Abstract
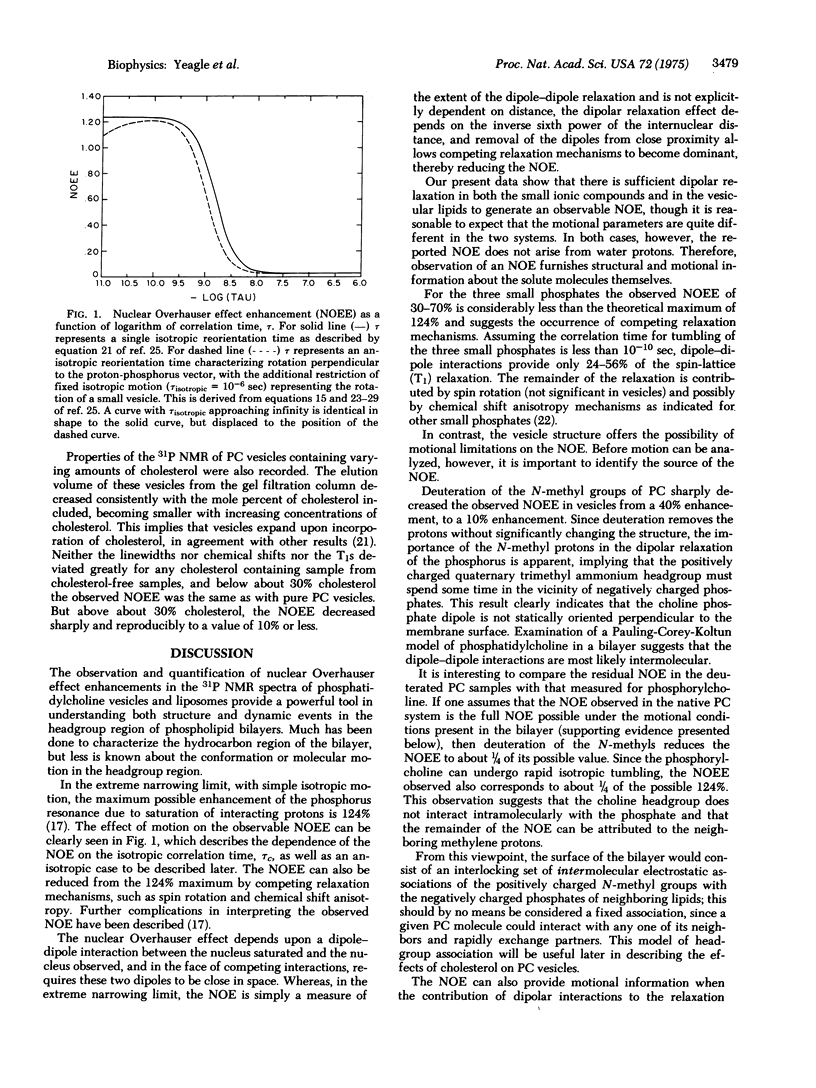
The nuclear Overhauser effect has been observed in the nuclear magnetic resonance spectra of 31P. The information content of the nuclear Overhauser effect has been applied to the structure and dynamic properties of phosphatidylcholine vesicles. In the vesicles only 1/3 of the theoretical maximum nuclear Overhauser effect enhancement is observed. This result is accounted for by dipolar interactions between the N-methyl protons and the phosphate of phosphatidylcholine, and a correlation time for internal motion of 1.4 X 10(-9) sec. Addition of up to 30% cholesterol does not change the nuclear Overhauser effect enhancement or spin-lattice relaxation time of the vesicles. It is argued that the OH group of cholesterol is hydrogen bonded to the ester carbonyl oxygen of the phosphatidylcholine molecules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aneja R., Chadha J. S., Davies A. P. A general synthesis of glycerophospholipids. Biochim Biophys Acta. 1970 Oct 6;218(1):102–111. doi: 10.1016/0005-2760(70)90097-4. [DOI] [PubMed] [Google Scholar]
- Assmann G., Highet R. J., Sokoloski E. A., Brewer H. B., Jr 13C nuclear magnetic resonance spectroscopy of native and recombined lipoproteins. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3701–3705. doi: 10.1073/pnas.71.9.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berden J. A., Cullis P. R., Hoult D. I., McLaughlin A. C., Radda G. K., Richards R. E. Frequency dependence of 31P NMR linewidths in sonicated phospholipid vesicles: effects of chemical shift anisotropy. FEBS Lett. 1974 Sep 15;46(1):55–58. doi: 10.1016/0014-5793(74)80333-9. [DOI] [PubMed] [Google Scholar]
- Bittman R., Blau L. The phospholipid-cholesterol interaction. Kinetics of water permeability in liposomes. Biochemistry. 1972 Dec 5;11(25):4831–4839. doi: 10.1021/bi00775a029. [DOI] [PubMed] [Google Scholar]
- Davis D. G., Inesi G. Phosphorus and proton nuclear magnetic resonance studies in sarcoplasmic reticulum membranes and lipids. A comparison of phosphate and proton group mobilities in membranes and lipid bilayers. Biochim Biophys Acta. 1972 Sep 1;282(1):180–186. doi: 10.1016/0005-2736(72)90322-7. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Rothman J. E. The planar organization of lecithin-cholesterol bilayers. J Biol Chem. 1972 Jun 10;247(11):3694–3697. [PubMed] [Google Scholar]
- Gent M. P., Prestegard J. H. Cholesterol-phosphatidylcholine interactions in vesicle systems. Implication of vesicle size and proton magnetic resonance line-width changes. Biochemistry. 1974 Sep 10;13(19):4027–4033. doi: 10.1021/bi00716a033. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric investigation of the influence of cholesterol on the transition properties of bilayers formed from synthetic L- -lecithins in aqueous suspension. J Biol Chem. 1972 Jun 10;247(11):3697–3700. [PubMed] [Google Scholar]
- Horwitz A. F., Klein M. P. Magnetic resonance studies on membrane and model membrane systems. II. Phosphorus spectra and relaxation rates in dispersions of lecithin. J Supramol Struct. 1972;1(1):19–28. doi: 10.1002/jss.400010104. [DOI] [PubMed] [Google Scholar]
- Huang C. H., Sipe J. P., Chow S. T., Martin R. B. Differential interaction of cholesterol with phosphatidylcholine on the inner and outer surfaces of lipid bilayer vesicles. Proc Natl Acad Sci U S A. 1974 Feb;71(2):359–362. doi: 10.1073/pnas.71.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Keough K. M., Oldfield E., Chapman D. Carbon-13 and proton nuclear magnetic resonance of unsonicated model and mitochondrial membranes. Chem Phys Lipids. 1973 Jan;10(1):37–50. doi: 10.1016/0009-3084(73)90039-x. [DOI] [PubMed] [Google Scholar]
- Lee A. G., Birdsall N. J., Levine Y. K., Metcalfe J. C. High resolution proton relaxation studies of lecithins. Biochim Biophys Acta. 1972 Jan 17;255(1):43–56. doi: 10.1016/0005-2736(72)90006-5. [DOI] [PubMed] [Google Scholar]
- Lundberg B. An x-ray and vapour pressure study on lecithin--cholesterol--water interactions. Acta Chem Scand B. 1974;28(6):673–680. doi: 10.3891/acta.chem.scand.28b-0673. [DOI] [PubMed] [Google Scholar]
- Marcelja S. Chain ordering in liquid crystals. II. Structure of bilayer membranes. Biochim Biophys Acta. 1974 Oct 29;367(2):165–176. doi: 10.1016/0005-2736(74)90040-6. [DOI] [PubMed] [Google Scholar]
- Marsh D., Smith I. C. An interacting spin label study of the fluidizing and condensing effects of cholesterol on lecithin bilayers. Biochim Biophys Acta. 1973 Mar 16;298(2):133–144. doi: 10.1016/0005-2736(73)90345-3. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Molecular dynamics of cerebroside-cholesterol and sphingomyelin-cholesterol interactions: Implications for myelin membrane structure. FEBS Lett. 1972 Apr 1;21(3):303–306. doi: 10.1016/0014-5793(72)80189-3. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Engelman D. M. Molecular mechanism for the interaction of phospholipid with cholesterol. Nat New Biol. 1972 May 10;237(71):42–44. doi: 10.1038/newbio237042a0. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Chan S. I. Effect of sonication on the structure of lecithin bilayers. Biochemistry. 1972 Nov 21;11(24):4573–4581. doi: 10.1021/bi00774a024. [DOI] [PubMed] [Google Scholar]
- de Kruyff B., Demel R. A., van Deenen L. L. The effect of cholesterol and epicholesterol incorporation on the permeability and on the phase transition of intact Acholeplasma laidlawii cell membranes and derived liposomes. Biochim Biophys Acta. 1972 Jan 17;255(1):331–347. doi: 10.1016/0005-2736(72)90032-6. [DOI] [PubMed] [Google Scholar]



