Abstract
Development of temozolomide (TMZ) resistance contributes to the poor prognosis for glioblastoma multiforme (GBM) patients. It was previously demonstrated that delivery of exogenous wild-type tumor suppressor gene p53 via a tumor-targeted nanocomplex (SGT-53) which crosses the blood-brain barrier could sensitize highly TMZ-resistant GBM tumors to TMZ. Here we assessed whether SGT-53 could inhibit development of TMZ resistance. SGT-53 significantly chemosensitized TMZ-sensitive human GBM cell lines (U87 and U251), in vitro and in vivo. Furthermore, in an intracranial GBM tumor model, two cycles of concurrent treatment with systemically administered SGT-53 and TMZ inhibited tumor growth, increased apoptosis and most importantly, significantly prolonged median survival. In contrast TMZ alone had no significant effect on median survival compared to a single cycle of TMZ. These results suggest that combining SGT-53 with TMZ appears to limit development of TMZ resistance, prolonging its anti-tumor effect and could be a more effective therapy for GBM.
Keywords: p53, chemosensitization, systemic nanodelivery, temozolomide, glioblastoma multiforme, limiting TMZ-resistance
Background
Glioblastoma multiforme (GBM) is the most aggressive and lethal brain tumor in adults.1 Despite intensive multimodal treatments comprising surgical resection and chemoradiation therapy, the current prognosis of GBM patients remains poor in terms of median survival (14.6 months) and survival rate (below 4% for 5 year survival).2,3 This poor prognosis is mainly because of the highly aggressive and infiltrative nature of GBM tumors, as well as frequently observed therapeutic resistance, both inherent and acquired, which eventually result in tumor recurrence.3 Treatment with first-line alkylating agent temozolomide (TMZ) concurrent with ionizing radiation has demonstrated only a limited efficacy in newly diagnosed GBM patients with an extension of median survival by 2.5 months compared to radiation therapy alone.4 Moreover, even in patients with strong initial responses, the majority later develop TMZ-resistant recurrences.5
In GBM tumors, resistance to TMZ is predominantly linked to an elevated expression of O6-methylguanine-DNA methyltransferase (MGMT), an enzyme that repairs TMZ-induced DNA damage.6-8 There have been efforts to overcome TMZ resistance by depleting MGMT using O6-benzylguanine, a pseudo-substrate of MGMT9,10 or inhibiting MGMT using interferon (IFN)-β,11-13 a STAT3 inhibitor,14 levetiracetam15 or the wtp53 tumor suppressor gene.16,17
Mutations in p53 are found frequently in GBM tumors (~30% and ~65% of primary and secondary GBM, respectively)18 and the p53 pathway is functionally inactivated in almost all GBM.19 Thus, p53 gene therapy has potential for use as an adjunct to conventional cancer treatments.20
We have developed a tumor-targeting nanocomplex platform technology (designated as “scL”) for delivery of molecular medicines - including plasmid DNA,21-26 small interfering RNA,27,28 and small molecules29 - selectively to both bulk tumor cells and cancer stem cells (CSCs) of primary and metastatic tumors.30 In the prototype of this platform nanodelivery system, scL-p53, the wtp53 plasmid DNA payload is encapsulated within scL - a cationic liposome, the surface of which is decorated with an anti-transferrin receptor (TfR) single-chain antibody fragment (scFv) as the tumor-targeting moiety (henceforth referred to as “SGT-53”). SGT-53 is designed to target tumor cells via the TfR, which is highly expressed on surface of many tumor cells. The nanocomplex is efficiently internalized via receptor-mediated endocytosis.30 Importantly, since high levels of the TfR are also present on the cerebral endothelium of the blood-brain barrier (BBB),31 this nanodelivery system can cross the BBB via TfR-mediated transcytosis.17 We have shown that systemic administration of the SGT-53 nanocomplex enhances the efficacy of chemo- and radio-therapy in various pre-clinical models of human tumors.17,21,32,33 Moreover, SGT-53 is in human clinical trials where it has been shown to be well tolerated at the therapeutic doses tested and has demonstrated anti-cancer effects.34
In addition, we have previously demonstrated that abrogation of MGMT activity mediated by SGT-53 was able to reverse TMZ resistance and enhance the anti-cancer efficacy of TMZ in MGMT-proficient and inherently TMZ-resistant GBM cells both in vitro and in vivo.17
In this study, we have explored whether this wtp53-mediated effect could be further extended to TMZ-sensitive GBM cells and more, importantly, if treatment with SGT-53 could abrogate or delay the development of TMZ resistance, thus extending the efficacy of TMZ treatment.
Methods
Cell lines
Human GBM cell lines used were U87, U87-luc2, and U251. U87R, a TMZ-resistant subclone of U87, was established by treating TMZ-sensitive U87 cells with 100 μM TMZ in culture media for 3 weeks as previously described.14
Preparation of SGT-53 nanocomplex
TfRscFv/Liposome/p53 (scL-p53 or SGT-53) complex was prepared as previously described.23 The mean particle size of SGT-53 in water was 114.4 ± 8.4 nm, and the mean zeta potential 28.2 ± 1.2 mV.
XTT assay
The XTT assay was performed as previously described.17 The IC50 values, the drug concentration resulting in 50% cell death, was interpolated from the graph of the log of TMZ concentration versus the fraction of surviving cells using SigmaPlot (Systat Softwate Inc, San Jose, CA).
In vitro sensitization study
TMZ sensitization was evaluated by assessing the level of apoptosis in cancer cells as determined by the percent of cells in the sub-G1 population of the cell cycle.
Animal model
All animal experiments were performed in accordance with and under approved Georgetown University GUACUC protocols. For the orthotopic intracranial GBM tumor model, 5-6 week old female athymic nude mice (Harlan Sprague-Dawley, Indianapolis, IN) were stereotactically inoculated with U87-luc2 cells (5.0×105 cells per mouse) as described previously.17
In vivo animal survival studies
Mice with established intracranial U87-luc2 tumor xenografts were systemically injected (i.v. tail vein) with either SGT-53 (30 μg DNA/injection/mouse), with 7.5-75 mg/m2 TMZ, or the combination of both, following the indicated treatment schedules.
Bioluminescence imaging
To measure intracranial U87-luc2 tumor growth and to assess therapeutic efficacy, non-invasive bioluminescence imaging was performed with the Xenogen IVIS® in vivo imaging system (Caliper Life Sciences, Hopkinton, MA).
Magnetic resonance imaging
To visualize and measure the intracranial tumors, animals were MR imaged and tumor volumes determined as described previously.17
In vivo efficacy study
The in vivo response to the combination of SGT-53 and TMZ was assessed by determining the level of apoptosis in intracranial tumors. Fractionated cells were subjected to Annexin V, cell cycle, and cleaved Caspase-3 (cCAS3) assays as described previously.17
Western blot analysis
Western blot analysis was used to determine protein expression in SGT-53 treated and untreated U87R cells. ImageJ software (http://rsb.info.nih.gov/ij/) was used for quantification of the protein bands.
Statistical analysis
The statistical significance was determined by one-way analysis of variance (ANOVA). P values of <0.05 were considered significant. All graphs and statistical analysis were prepared using SigmaPlot.
Results
In vitro sensitization of GBM cells to TMZ by the SGT-53 nanocomplex
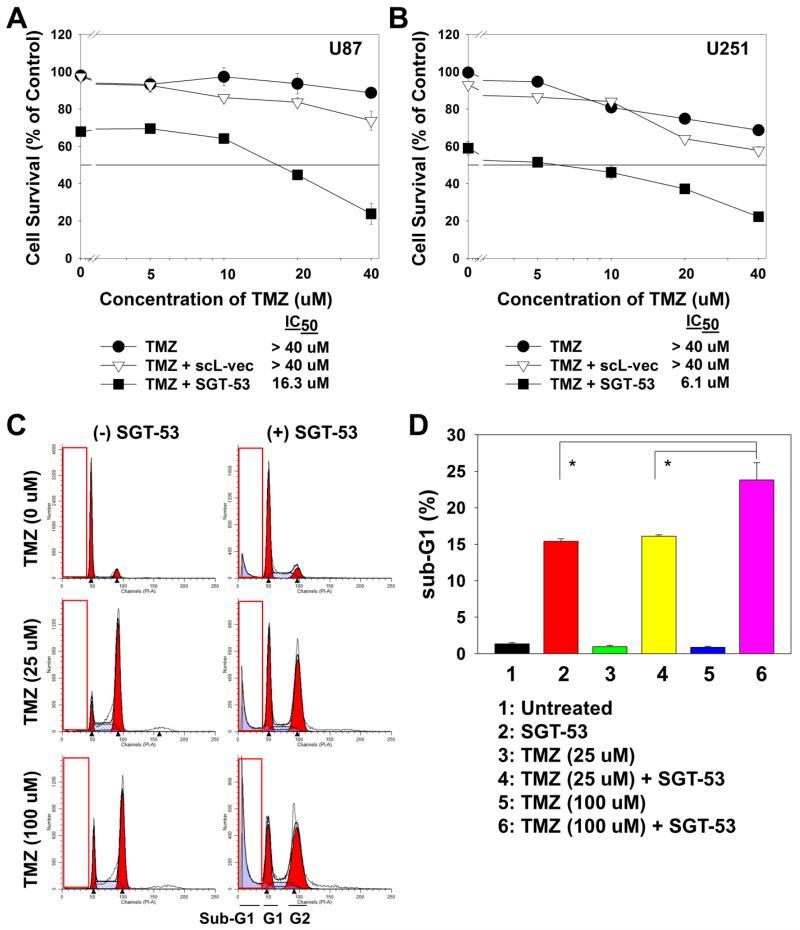
To determine the effect of SGT-53 on the response to TMZ in vitro, two different TMZ-responsive human GBM cell lines (U87 and U251) were tested. Initially we assessed the ability of SGT-53 to sensitize GBM cells to TMZ using XTT assay. U87 and U251 cells were transfected with SGT-53 (100 ng DNA/well, which is equivalent to 50 pg DNA/cell) for 24 h, and then treated with increasing concentrations of TMZ (0 to 40 μM) for an additional 72 h. Cells transfected with TMZ, either alone or in combination with scL-vec, the nanocomplex carrying the empty plasmid vector, were used as controls (Figure 1, A and B). In both cell lines, transfection with SGT-53 resulted in significantly increased response to TMZ compared to TMZ treatment alone or TMZ in combination with scL-vec. In the U87 cells (Figure 1, A), transfection with SGT-53 decreased the IC50 from >40 μM to 16.3 μM, at least a 2.5-fold increase in sensitivity to TMZ. In U251, the effect of SGT-53 on response to TMZ was even greater. Here the IC50 decreased from >40 μM to 6.1 μM, a close to 7-fold increase in sensitization (Figure 1, B). In contrast, transfection with the control scL-vec nanocomplex did not result in any increase in response to TMZ. In both cell lines the survival curves after treatment with scL-vec are comparable to those after treatment with TMZ alone, demonstrating that the response in these cell lines is likely due to the presence of exogenous wtp53 and not a result of non-specific cytotoxicity of the delivery system.
Figure 1.
SGT-53 mediated in vitro sensitization of GBM cells to TMZ. Human GBM cell lines, U87 (A) and U251 (B) were transfected with SGT-53 for 24 h, and then treated with increasing concentrations of TMZ for an additional 72 h. Cells were also treated with TMZ alone or in combination with scL-vec, the nanocomplex carrying the empty plasmid vector. Cell viability was measured by XTT assay. (C) Cell cycle profiles of U87 cells 72 h post-treatment with TMZ, either alone or in combination with SGT-53. The inset boxes indicate sub-G1 population. (D) Quantification of the percent of cells in the sub-G1 population in C. *p < 0.05.
To further study the SGT-53 mediated potentiation of TMZ, U87 cells were treated with TMZ at concentrations of either 25 or 100 μM, alone or in combination with SGT-53 transfection for 24 h, and assessed for apoptosis by measuring the percent of cells in sub-G1 populations. As expected, 72 h after TMZ treatment an increase in G2 population was observed in all TMZ-treated groups (Figure 1, C). More importantly, there was a significant increase in the sub-G1 population, an indicator of apoptosis, observed in the cells treated with the combination of SGT-53 and TMZ. These results demonstrate that addition of SGT-53 resulted in an SGT-53-mediated TMZ increase in apoptotic response in a dose-dependent manner (Figure 1, D). The combination of SGT-53 and 100 μM TMZ treatment significantly increased the sub-G1 population compared to either TMZ or SGT-53 treatment alone.
Tumor growth inhibition by the combination of SGT-53 and TMZ
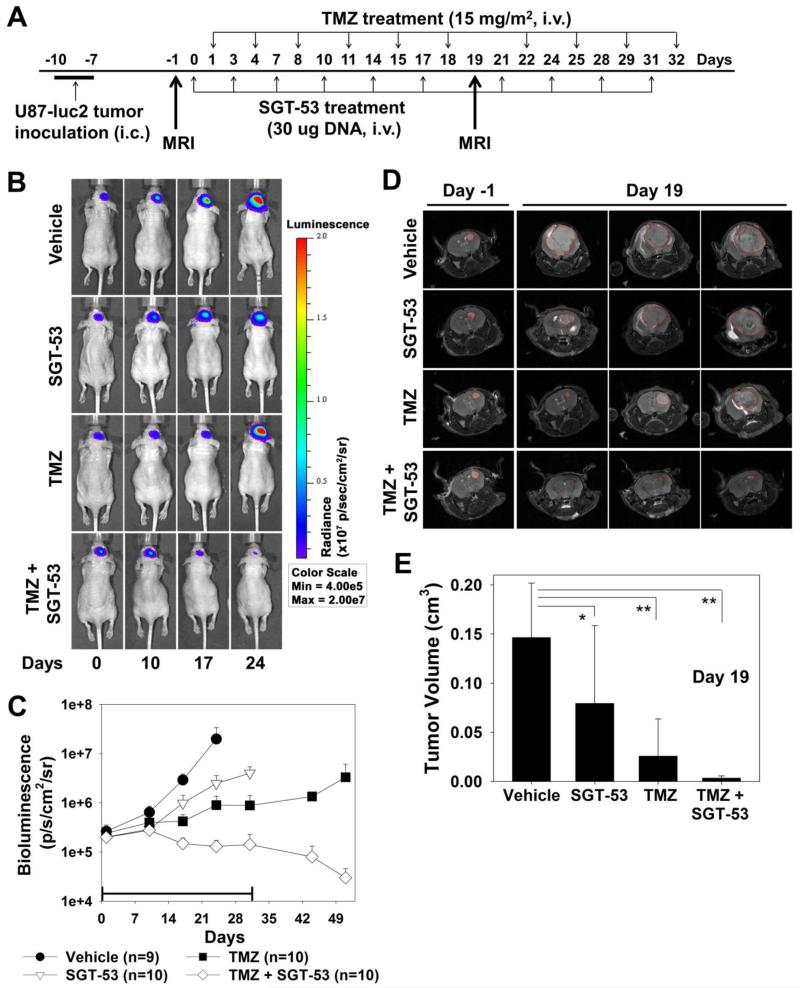
When systemically administered, the tumor-targeting scL nanodelivery system has been shown to specifically target, and efficiently deliver the payload (e.g. wtp53), to tumors wherever they occur in the body, including the brain. The tumor-targeted sensitization of intracranial U87-luc2 tumors to TMZ by systemically administred SGT-53 was examined in vivo. Mice with established intracranial U87-luc2 xenograft tumors were randomized and grouped (7-8 mice/group) for treatment with TMZ (15 mg/m2/injection), either alone or in combination with i.v. (tail vein) administered SGT-53 (30 μg DNA/mouse/injection) according to the treatment schedule shown in Figure 2, A. The vehicle control group was injected with liposome only. Tumor response was assessed by monitoring the bioluminescence signal which correlates to tumor size (Figure 2, B). The intensity of the bioluminescence signal was plotted. As shown in Figure 2, C, while TMZ alone and SGT-53 alone had some minimal inhibitory effect on intracranial tumor growth, the tumors in both groups rapidly increased in size during and after treatment. In contrast, the tumors in the group of mice that received the combination of SGT-53 and TMZ displayed not only inhibition of tumor growth, but also tumor regression during treatment. More significantly, this regression continued for approximately 3 weeks after the end of treatment (Figure 2, C). The tumor regression observed by bioluminescence in the combination group during treatment was also evident when animals were imaged by MRI (Figure 2, D). Instead of increasing size, as is the case in the treatment groups that received single agents, residual tumor is barely detectable after 19 days of treatment in the animals that received the combination therapy (Figure 2, D and E). Therefore, this experiment demonstrates that tumor-targeted delivery of wtp53 via the scL nanodelivery system (SGT-53) can further sensitize even TMZ-responsive GBM tumors to TMZ leading to enhanced tumor response (regression), not just tumor growth inhibition.
Figure 2.
Enhanced tumor responses by the combination of SGT-53 plus TMZ in a GBM tumor mouse model. (A) Treatment schedule. (B) Representative bioluminescence images of intracranial U87-luc2 tumors shown over time. The same animal from each of the treatment groups is shown at each time point. Bioluminescence signals, shown in a color map, correlate with tumor sizes. Red color: stronger signal, Violet color: weaker signal. (C) Bioluminescence intensity of tumors in the treatment groups plotted as a function of time. The bar indicates duration of treatment. (D) Representative MR images of brain tumors collected before treatment and on day 19 of treatment. Red lines indicate the outline of the brain tumors. (E) Quantification of tumor volume by MRI. *p < 0.05, **p < 0.001.
Enhanced apoptosis in vivo with the combination of SGT-53 and TMZ
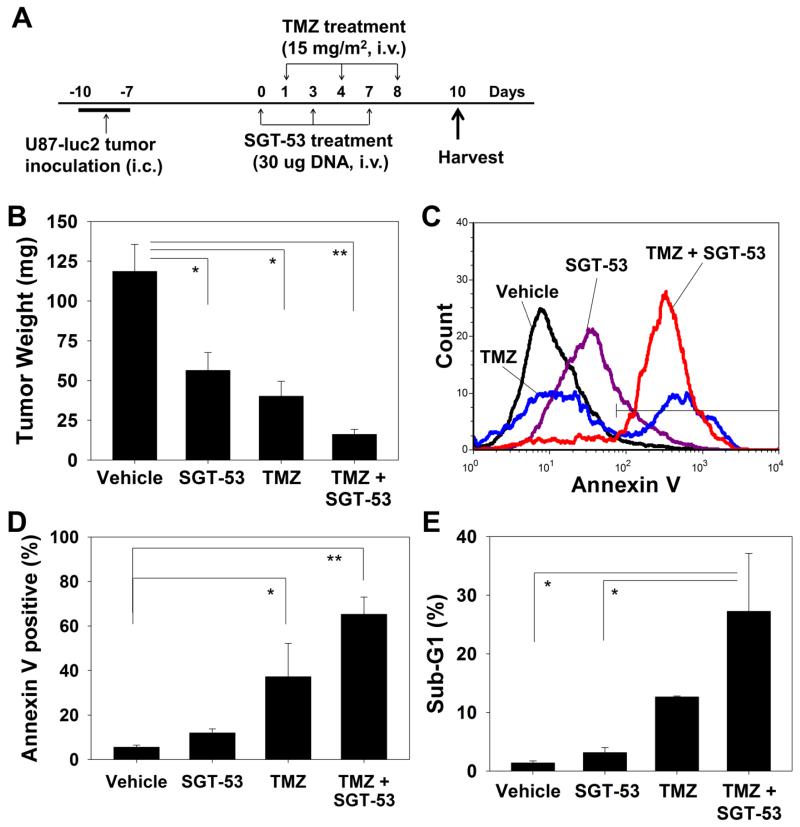
After observing the above tumor growth inhibition and regression, we evaluated the apoptotic response of GBM cells in vivo to the combination of SGT-53 and TMZ treatment with the schedule shown in Figure 3, A. The level of apoptosis induced in intracranial U87-luc2 brain tumors after 3 injections of SGT-53 and/or TMZ was determined by either Annexin V or the cell cycle assay. At harvest, the brain tumors were weighed and the average compared among the treatment groups. The tumors from animals that received both SGT-53 and TMZ showed significantly decreased size (16.2 ± 3.1 mg) compared to those which received vehicle control treatment (118.6 ± 16.9 mg) or single agent treatments (56.4 ± 11.2 and 40.1 ± 9.4 mg for SGT-53 and TMZ, respectively) (Figure 3, B). In addition, as shown in Figure 3, C, analysis of the level of apoptosis using the Annexin V assay showed an increase in the percent of Annexin V+ tumor cells after treatment with SGT-53 and TMZ combination (65.3 ± 7.7%) compared to vehicle-treated control (5.5 ± 1.0%) or either treatment alone (11.9 ± 1.8% and 37.2 ± 15.0% for SGT-53 and TMZ, respectively) (Figure 3, D). Furthermore, we also observed an increase in the percent of cells in sub-G1 confirming increased apoptosis in tumor treated with SGT-53 plus TMZ (27.2 ± 9.9%) compared to those treated with vehicle (1.4 ± 0.3%) or single agent (3.1 ± 0.9% and 12.6 ± 0.2% for SGT-53 and TMZ, respectively) (Figure 3, E). Thus, these results indicate that uptake of systemically administered SGT-53 by these TMZ-responsive intracranial tumors results in an enhanced apoptotic response to TMZ.
Figure 3.
Enhanced apoptosis in a mouse model of GBM tumors by the combination of SGT-53 plus TMZ. (A) Treatment schedule. (B) Quantification of the weight of brain tumors at harvest on day 10. (C) Representative histogram of Annexin V staining. (D) Quantification of Annexin V+ cells to assess apoptosis in tumors. (E) Quantification of the apoptotic response represented by the percent of cells in the sub-G1 population. *p < 0.05, **p < 0.001.
Prolonged survival with the combination treatment of SGT-53 and TMZ
As the above experiments demonstrated significant tumor responses with the combination of SGT-53 and TMZ, we assessed the effect of this combination therapy on animal survival. Mice with established intracranial U87-luc2 orthotopic xenografts were randomized and grouped (5-8 mice per group) for treatment with TMZ, either alone or in combination with i.v. administered SGT-53 (30 μg DNA/injection/mouse). Two doses of TMZ were employed in this study: a low dose of 15 mg/m2 and a high dose of 75 mg/m2 using the same treatment schedule shown in Figure 2, A.
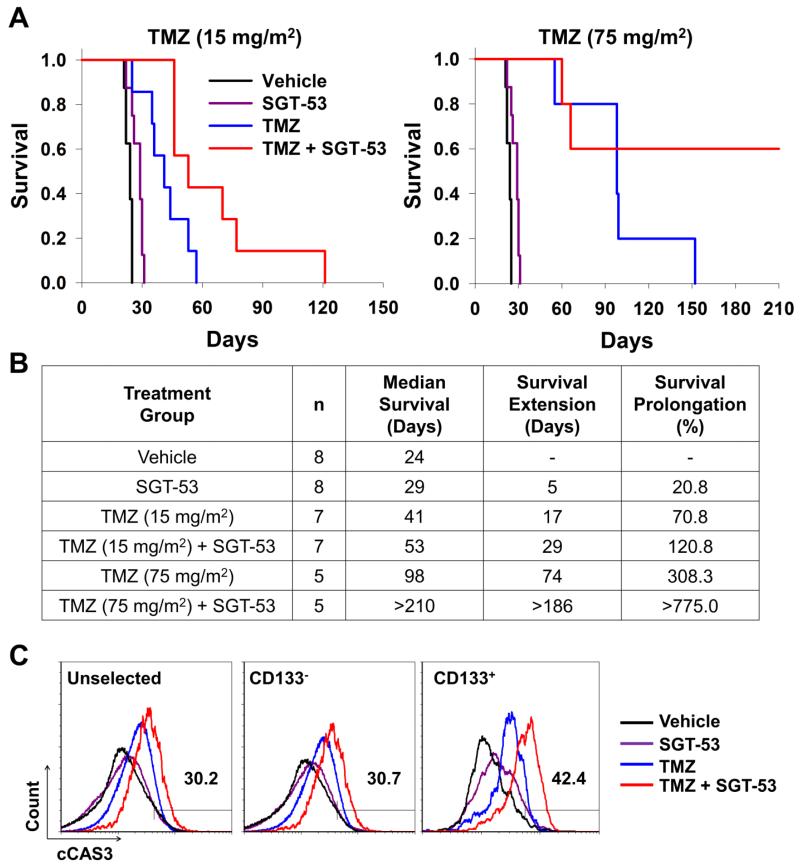
At the 15 mg/m2 TMZ dose, both of the single agent treatments showed some increase in survival compared to the vehicle treated group (Figure 4, A, left panel). The mice treated with SGT-53 alone had a median survival time of 29 days, 5 days (20.8%) longer than the control mice (Figure 4, B). Not unexpectedly for these TMZ-responsive tumors, TMZ treatment alone resulted in 41 days median survival, which is 17 days (70.8%) prolongation in median survival relative to the vehicle-treated control animals. However, both of these groups of animals succumbed to their disease by day 60. In contrast, the combination of TMZ and SGT-53 clearly showed a strong, statistically significant survival benefit with a median survival of 53 days, which is prolongation of 29 days compared to the vehicle-treated controls. This is approximately twice the life-span of the vehicle control animals and a 1.7-fold prolongation relative those treated with TMZ alone.
Figure 4.
Increased survival by combination of SGT-53 plus TMZ in a GBM tumor mouse model. Identical treatment schedule to that shown in Figure 2, A. (A) Kaplan-Meier survival curves of mice treated with either 15 mg/m2 (left panel) or 75 mg/m2 (right panel) of TMZ in combination with SGT-53. (B) Summary of survival analysis. n = number of animals. Survival prolongation was determined as % change in median survival compared to vehicle control group. (C) Quantification of apoptosis in the CD133+ CSCs and CD133− bulk tumor cells by cCAS3 assay. Numbers indicate the percentages of cCAS3 positive cells in tumors from mice treated with 15 mg/m2 TMZ in combination with SGT-53 for 20 days.
At the higher dose of 75 mg/m2, TMZ treatment alone was able to significantly prolong survival (74 days increase in median survival) compared to the vehicle-treated controls (Figure 4, A, right panel). Nevertheless, all of the mice still succumbed to their tumor by approximately 150 days. However, the survival was considerably extended by the addition of SGT-53 to the treatment regimen. In this combination treatment group 60% of the mice were still surviving at day 210, where the experiment was ended, resulting in a median survival of >210 days (Figure 4, B). This was a greater than 7.5-fold increase in survival compared to the vehicle controls and more than twice that of TMZ alone. Thus, the combination of SGT-53 and TMZ resulted in a significant increase in long-term survival in this mouse model.
Induction of apoptosis in CSCs by the combination of SGT-53 and TMZ
We have previously shown that SGT-53 can target and transfect cancer stem cells (CSCs), as well as bulk tumor cells, in inherently TMZ resistant GBM tumor cells resulting in the induction of apoptosis and tumor growth inhibition.17 Thus, it is possible that results observed above in U87 tumors could be due, at least in part, to an effect on CSCs in the tumors. Thus we assessed the effect of SGT-53 on the response to TMZ of CSCs from intracranial U87-luc2 tumor xenografts. We found that SGT-53 treatment also sensitized CD133+ CSCs to TMZ treatment (Figure 4, C). Quantification of apoptosis by cCAS3 assay double labeled with the CSC marker, CD133, demonstrated increased apoptosis in both CD133+ CSCs (42.4%) and CD133− bulk tumor cells (30.7%) when treated with the combination of SGT-53 plus TMZ. In contrast, in the mice treated with SGT-53 alone the percent of apoptotic cells was only 6.7 and 6.0% for CD133+ and CD133−, respectively, while in those treated with TMZ alone it was just 7.7 and 9.8% for CD133+ and CD133− cells, respectively. CSCs have been shown to play a significant role in chemoresistance and tumor recurrence. Therefore, the observed increased response to TMZ in CSCs after systemic treatment with SGT-53 demonstrates that this combination approach may also be able to decrease the potential for development of drug resistance and recurrence in these tumors.
Effect of multiple treatment cycles with the combination of SGT-53 and TMZ
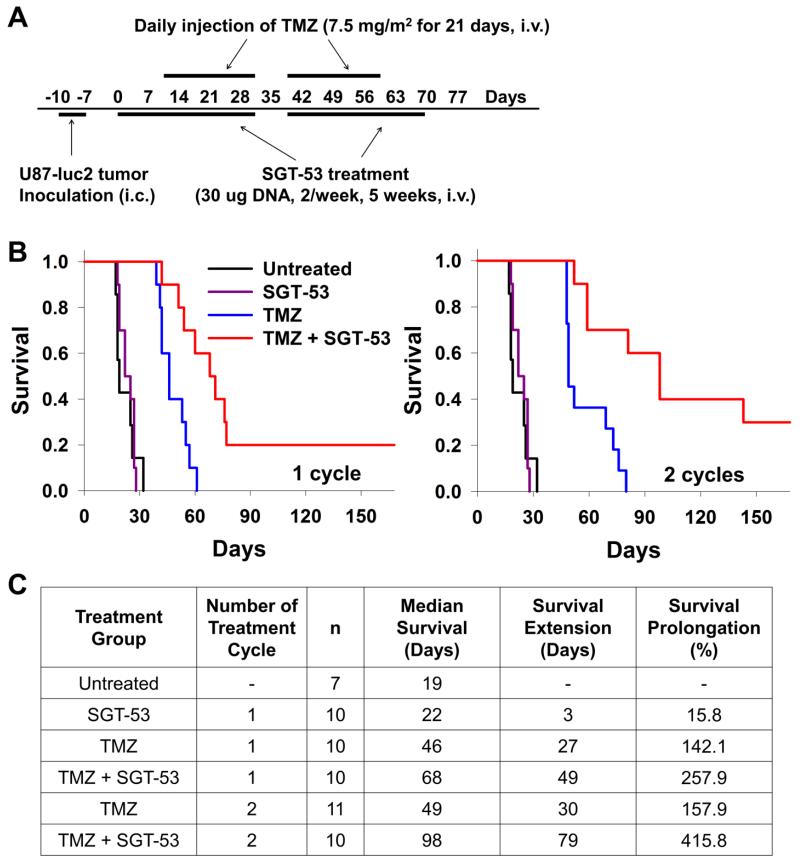
Since the development of TMZ resistance is observed in patients after receiving multiple treatment cycles of TMZ, we evaluated if the combination of SGT-53 and TMZ could overcome the potential development of therapeutic resistance. To assess the effect of repeated treatment cycles of SGT-53 plus TMZ, compared with TMZ alone, mice with established IC U87-luc2 tumors received either one or two cycles of SGT-53 plus TMZ, at a dose of only 7.5 mg/m2, an extremely low dose, as the standard dose for use in patients is 150 mg/m2. This low dose was employed in order to detect synergistic effects of treatment with the combination of SGT-53 and TMZ. The TMZ treatment schedule used was similar to that currently employed in the clinic for human patients. Each cycle of TMZ was administered daily for 21 consecutive days, with one week without treatment between cycles (Figure 5, A). Three doses of SGT-53 were administered prior to the initiation of the first cycle of TMZ. Thereafter, SGT-53 was administered twice weekly throughout TMZ treatment. SGT-53 was not administered during the week between TMZ cycles. As controls, groups of mice also received SGT-53 only, TMZ only, or remained untreated.
Figure 5.
Limiting TMZ resistance by combination of SGT-53 and TMZ. (A) Treatment schedule. (B) Kaplan-Meier survival curves of mice treated for either one cycle (left panel) or two cycles (right panel) of TMZ and SGT-53. The untreated and SGT-53 only treated groups were used as controls. (C) Summary of survival analysis. n = number of animals. Survival prolongation was determined as % change in median survival compared to untreated group.
Although the U87-luc2 tumors responded well to TMZ alone in the first cycle of treatment, with a median survival extension of 27 days compared to the untreated mice, the additional subsequent cycle of TMZ was significantly less effective with the median survival extended for only additional 3 days indicating the possible development of resistance to TMZ (Figure 5, B and C). However, the addition of SGT-53 to the TMZ treatment regimen did provide a significant survival benefit. After completing one cycle of SGT-53 plus TMZ treatment, a median survival extension of 49 days compared to untreated mice was observed. This is a 116% increase in survival compared to TMZ alone treatment (Figure 5, C). In mice that received two cycles of combination treatment, this median survival benefit was further extended by an additional 30 days to 98 days, which is a 258% increase compared to TMZ alone. This increase in survival was at least five times that of the median survival of the untreated mice.
More importantly, the life-span of the animals that received either one or two cycles of the combination treatment was significantly longer than the life-span of mice receiving TMZ alone, with 20% (one cycle) and 40% (two cycles) of the SGT-53 plus TMZ-treated animals surviving beyond 100 days, demonstrating a significant extension of life-span in these intracranial U87-luc2 tumor bearing mice even with this dose of TMZ which is 20-fold less than the standard human dose. It is possible that this increase in life span would be even more pronounced with higher doses of TMZ.
These findings, along with the fact that the SGT-53 alone resulted in no major change in survival (survival extension of 3 days), indicates the synergistic effect of the combination of SGT-53 and TMZ.
Overcoming TMZ resistance by the SGT-53 nanocomplex
In the experiment described above, development of TMZ resistance was not observed with the combination treatment as evidenced by prolongation of survival in the second cycle comparable to that observed with the first cycle. These results suggested that the addition of SGT-53 to the TMZ treatment regimen could help to abrogate or delay the development of TMZ resistance.
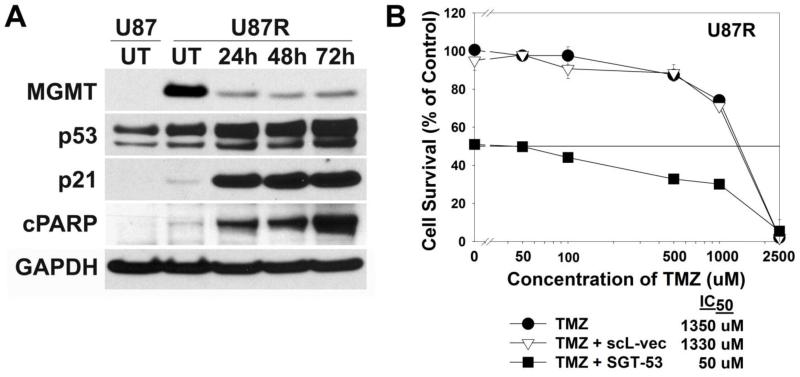
We assessed the ability of SGT-53 to sensitize GBM cells to TMZ using a TMZ-resistant subclone of U87 cells (U87R) that was established by exposure to 100 μM TMZ for 21 days.16 Similar to a previous report,16 there was a dramatic increase in MGMT expression in these now TMZ-resistant cells (Figure 6, A). We subsequently assessed the ability of SGT-53 to down-modulate this elevated MGMT expression. U87R cells were transfected with SGT-53 (10 μg DNA/dish, which is equivalent to 16.7 pg DNA/cell) and examined for changes in expression of MGMT, p53 and other proteins in the downstream p53 signaling pathway by western blot analysis (Figure 6, A). Compared to untreated U87R cells, transfection with SGT-53 resulted in significant silencing of MGMT expression which inversely correlated with p53 expression. Within 24 h post-transfection, there was a 50% increase in p53 levels relative to the untreated U87R cells, with a simultaneous 75% decrease in MGMT expression. The change was even more dramatic by 48 h when MGMT expression was decreased by 80%. This down-modulation lasted to at least 72 h post-SGT-53 transfection. Increase in the expression of p21, a downstream target of p53, was shown to directly correlate with p53 levels demonstrating signaling through this pathway. More importantly, elevated expression of cleaved PARP, an indicator of apoptosis, was detected as early as 24 h post-SGT-53 transfection, with a 23-fold increase relative to the level in untreated U87R cells by 72 h.
Figure 6.
In vitro sensitization of TMZ-resistant clone U87R to TMZ by SGT-53. (A) Western blot analysis assessing changes in MGMT, p53, p21, and cleaved PARP protein levels in U87R cells over time after transfection with the SGT-53 nanocomplex. (B) In vitro sensitization of U87R to the combination of SGT-53 and TMZ. U87R cells were transfected with SGT-53 for 24 h, then treated with increasing concentrations of TMZ for additional 72 h. As controls, cells were also treated with TMZ either alone or in combination with scL-vec. Cell viability was measured by XTT assay.
The elevated levels of MGMT observed in the U87R cells indicate that the acquired TMZ resistance of these cells may be due, at least in part, to high expression of this protein. Thus, we further assessed the potential of SGT-53 to reverse MGMT-mediated TMZ resistance in these cells by the XTT cell survival assay. U87R cells were transfected with SGT-53 (100 ng DNA/well, which is equivalent to 50 pg DNA/cell) for 24 h, and then treated with increasing concentrations of TMZ (0 to 2500 μM) for an additional 72 h. U87R cells either treated with TMZ alone or with TMZ after transfection with scL-vec were used as controls. Mirroring the results with the western blot analysis showing MGMT down-modulation, transfection with SGT-53 resulted in a 27-fold increase in sensitization to TMZ in the U87R cells, compared to treatment with TMZ alone (IC50 values of 50 μM and 1350 μM, respectively) (Figure 6, B).
Discussion
Although use of TMZ as the first-line treatment has extended the survival of GBM patients, the survival benefit is only moderate and response to TMZ is variable among patients. The most benefit was evident in a subset of GBM patients who have an aberrantly hypermethylated MGMT promoter,35 because they lack MGMT protein expression. Although the methylation status of the MGMT promoter does not always strongly correlate with MGMT protein levels,36 approximately 70% of gliomas have elevated levels of MGMT expression12 and thus would be expected to benefit less from TMZ-based therapy.
Furthermore, TMZ resistance can also develop during therapy. Following the completion of concomitant chemo-radiation therapy of newly diagnosed GBM patients, the standard of care is 6-12 cycles of adjuvant TMZ therapy for maintenance. However, the development of TMZ resistance during this adjuvant therapy occurs in more than 30% of patients.37 In the studies presented above, we observed increased expression of MGMT in a TMZ-resistant subclone (U87R). Analogous to the clinical situation, this clone was derived from TMZ responsive U87 cells by long-term exposure to TMZ, thus demonstrating a relationship between acquired TMZ resistance and induction of MGMT expression. However, it is uncertain if this process selects for a pre-existing subpopulation of cells that already possess elevated MGMT levels, or a population of cells arise in which MGMT expression has been induced in response to drug treatment. None-the-less, transfection of TMZ-resistant U87R cells with SGT-53 yielded a time dependent inverse correlation between p53 and MGMT protein levels demonstrating that this increase in MGMT expression could be reversed by p53. Moreover, in vitro studies showed that transfection of these TMZ-resistant U87R cells with SGT-53 also resulted in a dramatic increase in sensitivity to TMZ and an increased level of cell death. Such findings demonstrate that SGT-53 could overcome, at least in vitro, the TMZ-resistance of these U87R cells and suggest that this occurs through down-modulation of MGMT.
Furthermore, in the in vivo survival studies with mice bearing initially TMZ responsive U87 intracranial tumors, TMZ treatment became significantly less effective and without additional survival benefit during a second cycle of treatment, possibly indicating development of TMZ resistance, and mirroring what is observed in patients. In contrast, in the mice that received both SGT-53 and TMZ, survival benefit was maintained, and further extended, by adding a second treatment cycle. The median life-span of the animals that received two cycles of the combination therapy was significantly longer than those of mice that received TMZ alone or a single cycle of treatment. These findings suggest that SGT delivered p53 can, maintain U87 tumor sensitivity to TMZ during repeated cycles of treatments possibly through an effect on MGMT expression. Moreover, as multiple cycles (more than 2) of TMZ are currently administered to patients in the clinic, it is possible that multiple treatment cycles where SGT-53 is combined with TMZ could further prolong survival.
The tumor suppressor p53 is reported to suppress MGMT expression either by directly binding to MGMT or by sequestering specificity protein 1 (SP1), a transcription factor which binds to the MGMT promoter.8,38 In addition it has been shown that with all of the other potential MGMT inhibitors (IFN-β, STAT3 inhibitor, and levetiracetam) p53 is involved in the regulation of MGMT expression.11,12,15,39,40 Moreover, in our previous study using SGT-53 in inherently TMZ-resistant GBM tumors, we demonstrated the overexpression of exogenous wtp53 in intracranial xenografts reversed TMZ-resistance by abrogation of MGMT expression.17
In addition to its effect on MGMT, it is also likely that wtp53 replacement could operate through additional mechanisms to increase response to TMZ. The high levels of exogenous wtp53 expression in the target tumor cells induced by systemically delivered SGT-53 could affect downstream signaling through p53 to modulate apoptotic pathways such as induction of Fas receptor, caspase expression, or modulation of Bcl2 domain proteins.41
Importantly, this potentiation effect seems to exist independent of the endogenous p53 status as sensitization of both wild-type p53 (U87) and mutant p53 (U251), TMZ-sensitive, GBM cells was observed in vitro. Transfection of these cells with SGT-53 resulted in a dramatic increase in response and level of apoptosis after TMZ treatment. This enhanced therapeutic efficacy of TMZ in combination with SGT-53 continued in preclinical animal models. In vivo studies showed that mice with established intracranial U87-luc2 tumors benefited significantly from the combination of SGT-53 plus TMZ compared to TMZ alone or SGT-53 alone. In these mice, systemic administration of SGT-53 plus TMZ led not only an increase in apoptosis, but also to a substantial inhibition of tumor growth and even tumor regression. More importantly, SGT-53-mediated sensitization of U87 intracranial tumors to TMZ provided a significant prolongation of survival in these mice.
In these studies, this combination therapy demonstrated significantly enhanced anti-cancer effect and extended survival in TMZ-responsive tumors. As such tumors will likely develop TMZ-resistance during treatment, our results also suggest that even in patients who initially respond to TMZ, the combination of SGT-53 and TMZ may delay development of TMZ-resistance resulting in improved outcome compared to TMZ treatment alone, the current standard of care.
In summary, our data suggest that combining SGT-53, with standard TMZ treatment could be a more effective therapy for both TMZ-responsive and TMZ-resistant GBM tumors. Due to the tumor-targeting ability, BBB penetrance, and proven safety of SGT-53, we believe that the combination of SGT-53 plus conventional chemo- and/or radiotherapy could bring about a significant decrease in treatment resistance and recurrence, resulting in improvement in survival for GBM patients.
Supplementary Material
Acknowledgments
Statement of funding: This study was supported in part by NCI grant 5R01CA132012-02 (E.H.C.), National Foundation for Cancer Research grant HU0001 (E.H.C.), and a research grant from SynerGene Therapeutics Inc. (K.F.P.). This study was conducted in part using the Flow Cytometry & Cell Sorting Shared Resource, the Preclinical Imaging Research Laboratory, Microscopy & Imaging Shared Resource, Tissue Culture & Biorepository for Cell Lines and Biofluids Shared Resource, and Animal Core Facilities supported by NCI Cancer Center Support grant and U.S. Public Health Service grant 2P30-CA-51008 and 1 S10 RR 15768-01. This investigation was performed in part in a facility constructed with support from Research Facilities Improvement grant C06RR14567 from the National Center for Research Resources, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Drs. Chang and Pirollo are two of the inventors of the described technology, for which several patents owned by Georgetown University have been issued. The patents have been licensed to SynerGene Therapeutics, Inc. for commercial development. Dr. Chang owns equity interests in SynerGene Therapeutics, Inc. and serves as a non-paid scientific consultant to SynerGene Therapeutics, Inc.
References
- 1.Nagasawa DT, Chow F, Yew A, Kim W, Cremer N, Yang I. Temozolomide and other potential agents for the treatment of glioblastoma multiforme. Neurosurg Clin N Am. 2012;23(2):307–22. ix. doi: 10.1016/j.nec.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 3.Hou LC, Veeravagu A, Hsu AR, Tse VC. Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20(4):E5. doi: 10.3171/foc.2006.20.4.2. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain MC. Temozolomide: therapeutic limitations in the treatment of adult high-grade gliomas. Expert Rev Neurother. 2010;10(10):1537–44. doi: 10.1586/ern.10.32. [DOI] [PubMed] [Google Scholar]
- 5.Sun S, Lee D, Ho AS, Pu JK, Zhang XQ, Lee NP, et al. Inhibition of prolyl 4-hydroxylase, beta polypeptide (P4HB) attenuates temozolomide resistance in malignant glioma via the endoplasmic reticulum stress response (ERSR) pathways. Neuro Oncol. 2013;15(5):562–77. doi: 10.1093/neuonc/not005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma J, Murphy M, O’Dwyer PJ, Berman E, Reed K, Gallo JM. Biochemical changes associated with a multidrug-resistant phenotype of a human glioma cell line with temozolomide-acquired resistance. Biochem Pharmacol. 2002;63(7):1219–28. doi: 10.1016/s0006-2952(02)00876-6. [DOI] [PubMed] [Google Scholar]
- 7.Fruehauf JP, Brem H, Brem S, Sloan A, Barger G, Huang W, et al. In vitro drug response and molecular markers associated with drug resistance in malignant gliomas. Clin Cancer Res. 2006;12(15):4523–32. doi: 10.1158/1078-0432.CCR-05-1830. [DOI] [PubMed] [Google Scholar]
- 8.Bocangel D, Sengupta S, Mitra S, Bhakat KK. p53-Mediated down-regulation of the human DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT) via interaction with Sp1 transcription factor. Anticancer Res. 2009;29(10):3741–50. [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn JA, Desjardins A, Weingart J, Brem H, Dolan ME, Delaney SM, et al. Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2005;23(28):7178–87. doi: 10.1200/JCO.2005.06.502. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Gerson SL. Targeted modulation of MGMT: clinical implications. Clin Cancer Res. 2006;12(2):328–31. doi: 10.1158/1078-0432.CCR-05-2543. [DOI] [PubMed] [Google Scholar]
- 11.Natsume A, Ishii D, Wakabayashi T, Tsuno T, Hatano H, Mizuno M, et al. IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res. 2005;65(17):7573–9. doi: 10.1158/0008-5472.CAN-05-0036. [DOI] [PubMed] [Google Scholar]
- 12.Natsume A, Wakabayashi T, Ishii D, Maruta H, Fujii M, Shimato S, et al. A combination of IFN-beta and temozolomide in human glioma xenograft models: implication of p53-mediated MGMT downregulation. Cancer Chemother Pharmacol. 2008;61(4):653–9. doi: 10.1007/s00280-007-0520-x. [DOI] [PubMed] [Google Scholar]
- 13.Yoshino A, Ogino A, Yachi K, Ohta T, Fukushima T, Watanabe T, et al. Effect of IFN-beta on human glioma cell lines with temozolomide resistance. Int J Oncol. 2009;35(1):139–48. doi: 10.3892/ijo_00000322. [DOI] [PubMed] [Google Scholar]
- 14.Kohsaka S, Wang L, Yachi K, Mahabir R, Narita T, Itoh T, et al. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol Cancer Ther. 2012;11(6):1289–99. doi: 10.1158/1535-7163.MCT-11-0801. [DOI] [PubMed] [Google Scholar]
- 15.Bobustuc GC, Baker CH, Limaye A, Jenkins WD, Pearl G, Avgeropoulos NG, et al. Levetiracetam enhances p53-mediated MGMT inhibition and sensitizes glioblastoma cells to temozolomide. Neuro Oncol. 2010;12(9):917–27. doi: 10.1093/neuonc/noq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirose Y, Berger MS, Pieper RO. p53 effects both the duration of G2/M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res. 2001;61(5):1957–63. [PubMed] [Google Scholar]
- 17.Kim SS, Rait A, Kim E, Pirollo KF, Nishida M, Farkas N, et al. A Nanoparticle Carrying the p53 Gene Targets Tumors Including Cancer Stem Cells, Sensitizes Glioblastoma to Chemotherapy and Improves Survival. ACS Nano. 2014;8(6):5494–514. doi: 10.1021/nn5014484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biddlestone-Thorpe L, Sajjad M, Rosenberg E, Beckta JM, Valerie NC, Tokarz M, et al. ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin Cancer Res. 2013;19(12):3189–200. doi: 10.1158/1078-0432.CCR-12-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shchors K, Persson AI, Rostker F, Tihan T, Lyubynska N, Li N, et al. Using a preclinical mouse model of high-grade astrocytoma to optimize p53 restoration therapy. Proc Natl Acad Sci U S A. 2013;110(16):E1480–9. doi: 10.1073/pnas.1219142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steele RJ, Lane DP. P53 in cancer: a paradigm for modern management of cancer. Surgeon. 2005;3(3):197–205. doi: 10.1016/s1479-666x(05)80041-1. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Pirollo KF, Tang WH, Rait A, Chang EH. Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Hum Gene Ther. 1999;10(18):2941–52. doi: 10.1089/10430349950016357. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Tang WH, Huang CC, Alexander W, Xiang LM, Pirollo KF, et al. Systemic p53 gene therapy of cancer with immunolipoplexes targeted by anti-transferrin receptor scFv. Mol Med. 2001;7(10):723–34. [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L, Huang CC, Huang W, Tang WH, Rait A, Yin YZ, et al. Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes. Mol Cancer Ther. 2002;1(5):337–46. [PubMed] [Google Scholar]
- 24.Pirollo KF, Rait A, Zhou Q, Zhang XQ, Zhou J, Kim CS, et al. Tumor-targeting nanocomplex delivery of novel tumor suppressor RB94 chemosensitizes bladder carcinoma cells in vitro and in vivo. Clin Cancer Res. 2008;14(7):2190–8. doi: 10.1158/1078-0432.CCR-07-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu W, Pirollo KF, Yu B, Rait A, Xiang L, Huang W, et al. Enhanced transfection efficiency of a systemically delivered tumor-targeting immunolipoplex by inclusion of a pH-sensitive histidylated oligolysine peptide. Nucleic Acids Res. 2004;32(5):e48. doi: 10.1093/nar/gnh049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Frederik P, Pirollo KF, Tang WH, Rait A, Xiang LM, et al. Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery. Hum Gene Ther. 2002;13(3):469–81. doi: 10.1089/10430340252792594. [DOI] [PubMed] [Google Scholar]
- 27.Pirollo KF, Zon G, Rait A, Zhou Q, Yu W, Hogrefe R, et al. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Hum Gene Ther. 2006;17(1):117–24. doi: 10.1089/hum.2006.17.117. [DOI] [PubMed] [Google Scholar]
- 28.Pirollo KF, Rait A, Zhou Q, Hwang SH, Dagata JA, Zon G, et al. Materializing the potential of small interfering RNA via a tumor-targeting nanodelivery system. Cancer Res. 2007;67(7):2938–43. doi: 10.1158/0008-5472.CAN-06-4535. [DOI] [PubMed] [Google Scholar]
- 29.Hwang SH, Rait A, Pirollo KF, Zhou Q, Yenugonda VM, Chinigo GM, et al. Tumor-targeting nanodelivery enhances the anticancer activity of a novel quinazolinone analogue. Mol Cancer Ther. 2008;7(3):559–68. doi: 10.1158/1535-7163.MCT-07-0548. [DOI] [PubMed] [Google Scholar]
- 30.Kim SS, Rait A, Rubab F, Rao AK, Kiritsy MC, Pirollo KF, et al. The clinical potential of targeted nanomedicine: delivering to cancer stem-like cells. Mol Ther. 2014;22(2):278–91. doi: 10.1038/mt.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathanson D, Mischel PS. Charting the course across the blood-brain barrier. J Clin Invest. 2011;121(1):31–3. doi: 10.1172/JCI45758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camp ER, Wang C, Little EC, Watson PM, Pirollo KF, Rait A, et al. Transferrin receptor targeting nanomedicine delivering wild-type p53 gene sensitizes pancreatic cancer to gemcitabine therapy. Cancer Gene Ther. 2013;20(4):222–8. doi: 10.1038/cgt.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pirollo KF, Hao Z, Rait A, Jang YJ, Fee WE, Jr., Ryan P, et al. p53 mediated sensitization of squamous cell carcinoma of the head and neck to radiotherapy. Oncogene. 1997;14(14):1735–46. doi: 10.1038/sj.onc.1201116. [DOI] [PubMed] [Google Scholar]
- 34.Senzer N, Nemunaitis J, Nemunaitis D, Bedell C, Edelman G, Barve M, et al. Phase I Study of a Systemically Delivered p53 Nanoparticle in Advanced Solid Tumors. Mol Ther. 2013;21(5):1096–103. doi: 10.1038/mt.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 36.Moen EL, Stark AL, Zhang W, Dolan ME, Godley LA. The Role of Gene Body Cytosine Modifications in MGMT Expression and Sensitivity to Temozolomide. Mol Cancer Ther. 2014;13(5):1334–44. doi: 10.1158/1535-7163.MCT-13-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke MJ, Mulligan EA, Grogan PT, Mladek AC, Carlson BL, Schroeder MA, et al. Effective sensitization of temozolomide by ABT-888 is lost with development of temozolomide resistance in glioblastoma xenograft lines. Molecular Cancer Therapeutics. 2009;8(2):407–14. doi: 10.1158/1535-7163.MCT-08-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohsaka S, Tanaka S. Chemotherapeutic Agent for Glioma. In: Lichtor T, editor. Clinical Management and Evolving Novel Therapeutic Strategies for Patients with Brain Tumors. InTech; Rijeka, Croatia: 2013. pp. 415–38. [Google Scholar]
- 39.Lin J, Tang H, Jin X, Jia G, Hsieh JT. p53 regulates Stat3 phosphorylation and DNA binding activity in human prostate cancer cells expressing constitutively active Stat3. Oncogene. 2002;21(19):3082–8. doi: 10.1038/sj.onc.1205426. [DOI] [PubMed] [Google Scholar]
- 40.Lant B, Storey KB. An overview of stress response and hypometabolic strategies in Caenorhabditis elegans: conserved and contrasting signals with the mammalian system. Int J Biol Sci. 2010;6(1):9–50. doi: 10.7150/ijbs.6.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26(2):186–97. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.