB did not normalize BDNF or CALB mRNA levels in OVX rats in the presence of P, as compared to sham controls, although mRNA changes in the EB-only group were not evaluated. All together, these data suggest that creatine interacts synergistically with EB+P in comparison to EB alone, and that this positive effect is not driven solely by EB.
The current literature provides a few possible interpretations of the positive and negative effects of creatine in intact and gonadectomized rats, which may help to reconcile these opposing outcomes and inspire directions for further research. First, creatine has an important role in regulating brain energy metabolism (Allen, 2012; Andres et al., 2008), and evidence indicates that creatine supplementation benefits individuals with significantly elevated energy demands or metabolic dysregulation (Ipsiroglu et al., 2001; McMorris et al., 2006; Watanabe et al., 2002). Gonadectomy is known to cause significant impairments in energy metabolism in brain and muscle tissue, which may account for the positive outcomes of creatine’s effects on plasticity-related mRNA expression in castrated and ovariectomized rats (Irwin et al., 2008; Kemper et al., 2013; Li et al., 2011; Oner et al., 2008; Ramamani et al., 1999; Shi and Xu, 2008). Castration advances mitochondrial degeneration and muscle atrophy (Oner et al., 2008), reduces ATP content and creatine kinase activity (Ramamani et al., 1999), and activates apoptotic pathways that cause mitochondrial dysfunction in male rats (Li et al., 2011), whereas androgen replacement improves or reverses damage to mitochondrial structure and function in castrates (Koenig et al., 1980a b; 1982; Ramamani et al., 1999). Similarly in females, ovarian hormone withdrawal precipitates impairments in energy metabolism by interfering with enzymes (including creatine kinase) and transcription factors that regulate mitochondrial biogenesis and efficiency in the brains of rodents, including hippocampal neurons, whereas replacement with EB, P, or EB+P restores mitochondrial function and metabolic rates (Irwin et al., 2008; Kemper et al., 2013; Ramamani et al., 1999; Shi and Xu, 2008). Creatine may have buffered against these negative effects in GDX and OVX rats when combined with sex steroids, as evidenced by alterations in gene expression and behavior.
Second, creatine metabolism must remain well-balanced to maintain healthy brain function. The finding that high dose creatine supplementation reduced the expression of neurotrophic- and neurogenesis-related mRNA levels in otherwise healthy rats is concerning given that creatine monohydrate is among the most popular dietary supplements used frequently by healthy individuals (Metzl et al., 2001). We hypothesize that there is an inverted U-shape response curve in healthy rats, meaning that optimal levels peak approximately five weeks in intact rats, based on previous studies using this time course (Allen et al., 2010, 2012), but may begin to oversaturate tissue in the absence of metabolic demand, thus leading to negative effects. This explanation is consistent with the U-dose response curve reported by Matthews et al. (1999), who have examined oral creatine supplementation in rats using a rodent model of Huntington’s Disease. Specifically, these authors found that 0.25 to 1% creatine administered orally to rats for 2 weeks provided significant protection against brain lesions caused by the neurotoxin MPTP, but rats fed 2% and 3% creatine diets were not protected against this brain damage and did not differ from controls.
On this basis, it is possible that hippocampal cells compensated for excess creatine by reducing the synthesis of ATP to maintain energy homeostasis (Allen, 2012). Briefly, a phosphate group can be donated from ATP to form ADP plus phosphocreatine (the stored form of creatine) following a simple one-step enzymatic process governed by creatine kinase. This system is summarized in the following equation: creatine + ATP ⇔ phosphocreatine + ADP (Wyss and Kaddurah-Daouk, 2000). Oversaturation of tissue with creatine (in otherwise healthy, non-exercising animals) may have disrupted energetic balance within the creatine-phosphocreatine system by reducing ATP availability and consequently downregulating neuroplasticity-related gene expression (Allen et al., 2010; Iosifescu et al., 2008; Lyoo et al., 2003). The present study did not assay metabolic markers aside from creatine, but previous human neuroimaging findings support this possibility. Creatine supplementation given to healthy young adults (2–4 weeks, 2–20 g/d) reduced brain levels of N-acetylcholine (NAA), choline, and ATP, which are markers of neuronal integrity and energy homeostasis, as determined by phosphorus magnetic resonance imaging (pMRS; Dechent et al., 1999, Lyoo et al., 2003). In contrast to healthy subjects, a recent double-blind, placebo-controlled trial found that 30g of creatine monohydrate daily (15 g, 2x/d, up to 18 months) slowed cortical and striatal neurodegeneration in patients with in Huntington’s Disease (HD) compared to non-HD controls (Rosas et al., 2014). Taken together, these studies support the idea that creatine is beneficial in metabolically challenged or distressed brain tissue but could impair energy metabolism when creatine monohydrate is taken chronically and in excess doses by otherwise healthy individuals.
Altogether, our findings are partially consistent with our hypothesis that sex hormones are necessary to observe the antidepressant properties of creatine. Creatine had no positive effects on gene expression or behavior in GDX and OVX rats without hormone replacement, indicating that sex hormones are necessary to observe the antidepressant properties of creatine. However, sex hormone replacement without dietary creatine did not normalize reduced gene expression in GDX and OVX rats, indicating that creatine and sex hormone replacement acted synergistically. Moreover, sham rats demonstrated that naturally occurring sex hormones were not sufficient to produce a positive effect of creatine on gene expression or behavior at a high dose following a chronic time course (5–7 weeks before behavior testing and euthanasia), though this may be related to the fact that they were not metabolically impaired. These data suggest sex steroids directly or indirectly interact with creatine metabolism, or vice versa, to buffer energy consumption. It is plausible that stimulation of mitochondrial and creatine kinase activity with sex or stress steroids may have promoted consumption of stored creatine (phosphocreatine) in gonadectomized rats. However, these findings are contrary to the prediction that creatine would only serve to benefit females only (Allen et al., 2010, 2012). The finding of a positive effect of combined creatine and T should encourage further rodent and clinical research to evaluate the potential benefits of creatine in men, such as those with symptomatic testosterone deficiency (hypogonadism), a condition in which depression and metabolic syndrome are frequently observed (Bhattacharya et al., 2011; Miner et al., 2013).
Conclusions
Biological sex, sex hormones, and metabolic status are factors that appear to mediate the behavioral and neurochemical effects of long-term creatine supplementation. In the present work, combined creatine and sex hormone treatment administered chronically had a normalizing effect on hippocampal neuroplasticity-related gene expression in gonadectomized male and female rats (with the most robust effects in males) as well as an antidepressant-like effect in females (with similar trends in males), highlighting a novel avenue for therapeutic drug development. In contrast, creatine supplementation produced negative effects on these same plasticity-related factors in otherwise healthy rats, raising concern over the clinical implications of this supplement given its widespread use by healthy individuals. Taken together, it may be that chronic creatine supplementation is most beneficial in individuals that exhibit significant metabolic impairments or elevated ATP demand, which is based on the fact that significant metabolic impairments are observed in OVX and GDX rats. Future studies are needed to address important questions regarding potential interaction effects of creatine with variables such as sex, stress, exercise, drugs of abuse (e.g., anabolic steroids), prescription medications and nutritional supplements that are often used in conjunction with creatine supplementation in healthy and ill populations. This work highlights how there is a clear need for more basic neuroscience studies to characterize creatine-induced changes in neurobiology and behavior using specific doses and time courses, as well as to identify hormonal and metabolic factors that alter the safety and usefulness of creatine.
Highlights.
Sex differences in creatine’s antidepressant properties were reported previously.
We studied the neural and behavioral effects of creatine ± sex hormones in rats.
Creatine reduced expression of hippocampal plasticity-related genes in sham rats.
Creatine + sex hormones partly normalized gene expression in gonadectomized rats.
Creatine interacts with sex steroids and its effects may depend on metabolic need.
Acknowledgments
Financial support was provided by award number M280251 from the Tufts Center for Neuroscience Research, award number F31AT006292 from the National Center of Complementary and Alternative Medicine (NCCAM), and award number T32MH016259 from the Stuart T. Hauser, M.D., Ph.D. Harvard Clinical Research Training program in Biological and Social Psychiatry and the National Institute of Mental Health (NIMH).
Footnotes
Disclosure/Conflict of Interest
PJ Allen, JF DeBold, RB Kanarek, and M Rios have no financial or competing interests to declare.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NCCAM, NIMH, or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Patricia J. Allen, Email: pallen1@bidmc.harvard.edu.
Joseph F. DeBold, Email: joe.debold@tufts.edu.
Maribel Rios, Email: maribel.rios@tufts.edu.
Robin B. Kanarek, Email: robin.kanarek@tufts.edu.
References
- Allen PJ. Creatine metabolism and psychiatric disorders: Does creatine supplementation have therapeutic value? Neurosci Biobehav Rev. 2012;36:1442–62. doi: 10.1016/j.neubiorev.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PJ, D’Anci KE, Kanarek RB, Renshaw PF. Chronic Creatine Supplementation Alters Depression-like Behavior in Rodents in a Sex-Dependent Manner. Neuropsychopharmacology. 2009;35:534–46. doi: 10.1038/npp.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PJ, D’Anci KE, Kanarek RB, Renshaw PF. Sex-specific antidepressant effects of dietary creatine with and without sub-acute fluoxetine in rats. Pharmacology Biochemistry and Behavior. 2012;101:588–601. doi: 10.1016/j.pbb.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre C, Jayaraman A, Pike C, Baudry M. Progesterone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-β. J Neurochem. 2010;115:1277–1287. doi: 10.1111/j.1471-4159.2010.07038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres R, Ducray A, Schlattner U, Wallimann T, Widmer H. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76:329–43. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Baudry M, Bi X, Aguirre C. Progesterone–estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Beckers J, Schneider I, Hölter SM, Haack T, Ruthsatz T, et al. Creatine improves health and survival of mice. Neurobiol Aging. 2008;29:1404–11. doi: 10.1016/j.neurobiolaging.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Bhattacharya RK, Khera M, Blick G, Kushner H, Nguyen D, Miner MM. Effect of 12 months of testosterone replacement therapy on metabolic syndrome components in hypogonadal men: data from the Testim Registry in the US (TRiUS) BMC Endocrine Disorders. 2011;11:18. doi: 10.1186/1472-6823-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braissant O, Henry H. AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: A review. J Inherit Metab Dis. 2008 doi: 10.1007/s10545-008-0826-9. [DOI] [PubMed] [Google Scholar]
- Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, et al. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Molecular and Cellular Neuroscience. 2003;24:603–13. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-Derived Neurotrophic Factor Regulates Hedonic Feeding by Acting on the Mesolimbic Dopamine System. J Neurosci. 2010;30:2533–41. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182:335–44. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Cunha MP, Machado DG, Capra JC, Jacinto J, Bettio LE, Rodrigues ALS. Antidepressant-like effect of creatine in mice involves dopaminergic activation. J Psychopharmacol (Oxf) 2012;26:1489–501. doi: 10.1177/0269881112447989. [DOI] [PubMed] [Google Scholar]
- Cunha MP, Pazini FL, Oliveira A, Bettio LE, Rosa JM, Machado DG, et al. The activation of alpha1-adrenoceptors is implicated in the antidepressant-like effect of creatine in the tail suspension test. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:39–50. doi: 10.1016/j.pnpbp.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Cunha MP, Pazini FL, Oliveira Á, Machado DG, Rodrigues ALS. Evidence for the involvement of 5-HT1A receptor in the acute antidepressant-like effect of creatine in mice. Brain Res Bull. 2013 doi: 10.1016/j.brainresbull.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Damassa DA, Smith ER, Tennent B, Davidson JM. The relationship between circulating testosterone levels and male sexual behavior in rats. Horm Behav. 1977;8:275–86. doi: 10.1016/0018-506x(77)90002-2. [DOI] [PubMed] [Google Scholar]
- Dechent P, Pouwels PJ, Wilken B, Hanefeld F, Frahm J. Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. Am J Physiol. 1999;277:R698–704. doi: 10.1152/ajpregu.1999.277.3.R698. [DOI] [PubMed] [Google Scholar]
- des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–12. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Dijk F, van Leeuwen S, Kamphuis W. Differential effects of ischemia/reperfusion on amacrine cell subtype-specific transcript levels in the rat retina. Brain Res. 2004;1026:194–204. doi: 10.1016/j.brainres.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Frey BN, Walss-Bass C, Stanley JA, Nery FG, Matsuo K, Nicoletti MA, et al. Brain-derived neurotrophic factor val66met polymorphism affects prefrontal energy metabolism in bipolar disorder. Neuroreport. 2007;18:1567–70. doi: 10.1097/WNR.0b013e3282ef7082. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bock BC, Kanarek RB. Hormonal milieu affects tailflick latency in female rats and may be attenuated by access to sucrose. Physiol Behav. 1992;52:699–706. doi: 10.1016/0031-9384(92)90400-v. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–15. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–26. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Schubert F, Brühl R, Hellweg R, Klär AA, Kehrer C, et al. Met carriers of BDNF Val66Met genotype show increased N-acetylaspartate concentration in the anterior cingulate cortex. Neuroimage. 2010;49:767–71. doi: 10.1016/j.neuroimage.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Huang Y, Shi X, Xu H, Yang H, Chen T, Chen S, et al. Chronic Unpredictable Stress Before Pregnancy Reduce the Expression of Brain-Derived Neurotrophic Factor and N-Methyl-D-Aspartate Receptor in Hippocampus of Offspring Rats Associated with Impairment of Memory. Neurochem Res. 2010;35:1038–49. doi: 10.1007/s11064-010-0152-0. [DOI] [PubMed] [Google Scholar]
- in ‘t Zandt HJ, Renema WK, Streijger F, Jost C, Klomp DW, Oerlemans F, et al. Cerebral creatine kinase deficiency influences metabolite levels and morphology in the mouse brain: a quantitative in vivo 1H and 31P magnetic resonance study. J Neurochem. 2004;90:1321–30. doi: 10.1111/j.1471-4159.2004.02599.x. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, Bolo NR, Nierenberg AA, Jensen JE, Fava M, Renshaw PF. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry. 2008;63:1127–34. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Ipsiroglu OS, Stromberger C, Ilas J, Hoger H, Muhl A, Stöckler-Ipsiroglu S. Changes of tissue creatine concentrations upon oral supplementation of creatine-monohydrate in various animal species. Life Sci. 2001;69:1805–15. doi: 10.1016/s0024-3205(01)01268-1. [DOI] [PubMed] [Google Scholar]
- Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. Progesterone and Estrogen Regulate Oxidative Metabolism in Brain Mitochondria. Endocrinology. 2008;149:3167–75. doi: 10.1210/en.2007-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost CR, Van Der Zee CE, In ‘t Zandt HJ, Oerlemans F, Verheij M, Streijger F, et al. Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur J Neurosci. 2002;15:1692–706. doi: 10.1046/j.1460-9568.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- Kádár E, Lim LW, Carreras G, Genís D, Temel Y, Huguet G. High-frequency stimulation of the ventrolateral thalamus regulates gene expression in hippocampus, motor cortex and caudate–putamen. Brain Res. 2011;1391:1–13. doi: 10.1016/j.brainres.2011.03.059. [DOI] [PubMed] [Google Scholar]
- Kemper MF, Zhao Y, Duckles SP, Krause DN. Endogenous ovarian hormones affect mitochondrial efficiency in cerebral endothelium via distinct regulation of PGC-1 isoforms. J Cereb Blood Flow Metab. 2013;33:122–8. doi: 10.1038/jcbfm.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig H, Goldstone A, Blume G, Lu CY. Testosterone-mediated sexual dimorphism of mitochondria and lysosomes in mouse kidney proximal tubules. Science. 1980;209:1023–6. doi: 10.1126/science.7403864. [DOI] [PubMed] [Google Scholar]
- Koenig H, Goldstone A, Lu CY. Androgens regulate mitochondrial cytochrome c oxidase and lysosomal hydrolases in mouse skeletal muscle. Biochem J. 1980;192:349–53. doi: 10.1042/bj1920349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig H, Goldstone A, Lu CY. Testosterone-mediated sexual dimorphism of the rodent heart. Ventricular lysosomes, mitochondria, and cell growth are modulated by androgens. Circ Res. 1982;50:782–7. doi: 10.1161/01.res.50.6.782. [DOI] [PubMed] [Google Scholar]
- Kondo DG, Sung Y-H, Hellem TL, Fiedler KK, Shi X, Jeong E-K, et al. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorus magnetic resonance spectroscopy study. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Jiang B, Li Y, Xia F, Yu J, Yang Lz, et al. Mitochondrial Apoptotic Pathways: A Mechanism for Low Androgen-induced Vascular Endothelial Injury in Male Rats. Horm Metab Res. 2011;43:374–9. doi: 10.1055/s-0031-1271745. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kong SW, Sung SM, Hirashima F, Parow A, Hennen J, et al. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003;123:87–100. doi: 10.1016/s0925-4927(03)00046-5. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Yoon S, Kim TS, Hwang J, Kim JE, Won W, et al. A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. Am J Psychiatry. 2012;169:937–45. doi: 10.1176/appi.ajp.2012.12010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnick SD, Shaer A, Soreq H, Kaye AM. Estrogen-induced creatine kinase in the reproductive system of the immature female rat. Endocrinology. 1983;113:1907–9. doi: 10.1210/endo-113-5-1907. [DOI] [PubMed] [Google Scholar]
- McMorris T, Harris R, Howard A, Langridge G, Hall B, Corbett J, et al. Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol Behav. 2007;90:21–8. doi: 10.1016/j.physbeh.2006.08.024. [DOI] [PubMed] [Google Scholar]
- McMorris T, Harris RC, Swain J, Corbett J, Collard K, Dyson RJ, et al. Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology (Berl) 2006;185:93–103. doi: 10.1007/s00213-005-0269-z. [DOI] [PubMed] [Google Scholar]
- McMorris T, Mielcarz G, Harris RC, Swain JP, Howard A. Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:517–28. doi: 10.1080/13825580600788100. [DOI] [PubMed] [Google Scholar]
- Metzl JD, Small E, Levine SR, Gershel JC. Creatine Use Among Young Athletes. Pediatrics. 2001;108:421–5. doi: 10.1542/peds.108.2.421. [DOI] [PubMed] [Google Scholar]
- Miner M, Bhattacharya R, Blick G, Kushner H, Khera M. 12-Month Observation of Testosterone Replacement Effectiveness in a General Population of Men. Postgrad Med. 2013;125:8–18. doi: 10.3810/pgm.2013.03.2637. [DOI] [PubMed] [Google Scholar]
- Öner J, Öner H, Sahin Z, Demir R, Üstunel Melatonin is as Effective as Testosterone in the Prevention of Soleus Muscle Atrophy Induced by Castration in Rats. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2008;291:448–55. doi: 10.1002/ar.20659. [DOI] [PubMed] [Google Scholar]
- Petralia SM, DeBold JF, Frye CA. MK-801 infusions to the ventral tegmental area and ventromedial hypothalamus produce opposite effects on lordosis of hormone-primed rats. Pharmacol Biochem Behav. 2007;86:377–85. doi: 10.1016/j.pbb.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2001;Chapter 8(Unit 8 10A) doi: 10.1002/0471142301.ns0810as14. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Sullivan PG, Fugaccia I, Scheff SW. Creatine diet supplement for spinal cord injury: influences on functional recovery and tissue sparing in rats. J Neurotrauma. 2003;20:659–69. doi: 10.1089/089771503322144572. [DOI] [PubMed] [Google Scholar]
- Ramamani A, Aruldhas MM, Govindarajulu P. Impact of testosterone and oestradiol on region specificity of skeletal muscle-ATP, creatine phosphokinase and myokinase in male and female Wistar rats. Acta Physiol Scand. 1999;166:91–7. doi: 10.1046/j.1365-201x.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Doros G, Gevorkian S, Malarick K, Reuter M, Coutu JP, et al. PRECREST: a phase II prevention and biomarker trial of creatine in at-risk Huntington disease. Neurology. 2014;82:850–7. doi: 10.1212/WNL.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakellaris G, Kotsiou M, Tamiolaki M, Kalostos G, Tsapaki E, Spanaki M, et al. Prevention of Complications Related to Traumatic Brain Injury in Children and Adolescents With Creatine Administration: An Open Label Randomized Pilot Study. The Journal of Trauma: Injury, Infection, and Critical Care. 2006;61:322–9. doi: 10.1097/01.ta.0000230269.46108.d5. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Dhillon HS. Creatine-enhanced diet alters levels of lactate and free fatty acids after experimental brain injury. Neurochem Res. 2004;29:469–79. doi: 10.1023/b:nere.0000013753.22615.59. [DOI] [PubMed] [Google Scholar]
- Shi C, Xu J. Increased vulnerability of brain to estrogen withdrawal-induced mitochondrial dysfunction with aging. J Bioenerg Biomembr. 2008;40:625–30. doi: 10.1007/s10863-008-9195-1. [DOI] [PubMed] [Google Scholar]
- Smith ER, Damassa DA, Davidson JM. Hormone administration: Peripheral and intracranial implants. New York: Academic Press; 1977. [Google Scholar]
- Sömjen D, Katzburg S, Sharon O, Knoll E, Hendel D, Stern N. Sex specific response of cultured human bone cells to ERα and ERβ specific agonists by modulation of cell proliferation and creatine kinase specific activity. The Journal of Steroid Biochemistry and Molecular Biology. 2011;125:226–30. doi: 10.1016/j.jsbmb.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Sömjen D, Lundgren S, Kaye AM. Sex and depot-specific stimulation of creatine kinase B in rat adipose tissues by gonadal steroids. J Steroid Biochem Mol Biol. 1997;62:89–96. doi: 10.1016/s0960-0760(97)00011-3. [DOI] [PubMed] [Google Scholar]
- Sömjen D, Weisman Y, Harell A, Berger E, Kaye AM. Direct and sex-specific stimulation by sex steroids of creatine kinase activity and DNA synthesis in rat bone. Proc Natl Acad Sci U S A. 1989;86:3361–5. doi: 10.1073/pnas.86.9.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sömjen DWY, Mor Z, Harell A, Kaye AM. Regulation of proliferation of rat cartilage and bone by sex steroid hormones. J Steroid Biochem Mol Biol. 1991;40:717–23. doi: 10.1016/0960-0760(91)90296-h. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Galea LAM. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Ibler E, Inglis W, Curtis MG. Testosterone and social isolation influence adult neurogenesis in the dentate gyrus of male rats. Neuroscience. 2011;195:180–190. doi: 10.1016/j.neuroscience.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A, Savostyanova A, Goldman A, Barnett A, Vanderveen J, Callicott J, et al. Impact of the Brain-Derived Neurotrophic Factor Val66Met Polymorphism on Levels of Hippocampal N-Acetyl-Aspartate Assessed by Magnetic Resonance Spectroscopic Imaging at 3 Tesla. Biol Psychiatry. 2008;64:856–62. doi: 10.1016/j.biopsych.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streijger F, Jost CR, Oerlemans F, Ellenbroek BA, Cools AR, Wieringa B, et al. Mice lacking the UbCKmit isoform of creatine kinase reveal slower spatial learning acquisition, diminished exploration and habituation, and reduced acoustic startle reflex responses. Mol Cell Biochem. 2004;256–257:305–18. doi: 10.1023/b:mcbi.0000009877.90129.e3. [DOI] [PubMed] [Google Scholar]
- Streijger F, Oerlemans F, Ellenbroek B, Jost C, Wieringa B, Vanderzee C. Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit. Behav Brain Res. 2005;157:219–34. doi: 10.1016/j.bbr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Ann Neurol. 2000;48:723–9. [PubMed] [Google Scholar]
- Svensson A. Testosterone treatment induces behavioral disinhibition in adult male rats. Pharmacology Biochemistry and Behavior. 2003;75:481–90. doi: 10.1016/s0091-3057(03)00137-0. [DOI] [PubMed] [Google Scholar]
- Todkar K, Scotti AL, Schwaller B. Absence of the calcium-binding protein calretinin, not of calbindin D-28k, causes a permanent impairment of murine adult hippocampal neurogenesis. Front Mol Neurosci. 2012:5. doi: 10.3389/fnmol.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A Review and Update of Mechanisms of Estrogen in the Hippocampus and Amygdala for Anxiety and Depression Behavior. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281 (Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Kato N, Kato T. Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci Res. 2002;42:279–85. doi: 10.1016/s0168-0102(02)00007-x. [DOI] [PubMed] [Google Scholar]
- Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Xi D, Keeler B, Zhang W, Houle JD, Gao W-J. NMDA receptor subunit expression in GABAergic interneurons in the prefrontal cortex: Application of laser microdissection technique. J Neurosci Methods. 2009;176:172–81. doi: 10.1016/j.jneumeth.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainuddin MSA, Thuret S. Nutrition, adult hippocampal neurogenesis and mental health. Br Med Bull. 2012;103:89–114. doi: 10.1093/bmb/lds021. [DOI] [PubMed] [Google Scholar]