Abstract
Purpose
To investigate the influence of prior high intensity double poling (DP) on physiological and biomechanical responses during subsequent diagonal stride (DIA).
Methods
Eight well-trained male cross-country skiers (age 22 ± 3 yr; VO2max 69 ± 3 ml · kg−1 · min−1) roller-skied on a treadmill sequentially for 3 min at 90% DIA VO2max (DIA1), 3 min at 90% DP VO2peak and 3 min at 90% DIA VO2max (DIA2). Cardio-respiratory responses were monitored continuously and gases and metabolites in blood from the a. femoralis, v. femoralis and v. subclavia determined. Pole and plantar forces and EMG from 6 lower- and upper-body muscles were measured.
Results
VO2 decreased from DIA1 to DP and increased again to DIA2 (both P < 0.05), with no difference between the DIA sessions. Blood lactate rose from DIA1 to DP to DIA2. O2 extraction was attenuated during DP (P < 0.05), but was the same during DIA1 and DIA2. EMGRMS for arm muscles during poling phase, as well as peak pole force and cycle rate were higher, while leg muscle activity was lower during DP than both sessions of DIA (all P < 0.05). The ratio of upper-/whole-body EMGRMS correlated negatively with O2 extraction in the arms during both sessions of DIA (P < 0.05).
Conclusions
In well-trained skiers skiing at high-intensity DP prior to DIA did not influence VO2, muscle activation or forces in the latter. At race intensity DP does not influence the distribution of work between upper- and lower-body during a subsequent bout of DIA. O2 extraction is coupled to technical skills during skiing.
Keywords: EMG, Force, Oxygen extraction, Oxygen uptake
Background
Of the several different sub-techniques involved in classical cross-country skiing, diagonal stride (DIA) and double poling (DP) have been most frequently studied. With DIA, the skier utilizes both the arms and legs in a diagonal fashion for propulsion and this is considered to be whole-body exercise (Lindinger et al. 2009). In the case of DP, most propulsive force is supplied by the upper-body, although a dynamic leg movement also contributes to the application of body weight to pole forces (Holmberg et al. 2006; Rud et al. 2014). Consequently, in connection with both of these sub-techniques, and particularly DP, recovery of the upper-body muscles between strokes might be limited.
With regards to cardiovascular regulation, with a similar absolute pulmonary oxygen uptake (VO2), delivery of O2 to the arms and VO2 are higher, but extraction of O2 by the arms lower with DP than DIA (Calbet et al. 2005). In the case of DIA, when exercise intensity is reduced from high to moderate, O2 extraction in the arms is lowered to a greater extent than in the legs, probably due to a more pronounced decrease in the activation of arm muscles (Björklund et al. 2010). With DP the opposite is observed, i.e., O2 extraction in the arms is reduced to a lesser extent than in the legs as the intensity of exercise is diminished, apparently because strong activation of the muscles of the upper-body is maintained (Stöggl et al. 2013).
Furthermore, the skeletal muscles of the arms and legs differ with respect to the production and oxidation of lactate, with the arms producing more at a given workload (Ahlborg and Jensen-Urstad 1991). Thus, more extensive upper-body activation during DP compared to DIA should lead to a higher systemic concentration of lactate with the former sub-technique, as has, indeed, been shown by Van Hall et al. (2003) to be the case with exercise of moderate intensity (~76% VO2max). In that investigation and the study by Calbet et al. (2005), the cardiovascular and metabolic influence of DP on subsequent DIA were analyzed to a certain extent, but no direct comparisons between DIA before and after DP were made. Moreover, analysis of higher exercise intensities (race intensity) is also important, since the distinctly higher muscle activation and application of force involved might cause mechanical hindrance of O2 extraction and thereby elevate blood levels of lactate (Stöggl et al. 2013).
Whereas DIA and DP have both been characterized individually from a physiological and biomechanical perspective during simulated races (Larsson and Henriksson-Larsen 2005; Mognoni et al. 2001; Mygind et al. 1994; Ortenblad et al. 2011; Welde et al. 2003), the influence of these sub-techniques on one another remains to be examined. Previous investigations on different modes of exercises involving the legs have revealed that the biomechanical and physiological characteristics of running are influenced negatively by prior cycling in the case of moderately trained, but not elite triathletes (Bonacci et al. 2010, 2011). In addition, after this transition, only the latter preserve their running economy, with no change in VO2. With repeated sprints of cross-country skiing the fatigue experienced by the upper-body muscles already during the first sprint leads to a shift in body position and, consequently, less effective application of force during DP (Zory et al. 2009, 2011).
Accordingly, the upper-body muscle fatigue induced by high-intensity DP might alter the relative activation of arm and leg muscles, together with pole and plantar forces, during subsequent high-intensity DIA. Thus, our aim here was to examine the influence of DP on subsequent DIA at a simulated race intensity of ~90% of VO2max/peak from a biomechanical and physiological perspective. The hypothesis was that DP alters the distribution of work between the upper- and lower-body, i.e., decreases pole force and activation of arm muscles during subsequent DIA, thereby reducing the O2 extraction by the arms.
Methods
Subjects
The 8 well-trained male cross-country skiers who volunteered (Table 1) were informed about the test procedures and possible risks prior to providing their written consent to participate. The research techniques and experimental protocol were pre-approved by the Regional Ethical Review Board in Umeå, Sweden (#08-049M) and performed in accordance with the Declaration of Helsinki.
Table 1.
Anthropometric and physiological characteristics of the 8 well-trained male cross-country skiers who participated
| Variable | Mean ± SD |
|---|---|
| Age (yr) | 22 ± 3 |
| Height (cm) | 183 ± 4 |
| Weight (kg) | 79 ± 6 |
| BMI | 23.6 ± 2.1 |
| VO2max (l · min−1) | 5.4 ± 0.3 |
| VO2max (ml · kg−1 · min−1) | 69 ± 3 |
| VO2peak (l · min−1) | 4.8 ± 0.4 |
| VO2peak (ml · kg−1 · min−1) | 62 ± 3 |
| OBLA (%VO2max) | 82 ± 4 |
| OBLA (l · min−1) | 4.4 ± 0.4 |
| LT (%VO2max) | 76 ± 3 |
| LT (l · min−1) | 4.1 ± 0.4 |
| HRmax (beats · min−1) | 189 ± 5 |
VO2max, Maximal O2 uptake; OBLA, onset of blood lactate accumulation (4 mmol⋅l−1); LT, Lactate threshold; HRmax, Maximal heart rate.
The general experimental protocol
All tests were performed on a motor-driven treadmill (Rodby RL 3000, Vänge, Sweden) and, to minimize variations in rolling resistance, using the same pair of roller skis (Pro-Ski C2, Sterners, Nyhammar, Sweden). All of the participants were well accustomed to treadmill roller skiing, both as part of their regular training and from their involvement in previous testing. They visited the laboratory on two occasions: a) first, to carry out a preliminary test to determine VO2max, maximal heart rate (HRmax) and the linearity of the velocity-VO2 relationship for DIA and DP; and b) two days later to perform a continuous experimental protocol involving DIA and DP at race intensity.
The preliminary test
The velocity required to obtain 90% VO2max with DIA and 90% peak oxygen uptake (VO2peak) with DP were established employing separate linear graded protocols. In the case of DIA, VO2 was determined at several submaximal workloads separated by a one-minute break at a fixed incline of 6.5°, with an initial velocity set to 6 km∙h−1. The increase for the submaximal workloads was 2 km∙h−1 using four minute long bouts. During this submaximal protocol capillary blood samples were taken between workloads for determination of lactate and the onset of blood lactate accumulation (OBLA) (4.0 mmol · l−1) (Ivy et al. 1980; Sjödin and Jacobs 1981). VO2max values were established using a ramp protocol starting at 11 km∙h−1 at an incline of 4°, with an increase in gradient by 1° every minute. One day later the VO2peak for DP was obtained starting at a fixed inclination of 1°, initial velocity of 17 km · h−1, and a 2 km · h−1 increase in workload every fourth minute, until exhaustion. Using the means of the three highest consecutive VO2 values during the last minute at each submaximal workload, with a total sampling time of 30 s (mixing chamber), simple linear regression was applied to calculate the velocities required to achieve the targets of 90% VO2max (DIALIN) and 90% VO2peak (DPLIN).
Experimental procedures
The subjects reported to the laboratory two hours before starting the experimental protocol and were fitted with the EMG system and, thereafter, to normalize the amplitudes of the signals obtained, performed maximal voluntary contractions (MVC) with each muscle. Next, they rested in a supine position while the three catheters were inserted under local anesthesia (Carbocain 1%), utilizing an ultrasound system (Mindray DigiPrince DP-6600, Mindray Bio-Medical Electronics Co., Shenzhen, China) to visualize the vessels, as reported elsewhere (Calbet et al. 2005). For sampling arterial blood, a 16-gauge catheter (Arrow ES-04401, 300 mm, Arrow International Inc., Reading, PA) was inserted percutaneously using the Seldinger technique. Thereafter, a 20-gauge catheter (Arrow ES-04150, 120 mm) was inserted into the right femoral vein for collection of venous blood. Finally, another 16-gauge catheter (Arrow ES-04401, 300 mm) was inserted into an antecubital vein and then advanced into the subclavian vein for additional sampling of venous blood. (For further details, see Björklund et al. (2010)). All three catheters were sutured to the skin to minimize the risk of movement during exercise and connected to tubing (Alaris G420-B, 2000 mm, Cardinal Health, Switzerland) with a three-way stopcock and filled with isotonic saline containing heparin (100 E∙mL−1).
For the actual testing, inclines of 6.5° and 1° and velocities calculated to elicit 90% VO2max and VO2peak were used for DIA (11.2 ± 0.3 km∙h−1) and DP (24.3 ± 1.1 km∙h−1), respectively. The continuous experimental protocol was preceded by a 20 min warm-up with DIA and DP at 60 – 90% VO2max/VO2peak. The test itself consisted of 3 min of DIA at 90% of VO2max, followed by 3 min of DP at 90% VO2peak and, finally, a second 3-min bout of DIA at 90% VO2max.
Physiological measurements
Respiratory variables were monitored with the mixed expired procedure, employing an ergo-spirometry system (AMIS 2001 model C, Innovision A/S, Odense, Denmark) equipped with pneumotachograph to measure inspiratory flow. This system was calibrated with the standardized procedure described by Björklund et al. (2007, 2010). VO2, VCO2 and the inspired minute ventilation (VE) were monitored continuously and the average VO2 during the final 30 s at each workload calculated.
Heart rate (HR) was followed continuously using the Polar S610 monitor (Polar Electro Oy, Kempele, Finland) in combination with the metabolic cart. During the final minute of exercise at each bout, arterial and venous blood samples (1.5 ml) were collected anaerobically into syringes (PICO 50, Radiometer, Copenhagen, Denmark) containing heparin and then analyzed immediately for hemoglobin concentration [Hb], pH, PO2, PCO2, oxygen saturation (SO2) (ABL 80; Radiometer, Copenhagen, Denmark) and lactate (Biosen 5140, EKF-diagnostic GmbH, Magdeburg, Germany). The O2 content of arterial (CaO2) and venous (femoral: CfvO2; subclavian: CsvO2) blood was calculated from the SO2 and [Hb] as follows: (1.34 × [Hb] × SO2) + (0.003 × PO2) (Calbet et al. 2005).
Force measurements
Each participant used custom-made carbon-fiber racing poles adjusted to his preferred length. The ground reaction force was measured by a strain gauge force transducer mounted directly below the grips of these poles (Hottinger–Baldwin Messtechnik GmbH, Darmstadt, Germany). Plantar ski reaction forces were recorded at 100 Hz by a Pedar mobile system (Novel GmbH, Munich, Germany). These systems for determination of the pole and plantar forces were validated and calibrated utilizing procedures described previously (Holmberg et al. 2005).
EMG measurements
The EMG activity at the surface of the triceps brachii, latissimus dorsi, rectus abdominis, gluteus maximus, and gastrocnemius (medial head) muscles on the right side of the body were recorded employing pre-gelled bipolar Ag/AgCl surface electrodes (Skintact, Leonhard Lang GmbH, Innsbruck, Austria). Prior to fixation of these electrodes, the skin was shaved, abraded lightly, degreased, and disinfected with alcohol. The electrodes were positioned in parallel on the surface of the muscle belly in the direction of the fibers and 30 mm apart, in accordance with international standards (Hermens et al. 1999). The reference electrode was attached to the tibia. The active and reference electrodes for each muscle were connected to a single differential amplifier (base gain 500; input impedance >100 MΩ; common mode rejection ratio >100 dB; input range ±10 mV).
Processing the EMG signals
Prior to the calculation of EMG parameters, the raw signals were band-pass filtered digitally (10–400 Hz; Butterworth 2nd order) to remove both low- and high-frequency noise (Winter 1990). After full wave rectification of the signals the integrated and root mean square electromyography (IEMG and EMGRMS respectively) values for defined phases of exercise were calculated for all of these muscles. After training several times, MVC was performed with each muscle (Acierno et al. 1995; Holmberg et al. 2005). The recording and processing of MVC data were carried out according to Björklund et al. (2010). Statistical analysis (see further below) for IEMG and EMGRMS was performed using the sum, respectively the mean values for the arm (triceps brachii, and latissimus dorsi) and leg muscles (gastrocnemius, gluteus maximus, rectus femoris).
Collection and analysis of biomechanical data
EMG signals and pole forces were amplified with a telemetric recording system (TeleMyo 2400T G2, Noraxon, Scottsdale, AZ) and simultaneously stored (at a sampling rate of 3000 Hz) on a computer via an A/D converter card. Synchronization of the measurement of EMG signals and pole and plantar forces was achieved with a signal produced by the Pedar mobile system. The mean values for the ten successive cycles during the final 30 s of exercise at the three different bouts were subjected to statistical analysis.
Within each cycle of the DIA skiing, the following four phases were analyzed: 1–2) poling and recovery of the arms; 3) leg-gliding (starting from placement of the roller ski and ending when the leg force was minimal immediately prior to the increase in force associated with the push-off); and 4) the push-off and recovery of the legs. One such cycle was defined as extending from a pole plant to the next pole plant on the same side of the body. A cycle of DP skiing was divided into the poling and recovery phases. The average pole and plantar forces were calculated by dividing the integrated signal by the cycle time. All data were processed using the IKE-master software (IKE-Software Solutions, Salzburg, Austria).
Statistical analyses
All sets of data exhibited a Gaussian distribution, as confirmed by applying the Shapiro-Wilk test. Values are presented as means ± SD. A two way repeated-measures ANOVA (skiing technique × sampling site) was employed to test for differences between skiing techniques and sampling sites, as well as between biomechanical parameters associated with the upper- and lower-body. When a significant interaction was observed, paired sampled t-tests or a one-way ANOVA with repeated measures was performed. When only a single measurement at each workload was performed, a one-way ANOVA with repeated measures was used. A Bonferroni Post-Hoc test was performed on global effects identified by ANOVA. Pair-wise relationships between variables were compared using Pearson’s product moment correlation coefficient. A level of α < 0.05 was considered to be statistically significant in all cases. All statistical analyses were performed with the SPSS 18.0 program (IBM Corporation, Somers, NY).
Results
General observations
The calculated VO2 for DIA and the averaged measured VO2 during the first and second bout of DIA skiing and were 4.84 ± 0.95, 4.89 ± 0.13 and VO2 4.99 ± 0.11 l · min−1, respectively (P = 0.081) (Figure 1). Similarly, the calculated VO2 for DP and the averaged measured VO2 during DP the skiing did not differ (4.35 ± 0.36 and 4.36 ± 0.49 l · min−1, respectively; P = 0.94). The cardio-respiratory and biomechanical values are documented in Table 2.
Figure 1.
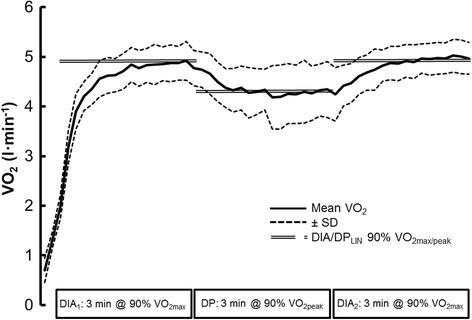
VO 2 values (means ± SD) during the three sessions of roller skiing on a treadmill. The mean calculated 90% VO2max (DIALIN) and 90% VO2peak (DPLIN) are illustrated by the double line.
Table 2.
Cardio-respiratory and biomechanical values for our 8 well-trained male cross-country skiers while roller-skiing with the diagonal stride (DIA) or double poling (DP) technique at 90% VO 2max/peak on a treadmill
| First session of DIA | DP | Second session of DIA | F -value | P -value | Power | |
|---|---|---|---|---|---|---|
| Cardio-respiratory parameters | ||||||
| VO2 (l⋅min−1) | 4.89 ± 0.36# | 4.36 ± 0.49*† | 4.99 ± 0.11# | F (1.2,8.6) = 35.3 | P < 0.001 | 1.000$ |
| Heart rate (beats · min−1) | 174 ± 4† | 168 ± 7† | 181 ± 4*# | F (2,14) = 14.7 | P < 0.001 | 0.994 |
| VE (l · min−1) | 152 ± 8† | 155 ± 18 | 172 ± 14* | F (2,14) = 8.52 | P = 0.004 | 0.921 |
| O2 pulse (ml per beat) | 28.1 ± 2.4# | 25.8 ± 3.1* | 27.3 ± 2.2 | F (1.1,7.8) = 5.77 | P = 0.041 | 0.581$ |
| Breaths · min−1 | 47 ± 2#† | 52 ± 3* | 53 ± 7* | F (1.2,8.3) = 6.79 | P = 0.027 | 0.672$ |
| VT (ml) | 3.24 ± 0.23# | 2.97 ± 0.39*† | 3.23 ± 0.27# | F (2,14) = 5.43 | P = 0.018 | 0.760 |
| Respiratory exchange ratio | 1.07 ± 0.06 | 1.11 ± 0.06 | 1.09 ± 0.04 | F (2,14) = 1.74 | n.s. | |
| VE/VO2 | 31.2 ± 1.9#† | 35.6 ± 2.1* | 34.9 ± 3.8* | F (2,14) = 14.7 | P < 0.001 | 0.994 |
| VE/VCO2 | 29.1 ± 1.1#† | 31.9 ± 1.4* | 32.0 ± 2.7* | F (2,14) = 10.7 | P = 0.002 | 0.966 |
| Biomechanical parameters | ||||||
| Speed (km∙h−1) | 11.2 ± 0.3 | 24.3 ± 1.1 | 11.2 ± 0.3 | |||
| Cycle rate (Hz) | 0.79 ± 0.02# | 0.87 ± 0.02*† | 0.78 ± 0.01# | F (2,14) = 13.1 | P = 0.001 | 0.985 |
| Cycle length (m) | 3.94 ± 0.06# | 7.82 ± 0.23*† | 4.00 ± 0.05# | F (2,14) = 211 | P = 0.001 | 1.000 |
| Cycle time (s) | 1.28 ± 0.07# | 1.16 ± 0.00*† | 1.30 ± 0.06# | F (2,14) = 12.3 | P = 0.001 | 0.979 |
| Recovery time for the arms (s) | 0.75 ± 0.06# | 0.80 ± 0.06*† | 0.75 ± 0.05# | F (2,14) = 4.91 | P = 0.028 | 0.693 |
| Relative arm recovery time (% cycle time) | 58.7 ± 2.5# | 68.9 ± 1.6*† | 57.9 ± 2.7# | F (2,14) = 155 | P = 0.001 | 1.000 |
| Peak pole force (N) | 101 ± 7.4# | 287 ± 45.2*† | 96.0 ± 5.5# | F (2,14) = 22.3 | P = 0.001 | 1.000 |
| Recovery time for the legs (s) | 0.50 ± 0.04 | 0.52 ± 0.03 | n.s. | |||
| Peak leg force (N) | 666 ± 87 | 673 ± 80 | n.s. | |||
| Impulse of pole force (Ns) | 33.6 ± 5.9 | 39.4 ± 9.6 | 30.9 ± 3.7 | F (2,14) = 4.49 | P = 0.035 | 0.651 |
| Impulse of foot force (Ns) | 481 ± 100 | 392 ± 54† | 482 ± 79# | F (2,14) = 9.47 | P = 0.003 | 0.937 |
The values are presented as means ± SD. VO2, oxygen uptake; VE, minute ventilation; VT, tidal volume; Bonferroni correction was used to check for differences between time-points. A One-Way repeated ANOVA was used to compare the responses and a paired Student’s t-test was when only two time-points were compared.
*Statistically different from DIA1.
#Statistically different from DP.
†Statistically different from DIA2.
$Greenhouse-Geisser-adjusted ANOVA, since the sphericity was violated.
n.s. not statistically significant.
Muscle activation
During the poling phase the EMGRMS for the arms was 36.7 and 49.7% higher with DP than during the first and second sessions of DIA skiing, respectively (both P < 0.05). Especially during DP high EMGRMS values of 99.7 ± 12.9 %MVC (range: 69–159 %MVC) were found. The relative EMGRMS for the arms (arms/whole body) for the entire cycle was 32.7% lower during DP (P < 0.001) than the first session of DIA (Table 3). The sums of the IEMG and average EMG (AEMG) for all of the muscles analyzed during both sessions of DIA were similar and greater than DP (IEMG: DIA1: 107 ± 24; DP: 86 ± 26; DIA2: 106 ± 25 %MVCs; AEMG: 93 ± 27, DP 72 ± 20, DIA2 79 ± 22 %MVC/s; P < 0.01 in both cases). The ratio of upper-/whole-body EMGRMS was inversely correlated with arm O2 extraction in the arms during both sessions of DIA (r = −0.918, P = 0.004 and r = −0.803, P = 0.03 respectively) (Figure 2A-B), whereas there was no such correlation in the case of DP.
Table 3.
The activities of arm and leg muscles during the entire cycle and the poling and recovery phases of the first and second sessions of DIA and the DP skiing at 90% of VO 2max/peak
| First session of DIA | DP | Second session of DIA | F -value | P -value | Power | |
|---|---|---|---|---|---|---|
| IEMG for the entire cycle arms (%MVCs) | 29.1 ± 9.2 | 25.7 ± 11.1 | 27.7 ± 8.0 | a F (2,14) = 9.29 | P = 0.004 | 0.932 |
| IEMG for the entire cycle legs (%MVCs) | 16.1 ± 3.5# | 11.6 ± 3.8*† | 16.0 ± 3.2# | b F (1,7) = 15.64 | P = 0.007 | 0.906 |
| c F (2,14) = 0.25 | n.s | |||||
| EMGRMS poling phase, arms (%MVC) | 72.9 ± 8.9# | 99.7 ± 12.9*† | 66.6 ± 10.7# | a F (1,7) = 6.66 | P = 0.042 | 0.580 |
| EMGRMS push-off phase legs (%MVC) | 44.6 ± 3.5 | 43.0 ± 3.6 | b F (1,7) = 7.44 | P = 0.034 | 0.626 | |
| c F (1.7) = 1.13 | n.s. | |||||
| EMGRMS recovery phase, arms (%MVC) | 14.2 ± 2.4 | 14.5 ± 3.1 | 15.1 ± 2.2 | a F (2,14) = 39.5 | P = 0.001 | 1.000 |
| EMGRMS recovery phase, legs (%MVC) | 31.4 ± 2.3# | 18.0 ± 2.8*† | 30.5 ± 2.0# | b F (2,14) = 51.1 | P = 0.001 | 1.000 |
| c F (4,28) = 5.24 | P = 0.023 | 0.723 | ||||
| EMGRMS ratio, entire cycle, arms/whole body (%) | 25.1 ± 1.6# | 16.9 ± 2.4*† | 24.2 ± 1.5# | F (1,7) = 13.0 | P = 0.001 | 0.984 |
The values presented are means ± SD. IEMG, integrated EMG; EMGRMS, EMG root mean squared; MVC, maximal voluntary contraction. A Two-Way repeated ANOVA (3 × 2) was used to compare the responses during the two sessions of DIA and DP. Since there was no push-off phase for the legs during DP, a 2 × 2 Two-Way repeated ANOVA was used to compare the EMGRMS for the poling and push-off phases. A One-Way repeated ANOVA was used in the case of the EMGRMS ratio and Bonferroni correction was used to compare different time-points, with paired Student’s t-test instead when only two time-points were compared.
*Significantly different from DIA1.
#Significantly different from DP.
†Significantly different from DIA2.
aMain effect: exercise intensity.
bMain effect: extremity.
cInteractive effect: exercise intensity x extremity.
n.s. not statistically significant.
Figure 2.
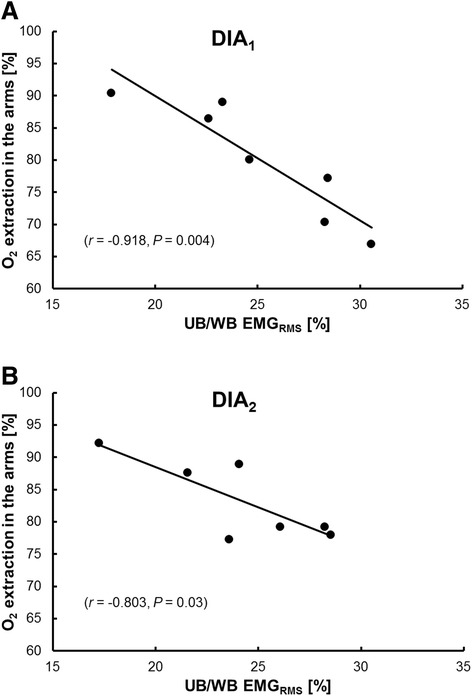
The relationship between extraction of O 2 in the arms and the ratio of upper- (UB) and whole-body EMG RMS during the first (A) (DIA 1 ) and second sessions (B) (DIA 2 ) of diagonal stride skiing.
Force and kinematics
Cycle rate and peak pole force were higher during DP than during both sessions of DIA (both P < 0.001), during which these values were similar. The impulse of pole forces was similar in all three sessions (P > 0.05). The absolute and relative (% cycle time) recovery times for the arms were longer with DP, with no difference between the two sessions of DIA (Table 2) (P > 0.05). During DIA, O2 extraction in the legs was negatively correlated to minimal leg force during ground contact (the end of the gliding phase) (r = −0.821, P = 0.024) and to the IEMG and EMGRMS for the entire cycle in the lower-body (r = −0.782, P = 0.038 and r = −0.794, P = 0.033, respectively). During DP, O2 extraction in the legs was negatively correlated to minimal leg force (r = −0.941, P = 0.002) and positively associated with cycle length (r = 0.862, P = 0.013). O2 extraction in the arms during DP was correlated to the cycle characteristics length (r = 0.941, P = 0.005), rate (r = −0.853, P = 0.031) and time (r = 0.865, P = 0.026), as well as absolute recovery time during the poling phase (r = 0.850, P = 0.032).
Overall extraction of O2
O2 extraction was higher in the legs than the arms at all workloads (F1,7 = 25.9, P = 0.002) and was lower in all limbs during DP than the two session of DIA (F2,14 = 10.3, P = 0.002 and F2, 14 = 10.4, P = 0.002, respectively), with no difference between the latter (leg P = 1.00 and arm P = 0.21). There was an interaction between skiing technique and sampling site (F2,14 = 3.89, P = 0.05), which might reflect the more pronounced reduction in O2 extraction in the arms during DP than both sessions of DIA (F2,14 = 13.31, P = 0.001) (Figure 3).
Figure 3.
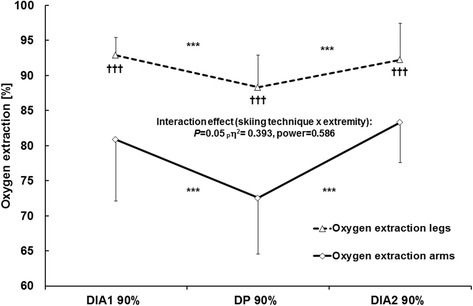
O 2 extraction in the arms and legs during the first and second sessions of diagonal stride skiing and the intervening double poling at 90% VO 2max/peak . The values presented are as means ± SD. The P-values, effect size (pη2), and observed power obtained with the two-way ANOVA (skiing technique × extremity) are presented. ***P < 0.001 in comparison to DP and ††† P < 0.001 in comparison to the arms.
Blood levels of lactate and pH
The arterial-venous difference in blood level of lactate in the legs was lowest (actually negative) during DP and similar during both sessions of DIA (F2,14 = 5.92, P = 0.014). In contrast, in the case of the arms this difference remained constant (F2,14 = 2.17, P = 0.163) (Figure 4). The absolute blood level of lactate rose from the first session of DIA to DP and again to the second session of DIA, being significantly higher in the subclavian vein than in either the femoral artery or vein throughout the entire test (P < 0.05).
Figure 4.
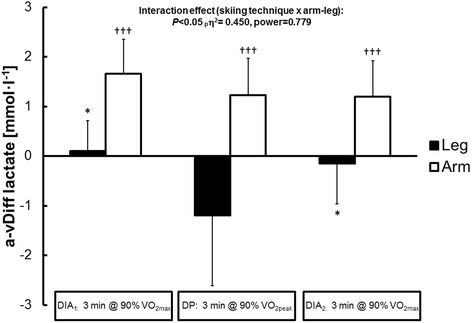
The arterial-venous difference in blood levels of lactate in the legs and arms during the first and second sessions of diagonal stride skiing and the intervening double poling at 90% VO 2max/peak . The values presented are means ± SD. The P-values, effect size (pη2), and observed power obtained with the two-way ANOVA (skiing technique × extremity) are presented. *P < 0.05 in comparison to DP and ††† P < 0.001 in comparison to the legs.
The blood level of bicarbonate fell to an equal extent at all three sampling sites for each successive workload (P < 0.05). The pH was lower in both the subclavian and femoral veins than the femoral artery during all three sessions, with the lowest values for all sampling sites occurring during the second session of DIA (P < 0.01). The PCO2 remained constant throughout the test and was higher in both the femoral and subclavian vein than the femoral artery (P < 0.05) (Table 4).
Table 4.
Lactate, pH and gases in the blood during roller-skiing with the diagonal stride (DIA) or double-poling (DP) technique at 90% VO 2max/peak
| Variable | Resting | First session of DIA | DP | Second session of DIA | F -values, P -values, observed power |
|---|---|---|---|---|---|
| [Lactate], mmol · l −1 | |||||
| Femoral artery | 0.8 (±0.1) | 4.6 (±0.9)¤† | 7.0 (±1.6)*† | 7.9 (±2.0)*¤† | a F (2,14) = 46.4, P < 0.001, power = 1.0 |
| Femoral vein | 0.7 (±0.2) | 4.7 (±0.8)¤† | 5.8 (±1.7)† | 7.8 (±1.8)*¤† | b F (2,14) = 29.4, P < 0.001, power = 1.0 |
| Subclavian vein | 0.8 (±0.3) | 6.3. (±1.4)¤$‡ | 8.2 (±1.6)*$‡ | 9.1 (±2.0)*$‡ | c F (4,28) = 5.17, P < 0.01, power = 0.94 |
| [Bicarbonate], mmol · l −1 | |||||
| Femoral artery | 23.6 (±1.9) | 19.2 (±1.0)¤ | 17.8 (±1.4)* | 16.9 (±1.3)* | a F (2,14) = 51.7, P < 0.001, power = 1.0 |
| Femoral vein | 23.5 (±1.6) | 19.4 (±1.3)¤ | 17.8 (±1.6)* | 16.6 (±1.2)*¤ | b F (2,14) = 0.081, n.s. |
| Subclavian vein | 23.7 (±1.3) | 19.4 (±1.1)¤ | 17.4 (±0.8) | 16.5 (±1.2)*¤ | c F (4,28) = 0.212, n.s. |
| pH | |||||
| Femoral artery | 7.42 (±0.01) | 7.37 (±0.02)¤‡† | 7.34 (±0.03)*†‡ | 7.31 (±0.04)*¤†‡ | a F (2,14) = 32.97, P < 0.001, power = 1.0 |
| Femoral vein | 7.38 (±0.02) | 7.27 (±0.02)¤$† | 7.23 (±0.04)*$ | 7.19 (±0.03)*¤$ | b F (2,14) = 384, P < 0.001, power = 1.0 |
| Subclavian vein | 7.37 (±0.03) | 7.24 (±0.03)¤$‡ | 7.21 (±0.03)*$ | 7.19 (±0.03)*¤$ | c F (4,28) = 5.68, P < 0.01, power = 0.95 |
| O 2 content, ml · l −1 | |||||
| Femoral artery | 190 (±15.2) | 193 (±15.3)†‡ | 192 (±27.8)†‡ | 200 (±11.0)†‡ | a F (2,14) = 1.51, n.s. |
| Femoral vein | 100 (±23.9) | 13.4 (±4.6)$† | 22.4 (±9.9)$† | 15.6 (±10.7)$† | b F (2,14) = 428, P < 0.001, power = 1.0 |
| Subclavian vein | 120 (±29.8) | 36.1 (±15.0)$‡ | 51.0 (±12.6)$‡ | 33.2 (±11.0)$‡ | c F (4,28) = 2.52, n.s. |
| SO 2 (%) | |||||
| Femoral artery | 98.3 (±0.8) | 94.8 (±2.0)¤†‡ | 96.0 (±1.7)*†‡ | 94.3 (±2.0)¤†‡ | a F (2,14) = 16.28, P < 0.001, power = 0.99 |
| Femoral vein | 51.1 (±11.1) | 6.5 (±2.4)¤$† | 11.2 (±5.2)*$† | 7.5 (±5.2)¤$† | b F (2,14) = 1294, P < 0.001, power = 1.0 |
| Subclavian vein | 57.0 (±17.9) | 16.9 (±7.5)$‡ | 22.7 (±6.1)$‡ | 14.7 (±5.3)¤ $‡ | c F (4,28) = 4.28, P < 0.01, power = 0.87 |
| PO 2 (mm Hg) | |||||
| Femoral artery | 111 (±15.2) | 77.3 (±10.8)¤†‡ | 88.1 (±11.4)*†‡ | 78.9 (±9.8)¤†‡ | a F (2,14) = 15.47, P < 0.001, power = 0.99 |
| Femoral vein | 27.5 (±4.8) | 8.8 (±2.3)¤$† | 12.6 (±3.6)*$† | 9.8 (±4.5)¤$† | b F (2,14) = 471, P < 0.001, power = 1.0 |
| Subclavian vein | 32.1 (±8.7) | 16.3 (±4.2)$‡ | 20.0 (±3.2)$‡ | 15.8 (±3.7)$‡ | c F (4,28) = 4.21, P < 0.05, power = 0.86 |
| PCO 2 (mm Hg) | |||||
| Femoral artery | 34.9 (±4.0) | 30.5 (±2.6)†‡ | 29.8 (±2.3)†‡ | 29.3 (±2.3)†‡ | a F (2,14) = 2.04, n.s. |
| Femoral vein | 43.5 (±4.2) | 55.1 (±4.1)$ | 54.8 (±3.2)$ | 59.0 (±4.9)$ | b F (2,14) = 179, P < 0.001, power = 1.0 |
| Subclavian vein | 45.5 (±5.3) | 60.3 (±7.0)$ | 57.9 (±5.1)$ | 59.8 (±2.9)$ | c F (4,28) = 2.26, n.s. |
The values shown are means (±SD). PO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; SO2, oxygen saturation.
The F- and P-values are for two-way ANOVA (3 × 3) analysis of exercise intensity (90% VO2max/peak) x sampling site.
aMain effect between intensities. $Significantly different to femoral artery. *Significantly different to DIA190%.
bMain effect between sampling sites. ‡Significantly different to femoral vein. ¤Significantly different to DP90%.
cIntensity by sampling site interactive effect. †Significantly different to subclavian vein.
n.s. not significant.
The breathing pattern
During DP the cycle and breathing rates were tightly coupled (i.e. entrainment), with 52 breaths · min−1 (0.87 Hz) being similar to the cycle rate (a ratio of 1:1) (r = 0.89, P = 0.007). In the case of DIA, the rate of breathing was similar to the cycle rate during the first session (approximately 0.79 Hz), but greater than cycle rate during the second (0.88 Hz vs. 0.78 Hz, P = 0.022). Furthermore, there was no correlation between the breathing and cycle rates during either session of DIA (r = 0.23 and r = 0.18 both P > 0.05).
Discussion
The major findings of the study using repeated high-intensity DIA bouts interspersed with high-intensity DP were: 1) DP did not alter the redistribution in muscle activation between arms and legs and any further biomechanical variables between DIA1 and DIA2. 2) O2 extraction in both arms and legs was unaffected by DP as O2 extraction was unchanged between DIA1 and DIA2. However, arm O2 extraction decreased during DP compared to DIA despite higher muscle activation, a three-fold increase in pole ground reaction force and an augmented blood lactate concentration in the arms. 3) Leg O2 extraction was related to a greater unloading of the lower body by a more dynamic lower body work during DP and both DIA, while arm O2 extraction was associated with cycle characteristics (e.g. cycle length, cycle rate, swing time) for DP only. Interestingly, a greater ratio of upper- to whole-body muscle activation at both DIA1 and DIA2 was associated to an attenuated arm O2 extraction. 4) Pulmonary VO2 did not increase over time as VO2 for DIA1 and DIA2 was similar. However, there was an apparent acid base disturbance from DIA1 to DIA2 reflected by a decrease in pH, bicarbonate and an increase in lactate both systemically and for venous arm and leg blood.
Effects of DP on upper to lower body muscle activation during DIA
Despite increased muscle activation and peak pole forces during DP compared to DIA (~ + 40%, +185%) no changes in regards of muscle activation distribution between upper- and lower-body and cycle characteristics for DIA2 compared to DIA1 were induced. Previous data from repeated sprint cross-country skiing on snow, using a higher exercise intensity than in the current study, demonstrated a decrease in upper body force and power output during DP (Zory et al. 2009). This decrease was accompanied by a decrease in performance. Our study was carried out on a treadmill using preset velocities where the skier was not able to adjust the velocity. However, this does not explain the absence in redistribution between upper and lower body as both arms and legs are used for propulsion during DIA. Also, it could be that the intensity was not strenuous enough for these well-trained skiers in accordance with a previous report that elite athletes withstand fatigue better to maintain neuromuscular control (Bonacci et al. 2011). Furthermore, the increased muscle activation during DP compared to DIA was specifically during the poling phase while for the entire movement cycle as well as the recovery phase no difference was observed. Therefore, even though the high demands on the upper body during high-intensity DP the upper-body contribution during DIA seems to be too small to necessitate a redistribution of upper to lower body work.
O2 extraction DP vs. DIA
One of the novel findings was the decreased arm O2 extraction during DP compared with DIA, although increased upper-body muscle activation (up to ~100% of MVC in DP vs. 70% of MVC in DIA). The result of a lower arm O2 extraction during DP than DIA have previously been demonstrated by Calbet et al. (2005) using a lower exercise intensity (76 vs. 90% of VO2max/peak). Furthermore, there was an interaction effect caused by a more pronounced decrease in O2 extraction in arms than legs when switching from DIA1 to DP and back to DIA2. The reason for a lower arm O2 extraction during DP has been shown, at least in part, to be due to a possible mechanical hindrance caused by high pole force generation and high arm muscle activity (~96% MVC) that hinders further elevation of O2 extraction (Stöggl et al. 2013). The current study supports this finding, based on the reduction in arm O2 extraction despite increased pole forces and upper body muscle activation when comparing DP with DIA.
Another explanation for the attenuated arm O2 extraction during DP could be the decrease in total activated muscle mass, as reflected by the increased sum IEMG and AEMG values during DIA compared to DP. Mortensen et al. (2008) demonstrated that when activating a larger muscle mass (two-legged supra maximal cycling compared with one-legged exercise), locomotor skeletal muscle perfusion leveled off with a concomitant larger increase in O2 extraction. Furthermore, comparisons between arm cycling vs. combined arm and leg cycling shows a decrease in arm blood flow when adding leg to arm exercise but at the same time increased O2 extraction in the arms (Volianitis and Secher 2002). More specifically, in a study by Calbet et al. (2004) arm blood flow was substantially higher using DP than DIA although at a similar cardiac output. Therefore, in the current study high-intensity DIA likely induce additional central cardiovascular stress due to an increased activated total muscle mass than DP – as demonstrated by the augmented sum IEMG and AMEG values - and thus induces a higher O2 extraction in both arms and legs to match the increased O2 demand.
Relationships between biomechanical variables and O2 extraction
Skiers who unloaded their legs to a greater extent (leg force minima) at the end of the gliding phase prior to leg push-off during DIA and prior to pole plant during DP extracted more O2 in the legs. The last finding is in accordance with a previous study from our group were leg force minima was related to both O2 extraction in the legs and arms at 90% and 70% of VO2peak during DP (Stöggl et al. 2013). Furthermore, this result fits nicely with the previous demonstration that modern DP involves more dynamic use of the lower body: a higher body position prior to pole plant; greater extension of the knee, hip and ankle joints; and distinct knee and hip flexion during the poling phase (Holmberg et al. 2005). In addition Stöggl et al. (2010) demonstrated that faster skiers had greater center of mass oscillation in vertical direction during DP and DIA being coupled with a more active and dynamic skiing technique. The aspect about coupling between greater unloading of the legs prior to the push-off phase during DIA and leg O2 extraction is novel. Therefore, more technically skilled skiers with more active and dynamic technique achieve a better O2 extraction.
Arm O2 extraction was negatively associated to cycle rate and positively related to cycle length and arm recovery time during DP, being in line with our previous findings during DP (Stöggl et al. 2013). This association between the decreased arm O2 extraction and cycle rate during DP might be influenced by coupling to limb blood flow. Accordingly, an increased cycle rate might increase the centrifugal force that amplifies the arm blood flow as shown in another study using arm exercise at a similar cycle rate as in the current study (0.75 Hz) (Sheriff et al. 2009). There is further support that an increased contraction frequency (in our case cycle rate) induces a greater muscle blood flow (Ferguson et al. 2001; Sheriff 2003). Possibly the increased cycle rate could induce a higher arterial pressure that increase or maintains a high blood flow although usually such a high muscle activity shown in the current study restricts blood flow (70–100% of MVC). For the legs on the other hand no relationship was noticed between cycle rate and leg O2 extraction which is in accordance with previous findings using isolated leg exercise (Ferguson et al. 2001).
Excess VO2, muscle activation and lactate
It is suggested that it is not possible to perform a constant work rate at higher intensities that refers to a specific percent of VO2max (Whipp 1994). One of the mechanisms proposed for this VO2 slow component is the magnitude and the time course of the increase in blood lactate, i.e. above the lactate threshold (Burnley and Jones 2007). In contrast to that, in the current study no changes in VO2 were found between DIA1 and DIA2 even though blood lactate steadily increased throughout the protocol (arterial: 4.6 to 7.9 mmol∙l−1). Comparable results were found in the study of Björklund et al. (2011) also demonstrating no increase in VO2 in world class cross-country skiers, when utilizing a variable intensity protocol containing 3 minutes exercise intensity of 90% of VO2max interspersed with 6 minutes of 70% of VO2max with a total duration of 24 min. One reason for the absence of a raise in VO2 from DIA1 to DIA2 in the current study might be due to the large amount of activated muscle mass and also already high O2 extraction (~90%) with DIA and DP making the potential for a further increase in VO2 diminutive.
Respiration locomotion coordination
While the exercise intensity was similar between the two DIA bouts there was a shift in breathing pattern. First, VE increased for the second DIA due to an increase in Bf (47 vs. 53 breaths · min−1) while no change in tidal volume. This is in accordance with previously shown data using DIA although at a lower exercise intensity (~76% VO2max) (Holmberg and Calbet 2007). Second, during the first DIA the Bf was similar as cycle rate (approximately 0.79 Hz), but at the second DIA workload there was a tachypneic shift, due to the elevated VE caused by an increased Bf with a mean ratio of 1.13:1, (range 1.:1 – 1.4:1) (Bf 0.88 Hz vs. cycle rate 0.78 Hz). The cause for this asynchronic Bf to cycle rate ratio during DIA2 could be explained by the increased metabolic acidosis as the arterial blood lactate concentration went from 4.6 to 7.9 mmol · l−1 and the pH dropped from 7.37 to 7.31 between DIA1 to DIA2. However the skiers PaCO2 remained fairly stable and did not show signs of hypercapnia, nor was there a relation between the magnitude of asynchronic breathing and PaCO2. Further, even though Bf increased between DIA1 and DIA2 the Bf remained unchanged between DP and DIA2 although the latter of the two was performed with a lower cycle rate. The coordination between respiration – locomotion was therefore not fixed for DIA. However the breathing pattern in the current study confirms that during the single bout of DP there was a tight coupling between cycle rate and Bf, i.e. entrainment, with a Bf of 52 breaths · min−1 equaling 0.87 Hz being similar to the cycle rate in DP (1:1). This 1:1 coupling could be exercise intensity dependent as shown previously during DP using a fixed cycle rate of 60 Hz were Bf and cycle rate was uncoordinated for lower speeds (12–18 km · h−1) while attaining a 1:1 ratio during 24 km · h−1 (Lindinger and Holmberg 2011).
Limitations
Due to the lack of blood flow measurements it cannot be definitively concluded whether the differences in O2 extraction between the arms and legs and between DP and DIA are due to differences in blood flow and/or metabolic demands. Further arterial blood pressure measurements could add information regarding the effect of arm swing and cycle rate influence of both O2 extraction and blood flow. This link was especially pronounced during mainly upper-body work, i.e. DP in the current study.
Conclusions
In well trained skiers, DP does not influence DIA VO2, at a simulated race intensity of ~90% of VO2max/peak. Furthermore, although high-intensity DP induce substantially higher arm muscle activation concurrent with a three-fold increase in pole force compared to DIA, it does not affect the redistribution of pole and foot forces, cycle characteristics and cardiorespiratory characteristics during the following DIA bout. This suggests that well trained skiers withstand the upper-body strain that high-intensity DP implies. The absence of an increase in VO2 from DIA1 to DIA2 despite a distinct increase of blood lactate questions the relation between blood lactate and excess VO2, and also strengthens the idea that VO2 slow component might not occur in well-trained athletes when using exercise modes where both legs and arms are at their upper limit of O2 extraction. Furthermore, the increase in blood lactate with no change in VO2 and measured biomechanical variables questions that lactate sampling from either leg or arm is an adequate method to establish arm or leg muscle activation during cross-country skiing. Furthermore, arm O2 extraction decreased during DP compared to DIA although both an increased pole force and muscle activation, pointing towards mechanical hindrance of blood flow during DP. Finally a greater ratio of upper- to lower-body muscle activation was associated with an attenuated arm O2 extraction in DIA, while increased cycle length, paralleled with increased recovery time and more active and dynamic lower body use increases O2 extraction especially in DP.
Acknowledgements
The authors thank the athletes and the trainers involved in this study for their participation, enthusiasm, and cooperation. This investigation was financially supported by the Swedish National Centre of Research in Sports (CIF).
Abbreviations
- AEMG
Average electromyography
- CaO2
O2 content of arterial blood
- CfvO2
Oxygen content of venous femoral blood
- CsvO2
Oxygen content of subclavian femoral blood
- DIA
Diagonal stride
- DIALIN
Workload required achieving 90% of VO2max during DIA
- DP
Double poling
- DPLIN
Workload required achieving 90% of VO2peak during DP
- EMGRMS
Root mean square electromyography
- HR
Heart rate
- HRmax
Maximal heart rate
- IEMG
Integrated electromyography
- MVC
Maximal voluntary contraction
- OBLA
Onset of blood lactate accumulation
- SO2
Oxygen saturation
- VCO2
Carbone dioxide production
- VE
Minute ventilation
- VO2
Oxygen uptake
- VO2max
Maximal oxygen uptake
- VO2peak
Peak oxygen uptake
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GB and HH participated in the critical conception and design of the work; GB and TS performed the acquisition of data and analysis (physiological and biomechanical data respectively); GB, HH and TS performed the interpretation of data, drafting the work and revising it critically for important intellectual content and final approval of the version to be published and agreement to be accountable for all aspects of the work.
Contributor Information
Glenn Björklund, Email: glenn.bjorklund@miun.se.
Hans-Christer Holmberg, Email: hc.holmberg@miun.se.
Thomas Stöggl, Email: thomas.stoeggl@sbg.ac.at.
References
- Acierno SP, Baratta RV, Solomonow M. A practical guide to electromyography for biomechansists. New Orleans: Louisiana State University; 1995. [Google Scholar]
- Ahlborg G, Jensen-Urstad M. Metabolism in exercising arm vs. leg muscle. Clin Physiol. 1991;11(5):459–468. doi: 10.1111/j.1475-097X.1991.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Björklund G, Pettersson S, Schagatay E. Performance predicting factors in prolonged exhausting exercise of varying intensity. Eur J Appl Physiol. 2007;99(4):423–429. doi: 10.1007/s00421-006-0352-0. [DOI] [PubMed] [Google Scholar]
- Björklund G, Stöggl T, Holmberg HC. Biomechanically influenced differences in O2 extraction in diagonal skiing: arm versus leg. Med Sci Sports Exerc. 2010;42(10):1899–1908. doi: 10.1249/MSS.0b013e3181da4339. [DOI] [PubMed] [Google Scholar]
- Björklund G, Laaksonen MS, Holmberg HC. Blood lactate recovery and respiratory responses during diagonal skiing of variable intensity. Eur J Sport Sci. 2011;11(5):317–326. doi: 10.1080/17461391.2010.521580. [DOI] [Google Scholar]
- Bonacci J, Green D, Saunders PU, Blanch P, Franettovich M, Chapman AR, Vicenzino B. Change in running kinematics after cycling are related to alterations in running economy in triathletes. J Sci Med Sport. 2010;13(4):460–464. doi: 10.1016/j.jsams.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Bonacci J, Saunders PU, Alexander M, Blanch P, Vicenzino B. Neuromuscular control and running economy is preserved in elite international triathletes after cycling. Sports Biomech. 2011;10(1):59–71. doi: 10.1080/14763141.2010.547593. [DOI] [PubMed] [Google Scholar]
- Burnley M, Jones AM. Oxygen uptake kinetics as a determinant of sports performance. Eur J Sport Sci. 2007;7(2):63–79. doi: 10.1080/17461390701456148. [DOI] [Google Scholar]
- Calbet JA, Jensen-Urstad M, van Hall G, Holmberg HC, Rosdahl H, Saltin B. Maximal muscular vascular conductances during whole body upright exercise in humans. J Physiol. 2004;558(Pt 1):319–331. doi: 10.1113/jphysiol.2003.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Holmberg HC, Rosdahl H, van Hall G, Jensen-Urstad M, Saltin B. Why do arms extract less oxygen than legs during exercise? Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1448–R1458. doi: 10.1152/ajpregu.00824.2004. [DOI] [PubMed] [Google Scholar]
- Ferguson RA, Ball D, Krustrup P, Aagaard P, Kjaer M, Sargeant AJ, Hellsten Y, Bangsbo J. Muscle oxygen uptake and energy turnover during dynamic exercise at different contraction frequencies in humans. J Physiol. 2001;536(Pt 1):261–271. doi: 10.1111/j.1469-7793.2001.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Merletti R, Stegeman DF, Blok JH, Rau G, Disselhorst-Klug C, Hägg G. European recommendations for surface electromyography. results of the SENIAM project. Enschede: Roessingh research and development; 1999. [Google Scholar]
- Holmberg HC, Calbet JA. Insufficient ventilation as a cause of impaired pulmonary gas exchange during submaximal exercise. Respir Physiol Neurobiol. 2007;157(2–3):348–359. doi: 10.1016/j.resp.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Holmberg HC, Lindinger S, Stöggl T, Eitzlmair E, Müller E. Biomechanical analysis of double poling in elite cross-country skiers. Med Sci Sports Exerc. 2005;37(5):807–818. doi: 10.1249/01.MSS.0000162615.47763.C8. [DOI] [PubMed] [Google Scholar]
- Holmberg HC, Lindinger S, Stöggl T, Björklund G, Müller E. Contribution of the legs to double-poling performance in elite cross-country skiers. Med Sci Sports Exerc. 2006;38(10):1853–1860. doi: 10.1249/01.mss.0000230121.83641.d1. [DOI] [PubMed] [Google Scholar]
- Ivy JL, Withers RT, Van Handel PJ, Elger DH, Costill DL. Muscle respiratory capacity and fiber type as determinants of the lactate threshold. J Appl Physiol. 1980;48(3):523–527. doi: 10.1152/jappl.1980.48.3.523. [DOI] [PubMed] [Google Scholar]
- Larsson P, Henriksson-Larsen K. Combined metabolic gas analyser and dGPS analysis of performance in cross-country skiing. J Sports Sci. 2005;23(8):861–870. doi: 10.1080/02640410400022078. [DOI] [PubMed] [Google Scholar]
- Lindinger SJ, Holmberg HC. How do elite cross-country skiers adapt to different double poling frequencies at low to high speeds? Eur J Appl Physiol. 2011;111(6):1103–1119. doi: 10.1007/s00421-010-1736-8. [DOI] [PubMed] [Google Scholar]
- Lindinger SJ, Göpfert C, Stöggl T, Müller E, Holmberg HC. Biomechanical pole and leg characteristics during uphill diagonal roller skiing. Sports Biomech. 2009;8(4):318–333. doi: 10.1080/14763140903414417. [DOI] [PubMed] [Google Scholar]
- Mognoni P, Rossi G, Gastaldelli F, Canclini A, Cotelli F. Heart rate profiles and energy cost of locomotion during cross-country skiing races. Eur J Appl Physiol. 2001;85(1–2):62–67. doi: 10.1007/s004210100432. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Damsgaard R, Dawson EA, Secher NH, Gonzalez-Alonso J. Restrictions in systemic and locomotor skeletal muscle perfusion, oxygen supply and VO2 during high-intensity whole-body exercise in humans. J Physiol. 2008;586(10):2621–2635. doi: 10.1113/jphysiol.2007.149401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mygind E, Andersen LB, Rasmussen B. Blood lactate and respiratory variables in elite cross-country skiing at racing speeds. Scand J Med Sci Sports. 1994;4(4):243–251. doi: 10.1111/j.1600-0838.1994.tb00435.x. [DOI] [Google Scholar]
- Ortenblad N, Nielsen J, Saltin B, Holmberg HC. Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J Physiol. 2011;589(Pt 3):711–725. doi: 10.1113/jphysiol.2010.195982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rud B, Secher NH, Nilsson J, Smith G, Hallen J. Metabolic and mechanical involvement of arms and legs in simulated double pole skiing. Scand J Med Sci Sports. 2014;24(6):913–919. doi: 10.1111/sms.12133. [DOI] [PubMed] [Google Scholar]
- Sheriff DD. Muscle pump function during locomotion: mechanical coupling of stride frequency and muscle blood flow. Am J Physiol Heart Circ Physiol. 2003;284(6 53–6):H2185–H2191. doi: 10.1152/ajpheart.01133.2002. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Mullin TM, Wong BJ, Ladouceur M. Does limb angular motion raise limb arterial pressure? Acta Physiol. 2009;195(3):367–374. doi: 10.1111/j.1748-1716.2008.01912.x. [DOI] [PubMed] [Google Scholar]
- Sjödin B, Jacobs I. Onset of blood lactate accumulation and marathon running performance. Int J Sports Med. 1981;2(1):23–26. doi: 10.1055/s-2008-1034579. [DOI] [PubMed] [Google Scholar]
- Stöggl T, Enqvist J, Müller E, Holmberg HC. Relationships between body composition, body dimensions, and peak speed in cross-country sprint skiing. J Sports Sci. 2010;28(2):161–169. doi: 10.1080/02640410903414160. [DOI] [PubMed] [Google Scholar]
- Stöggl T, Björklund G, Holmberg HC. Biomechanical determinants of oxygen extraction during cross-country skiing. Scand J Med Sci Sports. 2013;23(1):e9–e20. doi: 10.1111/sms.12004. [DOI] [PubMed] [Google Scholar]
- Van Hall G, Jensen-Urstad M, Rosdahl H, Holmberg HC, Saltin B, Calbet JA. Leg and arm lactate and substrate kinetics during exercise. Am J Physiol Endocrinol Metab. 2003;284(1):E193–E205. doi: 10.1152/ajpendo.00273.2002. [DOI] [PubMed] [Google Scholar]
- Volianitis S, Secher NH. Arm blood flow and metabolism during arm and combined arm and leg exercise in humans. J Physiol. 2002;544(Pt 3):977–984. doi: 10.1113/jphysiol.2002.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welde B, Evertsen F, Von Heimburg E, Ingulf Medbo J. Energy cost of free technique and classical cross-country skiing at racing speeds. Med Sci Sports Exerc. 2003;35(5):818–825. doi: 10.1249/01.MSS.0000064936.04725.FD. [DOI] [PubMed] [Google Scholar]
- Whipp BJ. The slow component of O2 uptake kinetics during heavy exercise. Med Sci Sports Exerc. 1994;26(11):1319–1326. doi: 10.1249/00005768-199411000-00005. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and motor control of human movement. 2. New York: John Wiley & Sons; 1990. [Google Scholar]
- Zory R, Vuillerme N, Pellegrini B, Schena F, Rouard A. Effect of fatigue on double pole kinematics in sprint cross-country skiing. Hum Mov Sci. 2009;28(1):85–98. doi: 10.1016/j.humov.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Zory R, Molinari F, Knaflitz M, Schena F, Rouard A. Muscle fatigue during cross country sprint assessed by activation patterns and electromyographic signals time-frequency analysis. Scand J Med Sci Sports. 2011;21(6):783–790. doi: 10.1111/j.1600-0838.2010.01124.x. [DOI] [PubMed] [Google Scholar]


