Abstract
Background
Diffuse interstitial lung diseases (DILDs) form a part of a heterogeneous group of respiratory diseases. Bronchoalveolar lavage (BAL) analysis has been used for differential diagnosis of DILDs, but their clinical usefulness is controversial. The aim of this study was to investigate the clinical usefulness of BAL cellular analysis with lymphocyte subsets for the differential diagnosis of DILDs.
Methods
A total of 69 patients diagnosed with DILDs were enrolled. Basic demographic data, BAL cellular analysis with lymphocyte subsets, histology, and high resolution computed tomogram (HRCT) findings were analyzed and compared as per disease subgroup.
Results
Significant differences were found between groups in the proportion of neutrophils (P=0.0178), eosinophils (P=0.0003), T cells (P=0.0305), CD4 cells (P=0.0002), CD8 cells (P<0.0001), and CD4/CD8 ratio (P<0.0001). These findings were characteristic features of eosinophilic pneumonia and sarcoidosis. Other parameters were not significantly different between groups. At the cut-off value of 2.16 for sarcoidosis, CD4/CD8 ratio showed sensitivity of 91.7% (95% CI, 61.5-98.6%) and specificity of 84.2% (95% CI, 72.1-92.5%).
Conclusions
Routine analysis of BAL lymphocyte subset may not provide any additional benefit for differential diagnosis of DILDs, except for conditions where BAL is specifically indicated, such as eosinophilic pneumonia or sarcoidosis.
Keywords: Bronchoalveolar lavage, Lymphocyte subsets, Sarcoidosis, Pulmonary eosinophilia, Interstitial lung diseases
INTRODUCTION
Diffuse interstitial lung diseases (DILDs) form a part of a heterogeneous group of non-neoplastic, non-infectious respiratory disorders resulting from damage to the lung parenchyma and present with similar clinical features [1]. DILDs include interstitial lung diseases (ILD) with a known cause (pneumoconiosis, ILD associated with connective tissue disease, hypersensitivity pneumonitis [HP]), sarcoidosis, and idiopathic interstitial pneumonia (IIP) including bronchiolitis obliterans organizing pneumonia (BOOP), eosinophilic pneumonia, nonspecific interstitial pneumonia (NSIP) and idiopathic pulmonary fibrosis, a form of usual interstitial pneumonia (UIP) according to the statement of the American Thoracic Society and the European Respiratory Society [2]. Although these diseases generally share common clinical features, an accurate diagnosis is crucial as treatment and prognosis differ [2]. Differential diagnosis of these disorders rests on clinical presentation combined with physical examination, pulmonary physiological testing, chest radiographic imaging, and lung biopsy [3].
Bronchoalveolar lavage (BAL) has been used as an ancillary diagnostic tool for DILDs, especially in situations where clinico-radiological findings fail to provide sufficient information for a definitive diagnosis or when a lung biopsy is not feasible [4, 5]. A number of previous studies have addressed the usefulness of BAL to evaluate patients with suspected ILD [6, 7, 8, 9, 10]. However, these were published prior to high-resolution computed tomogram (HRCT) becoming a routine diagnostic tool and also before the recognition of IIPs as distinct clinical entities [2]. With the use of HRCT becoming more widespread there is an increase in the skepticism surrounding the controversial practicality and diagnostic efficacy of BAL analysis in DILDs [2, 11].
Therefore, in this study, we investigated the clinical usefulness of BAL cellular analysis with lymphocyte subsets for the differential diagnosis of DILDs.
METHODS
1. Patients
This study enrolled 69 patients diagnosed with DILDs from 2007 through 2013, based on the combined information of clinical, radiological, and pathological findings at Ewha Woman's University School of Medicine, Mokdong Hospital, Seoul, Korea. A total of 58 patients were tested by using CT scan and 11 patients by using HRCT. Pathological diagnosis was confirmed in 22 patients. Detailed patient characteristics are presented in Table 1. This study was conducted by retrospective data review with Institutional Review Board exemption.
Table 1.
Patient characteristics
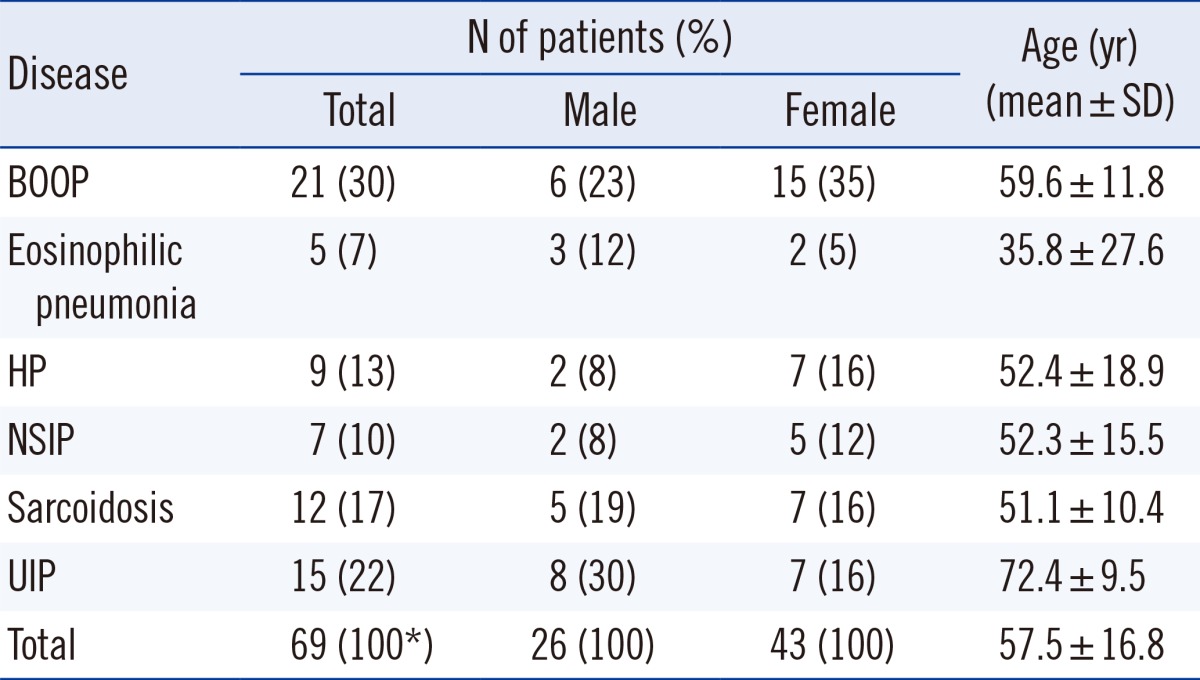
*Because of rounding, percentage may not total 100.
Abbreviations: BOOP, bronchiolitis obliterans organizing pneumonia; HP, hypersensitivity pneumonitis; NSIP, nonspecific interstitial pneumonia; UIP, usual interstitial pneumonia.
2. BAL cellular analysis with lymphocyte subsets
Fluid collected from BAL was cytocentrifuged and stained with Wright-Giemsa stain for total and differential cell counts. The cellular patterns for normal adult population differential cell counts according to the American Thoracic Society guideline [2] were followed: 85-100% of alveolar macrophages, 10-15% of lymphocytes, 0-3% of polymorphonuclear neutrophils, and 0-1% of eosinophils [2].
Immunophenotyping was performed using 2-color panel antibodies, consisting of IgG1-FITC, IgG1-PE (for isotype control), CD3-FITC, CD4-PE, CD8-PE, CD19-PE, and CD56-PE (IOTest®, Beckman Coulter Inc., Brea, CA, USA) in 5 tubes (IgG1/IgG1, CD3/CD4, CD3/CD8, CD3/CD19, CD3/CD56) and analyzed using Cytomics FC 500 flow cytometer (Beckman Coulter, Inc.). Data for a minimum of 5,000 cells were recorded for each tube. Lymphocytes were gated by forward scatter/side scatter (FSC/SSC) method, and the minimum requirement for FSC/SSC-gated lymphocyte recovery and purity was set to 90% and 95%, respectively.
3. Data analysis
Differences in BAL cellular analysis with lymphocyte subsets among groups were compare d by using the Kruskal-Wallis test, and a post-hoc analysis was performed by using the Steel-Dwass-Critchlow-Fligner test. For the parameters showing distinct differences for a particular disease, ROC curve analysis was performed to determine the cut-off value for diagnosis of that disease.
All statistical analyses were performed by using SPSS 20.0 trial version software for Windows (SPSS, Inc., Chicago, IL, USA) and Analyse-it (Analyse-it Software, Ltd., Leeds, UK). P value was considered statistically significant at P<0.05.
RESULTS
1. Characteristics of BAL cellular analysis with lymphocyte subsets among groups
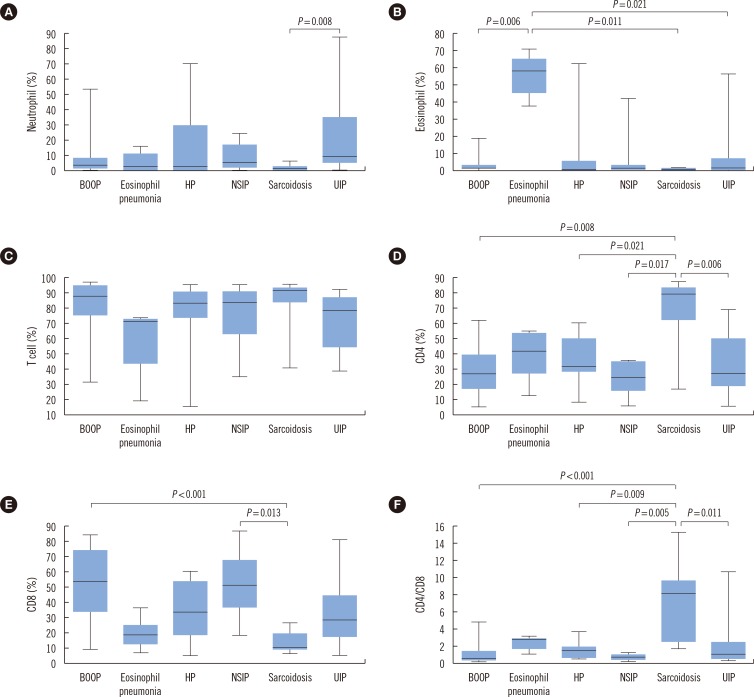
Significant differences were found between groups in the proportion of neutrophils (P=0.0178), eosinophils (P=0.0003), T cells (P=0.0305), CD4 cells (P=0.0002), CD8 cells (P<0.0001), and CD4/CD8 ratio (P<0.0001) (Table 2).
Table 2.
Characteristics of BAL cellular analysis and lymphocyte subsets among groups
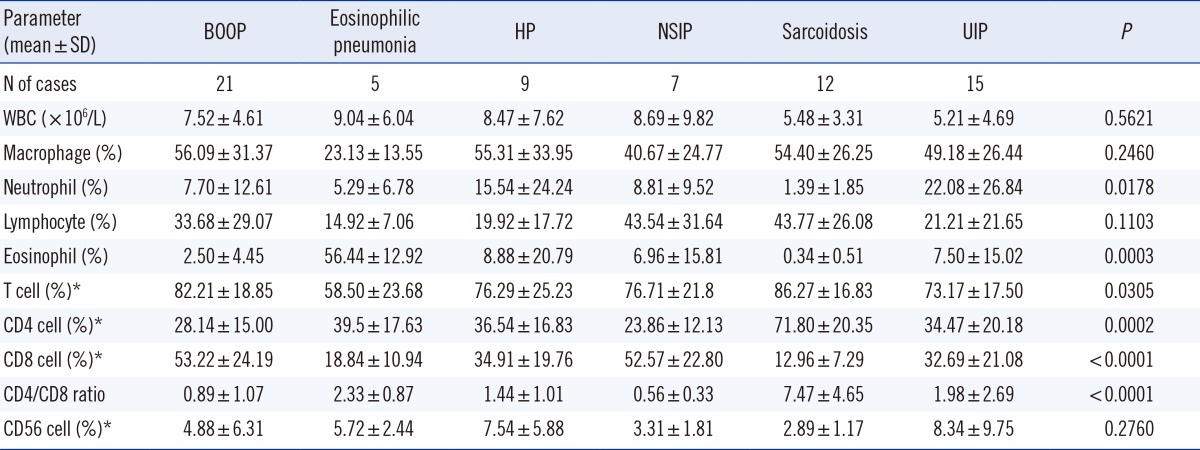
*Cell fractions were calculated based on gated lymphocytes.
Abbreviations: See Table 1. BAL, bronchoalveolar lavage; WBC, white blood cell.
As a result of post-hoc analysis on the variables with significant differences, the proportion of neutrophils was significantly different between UIP, including idiopathic pulmonary fibrosis (22.08±26.84%) and sarcoidosis groups (1.39±1.85%) (P=0.008). As for eosinophils, a significant increase was found in eosinophilic pneumonia, as compared to other groups (P=0.0003). Although there were significant differences in T cell proportion among groups (P=0.0305), no significant difference was found between groups in the post-hoc analysis. This result may be due to type I error. The sarcoidosis group showed a significant increase in the percentage of CD4 cells (P=0.0002), a decrease in that of CD8 cells (P<0.0001), and an increase in the CD4/CD8 ratio (P<0.0001), as compared to other groups. However, other parameters did not differ significantly between groups. Detailed results and statistical significance are shown in Table 2 and Fig. 1.
Fig. 1.
Post-hoc analysis of parameters between groups. (A) Neutrophil, (B) Eosinophil, (C) T cell, (D) CD4 cell, (E) CD8 cell, and (F) CD4/CD8 ratio. Post-hoc analysis between groups and statistical significance are shown. Eosinophil % showed a significant difference in the eosinophilic pneumonia group and CD4 cell %, CD8 cell %, and CD4/CD8 ratio in the sarcoidosis group.
Abbreviations: See Table 1.
2. ROC curve analysis for diagnosis of sarcoidosis
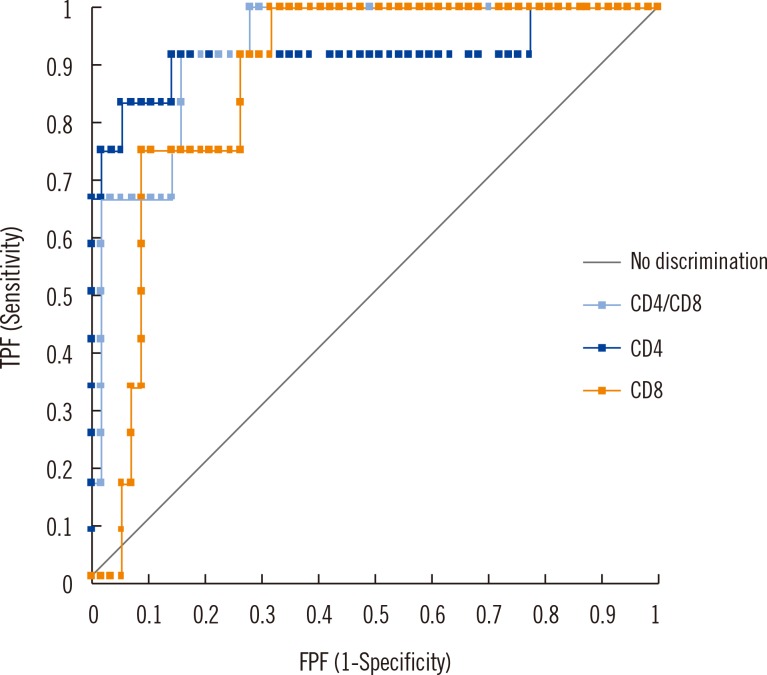
The ROC curves for diagnosis of sarcoidosis showed that the area under curve (AUC) was 0.918, 0.873, and 0.930 for CD4%, CD8%, and CD4/CD8 ratio, respectively, but no statistical differences were found between variables (P>0.13). Based on the Youden index, cut-off of CD4/CD8 ratio for sarcoidosis was determined at 2.16, with 91.7% (95% CI, 61.5-98.6%) and specificity of 84.2% (95% CI, 72.1-92.5%) (Fig. 2).
Fig. 2.
Comparison of ROC curve for sarcoidosis (CD4, CD8, and CD4/CD8). The ROC curve for sarcoidosis diagnosis shows that the area under curve (AUC) was 0.918, 0.873, and 0.930 for CD4 cell %, CD8 cell %, and CD4/CD8 ratio, respectively, but no statistical difference was found among the variables (P>0.13). Based on the Youden index, cut-off of CD4/CD8 ratio for sarcoidosis was determined at 2.16, with sensitivity of 91.7% (95% CI, 61.5-98.6%) and specificity of 84.2% (95% CI, 72.1-92.5%).
Abbreviations: TPF, true positive fraction; FPF, false positive fraction.
Non-sarcoidosis cases with CD4/CD8 ratio>2.16 included 4 cases of UIP, 3 cases of eosinophilic pneumonia, 1 case of BOOP, and 1 case of HP (Table 3, case nos. 1 through 9). Only 1 case of sarcoidosis showed CD4/CD8 ratio >2.16, with histologically confirmed diagnosis (Table 3, case no. 10). The patient was a 50-yr-old male, diagnosed as having stage II sarcoidosis, with lymphocytes 92%, CD4 cell 17%, CD8 cell 11%, and ratio of CD4/CD8 ratio 1.61.
Table 3.
Characteristics of non-sarcoidosis patients with CD4/CD8 ratio >2.16 and sarcoidosis patient with CD4/CD8 ratio ≤2.16
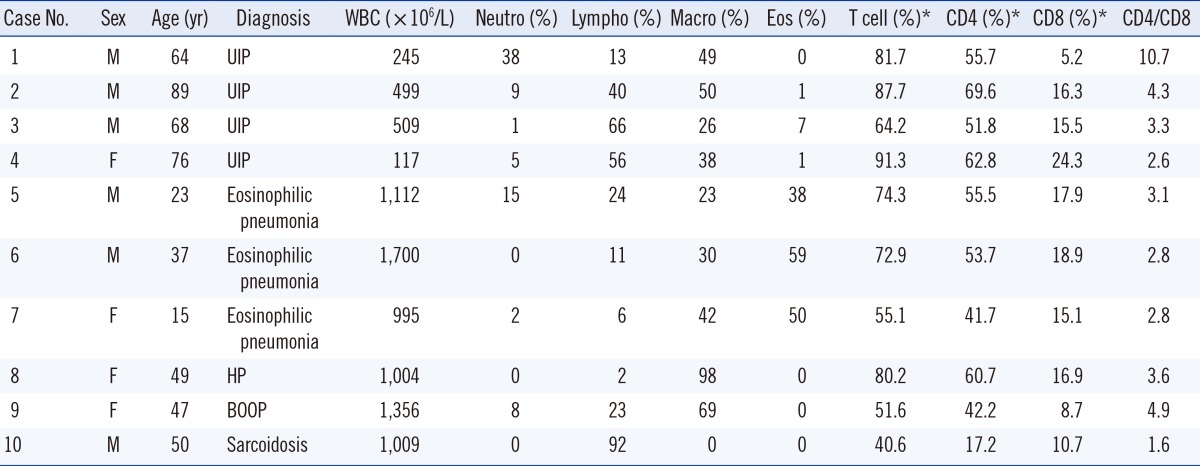
*Cell fractions were calculated based on gated lymphocytes.
Abbreviations: See Table 1. WBC, white blood cell; Neutro, neutrophil; Lympho, lymphocyte; Macro, macrophage; Eos, eosinophil; CD4, CD4 cell; CD8, CD8 cell; CD4/CD8, CD4/CD8 ratio.
DISCUSSION
In this study, the eosinophilic pneumonia group showed a significant increase in eosinophils and a significant decrease in T cell proportions, while sarcoidosis group showed a significant increase in CD4 cell %, a significant decrease in CD8 cell %, and a significant increase in CD4/CD8 ratio, as compared to the other groups. However, the other parameters of BAL analysis did not show significant differences between groups.
These findings suggest that BAL analysis may lack discriminative potential for a wide variety of ILD diseases; however, some studies reported that BAL analysis is useful for differential diagnosis of ILDs [1, 3, 11, 12].
When combined with other clinical data and imaging, BAL analysis can provide a confident diagnosis of a specific ILD group and may obviate the need to proceed to the more invasive procedure of surgical lung biopsy [13, 14]. However, BAL analysis findings may not be typical for a specific ILD diagnosis. The lack of diagnostic efficacy in BAL cellular analysis could be mainly due to overlapping features of these cellular components in different ILDs [5]. For example, lymphocytic cellular pattern (>15%) is usually associated with sarcoidosis, NSIP, BOOP, etc., while the neutrophilic pattern (>3%) is observed in bacterial infections, aspiration pneumonia, collagen vascular diseases, etc. [2]. Applying these criteria can be useful in differential diagnosis for heterogeneous disease groups. However, both cellular characteristics may be observed at the same time (e.g. lymphocyte counts 30% and neutrophil counts 50%), making the clinical utility of BAL cellular analysis controversial in many cases, which is a major drawback of BAL analysis for differential diagnosis.
In this study, BAL lymphocyte subsets showed characteristic patterns in eosinophilic pneumonia and sarcoidosis and may be useful to support and/or narrow down the differential diagnosis of ILDs. Previous studies showed that increased CD4 and decreased CD8 counts with an increased CD4/CD8 ratio are highly specific features of sarcoidosis [15, 16]. The cut-off of CD4/CD8 ratio ranges from 3.5 to 4, with sensitivity and specificity of 52% to 59% and 94% to 96%, respectively [15, 17]. In this study, ROC curve analysis of CD4, CD8, and CD4/CD8 ratio for diagnosis of sarcoidosis showed an excellent diagnostic efficacy (AUC>0.870) (Fig. 2). With the cutoff of CD4/CD8 ratio at 2.16, the highest diagnostic efficacy can be expected for diagnosis of sarcoidosis, namely, sensitivity of 91.7% and specificity of 84.2%. When we applied the cut-off range of 3.5 to 4 to our data, 67% sensitivity and 94% to 96% specificity were obtained. The cutoff point for CD4/CD8 ratio may be different for various manifestations of sarcoidosis. One study showed that sensitivity of the optimal cutoff point was lower in asymptomatic patients as compared to symptomatic patients, and the cutoff decreased with increasing stages of sarcoidosis [15]. Additionally, the BAL CD4/CD8 ratio varies with age and may be significantly increased in normal subjects [2, 15, 18]. In the present study, among 9 non-sarcoidosis patients with increased CD4/CD8 ratio (above 2.16 cutoff), 4 patients were more than 60 yr old. On the other hand, CD4/CD8 ratio may not be significantly increased in some cases of sarcoidosis, as seen in our study (Table 3, case no. 10) [2, 15, 19]. Taken together, the CD4/CD8 ratio in BAL is highly variable, and the results must be interpreted with caution.
In our study, excluding eosinophils, CD4 cell %, CD8 cell %, and CD4/CD8 ratio, no parameter showed a significant difference between groups. However, this result may be influenced by the small number of patients in some of the disease groups, which may have been inadequate to provide reliable statistical significance, and further studies are required using a larger cohort.
In conclusion, our study suggests that BAL analysis with cellular analysis and lymphocyte subsets can show characteristic patterns for eosinophilic pneumonia and sarcoidosis. Thus, it may be a useful adjunct to diagnostic evaluation, especially when a clinico-radiological profile is inadequate. However, the parameters of BAL analysis did not show significant differences between groups, except for eosinophilic pneumonia and sarcoidosis. Therefore, a routine use of BAL analysis may not provide additional diagnostic benefit for differential diagnosis of DILDs. Due to significant overlapping profiles of BAL cellular components among diseases, cellular profile is not always practically useful for differential diagnosis of DILDs, except for cases with high specificity, as seen for sarcoidosis and eosinophilic pneumonia. In this context, clinical usefulness of BAL cellular analysis in differential diagnosis of DILDs, as suggested by other studies, should be carefully reconsidered. Therefore, we suggest that BAL analysis is selectively recommended to support a diagnosis and/or narrow the specific differential diagnosis of eosinophilic pneumonia or sarcoidosis.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Kim DS. Diagnostic approaches to diffuse interstitial lung diseases. J Korean Med Assoc. 2009;52:5–13. [Google Scholar]
- 2.Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 3.Song KS, Heo WB, Won DI. Comparative analysis of bronchoalveolar lavages in interstitial lung diseases. Korean J Lab Med. 2007;27:221–227. doi: 10.3343/kjlm.2007.27.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Jara-Palomares L, Martín-Juan J, Gómez-Izquierdo L, Cayuela-Domínguez A, Rodríguez-Becerra E, Rodríguez-Panadero F. Bronchoalveolar lavage findings in patients with diffuse interstitial lung disease: prospective study of a cohort of 562 patients. Arch Bronconeumol. 2009;45:111–117. doi: 10.1016/j.arbres.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Ryu YJ, Chung MP, Han J, Kim TS, Lee KS, Chun EM, et al. Bronchoalveolar lavage in fibrotic idiopathic interstitial pneumonias. Respir Med. 2007;101:655–660. doi: 10.1016/j.rmed.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Report of the European Society of Pneumology Task Group. Eur Respir J. 1989;2:561–585. [PubMed] [Google Scholar]
- 7.Costabel U, Donner CF, Haslam PL, Rizzato G, Teschler H, Velluti G, et al. Clinical guidelines and indications for bronchoalveolar lavage (BAL): occupational lung diseases due to inhalation of inorganic dust. Eur Respir J. 1990;3:946–949. 961–969. [PubMed] [Google Scholar]
- 8.Clinical guidelines and indications for bronchoalveolar lavage (BAL): Report of the European Society of Pneumology Task Group on BAL. Eur Respir J. 1990;3:937–976. [PubMed] [Google Scholar]
- 9.Haslam PL, Baughman RP. Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur Respir J. 1999;14:245–248. doi: 10.1034/j.1399-3003.1999.14b01.x. [DOI] [PubMed] [Google Scholar]
- 10.The BAL Cooperative Group Streering Committee. Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. Am Rev Respir Dis. 1990;141:S169–S202. doi: 10.1164/ajrccm/141.5_Pt_2.S169. [DOI] [PubMed] [Google Scholar]
- 11.Veeraraghavan S, Latsi PI, Wells AU, Pantelidis P, Nicholson AG, Colby TV, et al. BAL findings in idiopathic nonspecific interstitial pneumonia and usual interstitial pneumonia. Eur Respir J. 2003;22:239–244. doi: 10.1183/09031936.03.00105202. [DOI] [PubMed] [Google Scholar]
- 12.Wells AU. The clinical utility of bronchoalveolar lavage in diffuse parenchymal lung disease. Eur Respir Rev. 2010;19:237–241. doi: 10.1183/09059180.00005510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer KC, Raghu G. Bronchoalveolar lavage for the evaluation of interstitial lung disease: is it clinically useful? Eur Respir J. 2011;38:761–769. doi: 10.1183/09031936.00069509. [DOI] [PubMed] [Google Scholar]
- 14.Meyer KC. The clinical utility of bronchoalveolar lavage in interstitial lung disease-is it really useful? Expert Rev Respir Med. 2014;8:133–135. doi: 10.1586/17476348.2014.879827. [DOI] [PubMed] [Google Scholar]
- 15.Danila E, Norkūniene J, Jurgauskiene L, Malickaite R. Diagnostic role of BAL fluid CD4/CD8 ratio in different radiographic and clinical forms of pulmonary sarcoidosis. Clinical Respir J. 2009;3:214–221. doi: 10.1111/j.1752-699X.2008.00126.x. [DOI] [PubMed] [Google Scholar]
- 16.Hyldgaard C, Kaae S, Riddervold M, Hoffmann HJ, Hilberg O. Value of s-ACE, BAL lymphocytosis, and CD4+/CD8+ and CD103+CD4+/CD4+ T-cell ratios in diagnosis of sarcoidosis. Eur Respir J. 2012;39:1037–1039. doi: 10.1183/09031936.00144311. [DOI] [PubMed] [Google Scholar]
- 17.Winterbauer RH, Lammert J, Selland M, Wu R, Corley D, Springmeyer SC. Bronchoalveolar lavage cell populations in the diagnosis of sarcoidosis. Chest. 1993;104:352–361. doi: 10.1378/chest.104.2.352. [DOI] [PubMed] [Google Scholar]
- 18.Meyer KC, Soergel P. Variation of bronchoalveolar lymphocyte phenotypes with age in the physiologically normal human lung. Thorax. 1999;54:697–700. doi: 10.1136/thx.54.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantrow SP, Meyer KC, Kidd P, Raghu G. The CD4/CD8 ratio in BAL fluid is highly variable in sarcoidosis. Eur Respir J. 1997;10:2716–2721. doi: 10.1183/09031936.97.10122716. [DOI] [PubMed] [Google Scholar]