Abstract
The basic connectivity from the vestibular labyrinth to the eye muscles (vestibular ocular reflex, VOR) has been elucidated in the past decade, and we summarise this in graphic format. We also review the concept of ‘velocity storage', a brainstem integrator that prolongs vestibular responses. Finally, we present new discoveries of how complex visual stimuli, such as binocular rivalry, influence VOR processing. In contrast to the basic brainstem circuits, cortical vestibular circuits are far from being understood, but parietal-vestibular nuclei projections are likely to be involved.
Introduction
In this contribution we will discuss a few clinical and scientific topics of potential interest relating to the present understanding of the vestibular ocular reflex (VOR). The vestibular system is connected to virtually all levels of the CNS. Simple introspection of your own reactions to spinning round or clinical observation of a patient with an acute vestibular episode will let you conclude that the vestibular system is connected to (1) the eye muscles (responsible for the nystagmus), (2) the spinal cord (responsible for the lateropulsion observed), (3) autonomic centres (responsible for the nausea), and (4) to the cortex (which mediates the conscious perception of turning and vertigo). Finally, the cerebellum, in particular the flocculo-nodular portion known as archi-cerebellum, is in overall modulatory control of these multisegmental vestibular projections.1
Of all the vestibular connections, the vestibular ocular is the best known and this is good news for clinicians given that the standard way to investigate patients with visuo-vestibular symptoms is via the VOR. The VOR, has a well-defined function—to maintain gaze stability and hence preserve visual acuity during head movements. It does so by generating slow-phase eye movements in exactly the opposite direction and of almost equal velocity to the head movement. Ophthalmologists are aware that apart from a variable degree of imbalance, the typical presentation in patients with bilateral vestibular failure is oscillopsia. As dictated by the physiology of the VOR, the oscillopsia in patients with bilateral vestibular failure presents only during head and body movements such as, walking, running, or driving on a bumpy road. Further details and the differential diagnosis of oscillopsia can be found in the article vision and vertigo.2
Basic clinical anatomy of the VOR
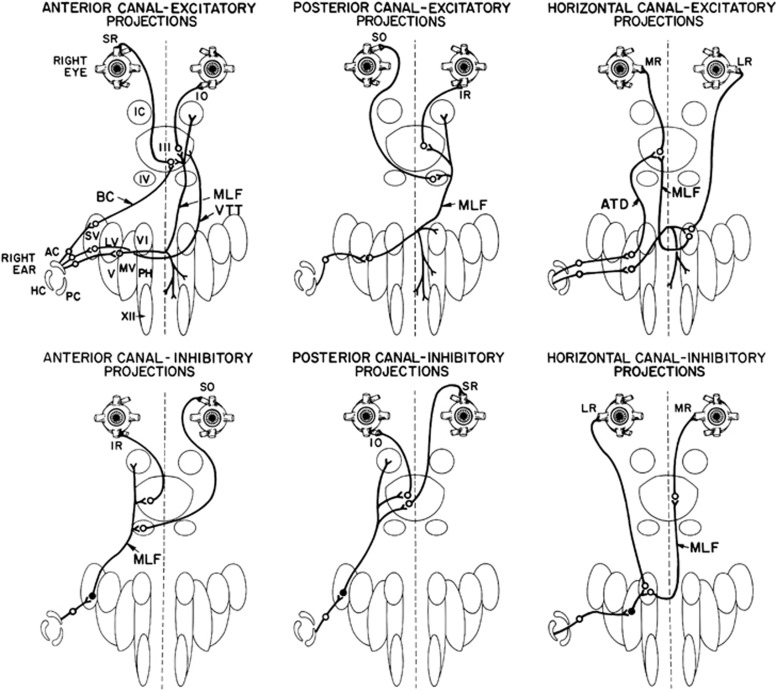
The basic neural connectivity of the VOR is provided by the ‘3-neuron arc', namely, a primary sensory afferent neuron whose body sits in the Scarpa's ganglion (the vestibular nerve), a vestibular nucleus neuron in the ponto-medullary region, and an oculomotor neuron in the III, IV, or VI nuclei in the brainstem. As the vestibular labyrinth consists of three semicircular canals (angular acceleration) and two otolith organs (utricle and sacculus, linear and gravitational acceleration sensors) the best way of describing the complete 3-neuron arc connectivity is by schematic figures, as shown in Figure 1. The general organisation comprises the vestibular nuclei on the same side of the labyrinth sending excitatory projections to the oculomotor nuclei on the opposite side and inhibitory projections to antagonistic oculomotor neurons on the same side of the brainstem. This scheme, easy to remember for the horizontal VOR (eg, contralateral abducens nucleus neurons and ipsilateral medial rectus motoneurons), is also preserved for vertically acting muscles (see Figure 1).
Figure 1.
Summary of the direct connections of the semicircular canals to the oculomotor neurons and muscles (from Leigh and Zee1 with permission). Each canal has excitatory projections (open circles) to the acting motoneurons and inhibitory projections (filled up circles) to the antagonistic motoneurons. Abbreviations: AC/PC/HC, anterior/posterior/horizontal canal; SR/MR/IR/LR, superior/medial/inferior/lateral rectus; SO/IO, superior/inferior oblique; III/IV/VI, third/fourth/sixth cranial nerve nucleus; IC, interstitial nucleus of Cajal; VTT, ventral tegmental tract; MLF, medial longitudinal fasciculus; ATD, ascending tract of Deiters; SV/LV/MV/V, superior/lateral/medial/inferior vestibular nucleus; BC, brachium conjunctivum; XII, hypoglossal nucleus; PH, prepositus hypoglossal nucleus.
Up to a few years ago, the only way to assess the VOR was with rotating chairs or by caloric ear stimulation. Recently, advances in understanding of vestibulo-ocular physiology, largely by Curthoys and Halmagyi in Sydney, have led to the development of, first, a bed-side clinical head thrust or impulse test (HIT)3 and subsequently video–image-based versions of the test that are now available commercially for clinical use (vHIT, or videoHIT).4
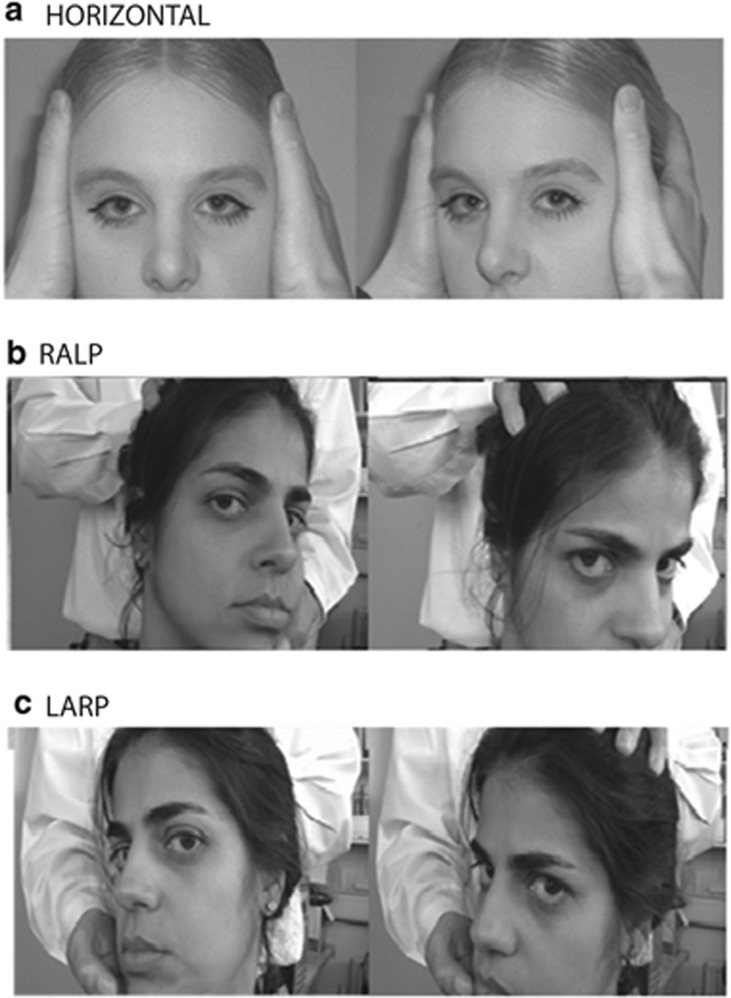
The principle of this procedure is that the VOR is capable of generating slow-phase eye movements of high velocity (to preserve gaze stability) in response to high acceleration head movements or impulses. Figure 2a shows how to conduct this test in the clinic. For examination of the horizontal semicircular canals (and accordingly the lateral and medial recti eye muscles) the head impulses are delivered in the horizontal (yaw) plane. Figure 2b also illustrates how to deliver oblique head acceleration impulses in the clinic so that right anterior–left posterior canals (hence the right superior rectus and the left inferior oblique muscles are activated during a nose-down oblique VOR). In vestibular jargon, this plane is called RALP and the plane of action of the left anterior–right posterior canals is called LARP (which recruits the left superior rectus and right inferior oblique during a nose-down VOR, see Figure 2c).
Figure 2.
The HIT. (a). (Top picture) the clinician instructs the patient to fixate a target straight ahead and then turns the head rapidly either to the left or right. A right head turn as shown tests the right horizontal canal. The normal response results in a slow-phase compensatory eye movement to the left, which occurs almost instantaneously (modified from, Bronstein and Lampert28 with permission). In (b) (middle picture), the clinician turns the patients head to the left by ∼30°, to align the right anterior and left posterior (RALP) semicircular canals along a vertical axis. The clinician then places one hand on the top of the patient's head and one hand below and rapidly thrusts the head either forwards (testing right anterior canal) or backwards (testing left posterior canal). In (c) (bottom picture), the clinician asks the patient to turn their head to the right by ∼30°, to align the left anterior and right posterior (LARP) semicircular canals along a vertical axis. The clinician then places one hand on top of the patient's head and one hand below and rapidly thrusts the head either forwards (testing left anterior canal) or backwards (testing right posterior canal).
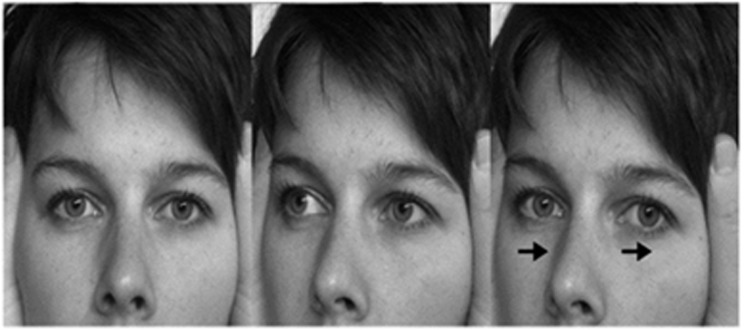
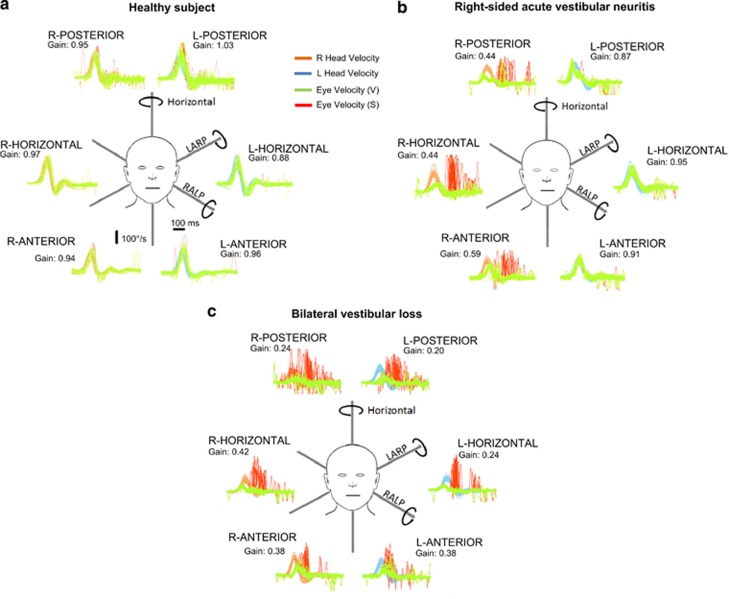
If the VOR is working normally, high acceleration impulses to the head are perfectly counteracted by compensatory slow-phase movements. Thus, fixation of the visual target (eg, the examiner's nose) is seamlessly preserved. However, if the VOR is substantially reduced (>50%) slow-phase vestibular eye movements are incapable of preserving fixation and therefore a small ‘catch-up' saccade is generated towards the visual target. With minimal practice, ophthalmologists are able to see the catch-up saccade by naked eye and hence establish that individual semicircular canals are dysfunctional. Figure 3 shows a photographic representation of a positive (ie, abnormal) head impulse test and Figure 4 shows two examples of abnormalities in the vHIT following either unilateral or bilateral loss of vestibular function.
Figure 3.
Head impulse test of the right horizontal canal in a simulated patient with loss of vestibular function on the right. Here, the eyes first move in the direction of the head turn (owing to absent VOR) before a corrective refixating saccade occurs in the opposite direction (from Bronstein and Lempert28 with permission).
Figure 4.
The plots represent time series showing superimposed recordings of the head velocity stimulus (brown for right canals and blue for left canals) and the slow-phase eye-velocity responses (eye velocity VOR—green traces) to 20 randomised (either direction) head turns, to sequentially test all the semicircular canals in turn in (a) a healthy subject, (b) a patient with right-sided acute vestibular neuritis and (c) a patient with bilateral vestibular loss. Overt or covert saccades are shown as red traces, and can be seen when testing the side of the lesion in the vestibular neuritis patient (ie, right) and when testing both the right and left sides of the patient with bilateral vestibular failure. Note that in this figure the eye velocity has been inverted to allow for a comparison with head velocity, and for illustration purposes only both leftward and rightward head movements are shown as positive. The average VOR gain value (eye velocity/head velocity) is shown next to each response.
The capacity of the HIT to detect substantial functional reduction of the semicircular canals, particularly the horizontal canal as this is the easiest to do, has given this procedure a prominent role in the diagnosis of acute vertigo in emergency rooms and stroke units. If a patient presents with acute vertigo and nystagmus, even in the absence of additional neurological features such as speech disorder, diplopia, or face numbness, the presence of a normal (negative) HIT raises a ‘red flag'. The normal HIT suggests that the vertigo may not be of peripheral labyrinthine origin and, therefore, an urgent MRI scan is warranted. Other ocular-motor signs are also becoming increasingly popular to help in the important distinction between acute peripheral vs central (‘stroke') origin of vertigo. In addition to the HIT (if positive, more likely peripheral), this includes testing for skew deviation (if present, more likely central) and whether the nystagmus is unidirectional (more likely vestibular) or not (more likely central). This forms the basis of the ‘HINTS' protocol (Head-impulse, Nystagmus, Test-skew) which has at least as much sensitivity to rule out posterior fossa stroke as MRI does.5, 6
Additional brainstem VOR mechanisms
The VOR is under powerful cerebellar control and this is reviewed by Zee in this issue. Indeed many ‘cerebellar' eye signs are actually vestibulo-cerebellar signs, including the common syndrome of downbeat nystagmus.7, 8 The cerebellum and interconnected brainstem nuclei, prominently the perihypoglosal nuclei in the medulla (Figure 1), are also part of a polysynaptic vestibular ocular network generally termed the ‘velocity storage system'. Essentially, this ‘velocity storage' integrator extends the duration of the horizontal vestibular ocular response, which allows for a better compensatory VOR response to rotational stimuli of low frequency (long duration). This function is exemplified by the fact that the time constant of decay (TC) following a post-rotational stimulus is 4–7 s but the TC of vestibular nystagmus is approximately 15–18 s.9, 10 It is in these additional cerebellar and brainstem circuits that a profound interaction between vestibular ocular-motor and visual-ocular-motor mechanisms, such as optokinetic nystagmic responses, takes place.
Visual input has profound influences on vestibular function. Up until recently, however, it was difficult to assess this interaction in patients with visual-motor disorders because the loss of visual input (blindness), excessive nystagmus, or reduced eye movements (ophthalmoplegia) interfered with obtaining reliable eye movement recordings, as required to measure the VOR. On the basis of the assumption that VOR and vestibulo-perceptual function (presumably mediated by the vestibulo-cortical projection) must be matched in normal man, we developed a technique to measure vestibular perception (Figure 5). Given that the latter technique does not rely on eye movement recordings we investigated vestibular function in three groups, patients with congenital nystagmus,9 severely reduced eye movements (chronic progressive external ophthalmoplegia,11) and congenital blindness who have no structured eye movements.12 On the basis of the measurement of the TC of the vestibulo-perceptual function we observed that in these patients perceptual TCs were shorter, that is, vestibulo-perceptual function was reduced. This somewhat suppressed vestibulo-perceptual function could be expected, given that long duration vestibular responses could create additional problems of disorientation in these patients when they move about in the environment. In any case, the findings testify to the interdependence of the two major motion processing systems of the brain, vestibular and visual, but whether this interaction takes place at lower (brainstem) or higher (cortical) levels is not fully understood.
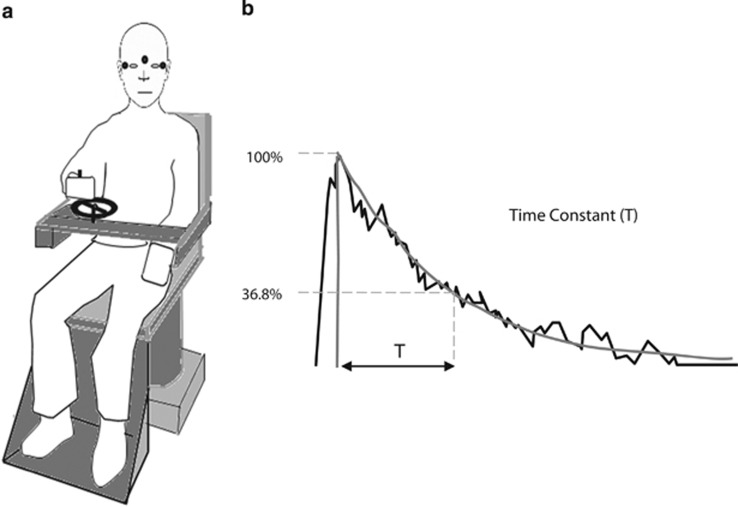
Figure 5.
Diagrammatic representation of the vestibular perception test. (a) Subject is shown holding the handle of a tachometer wheel, which the subject is instructed to turn as to indicate their subjective perception of the rotational stimuli during 90°/s chair rotation either to the right or left. EOG electrodes seen in the figure by small black circles simultaneously capture nystagmus thus allowing us to compare vestibulo-ocular and vestibulo-perceptual responses. Subjects are instructed to turn the wheel as fast as possible initially on chair acceleration or deceleration for the first couple of seconds. From then on, the subject is asked to slow down the turning of the tachometer wheel gradually to match their own perceived turning sensation and eventually stop turning the wheel at the point that they no longer feel the rotation. (b) Tachometer output in a normal subject during a rotational stopping response. This output is fitted with an exponential curve and the time constant of decay is measured (typically between 15–18 s).
Cortical processing and the VOR
Cortical modulation of the VOR has been known at least since the days of Charles Hallpike. In a series of experiments in the 1940s in London, Hallpike and colleagues provided arguably the first insights into the neurology of vertigo. In a series of patients with temporal lobe lesions, they found that the vestibular nystagmus elicited during caloric stimulation exhibited a strong directional preponderance, a phenomenon termed, ‘nystagmusberietschaft'.13
Much of the work conducted by the vestibular community since the above report has focussed primarily upon establishing the neural correlates using functional imaging techniques.14, 15, 16, 17 These data have implicated a widespread distributed network in the frontal and tempo-parietal areas that strongly overlaps with the neural networks found for spatial attention,18 with a right hemisphere dominance.19, 20
Behavioural observations in patients with posterior parietal cortical lesions provide further support for the suggested overlapping neural networks. Posterior parietal cortical lesions (particularly on the right) typically result in spatial neglect, a disorder of spatial attention.21 That is, despite having normal visual fields, patients typically tend to ignore salient visual stimuli presented to the contralateral side of space.22 Firstly, it was shown that this spatial bias can be temporally alleviated via the application of a caloric stimulus23 and secondly that these lesions result in an asymmetrical modulation of the VOR in response to velocity step rotations.21
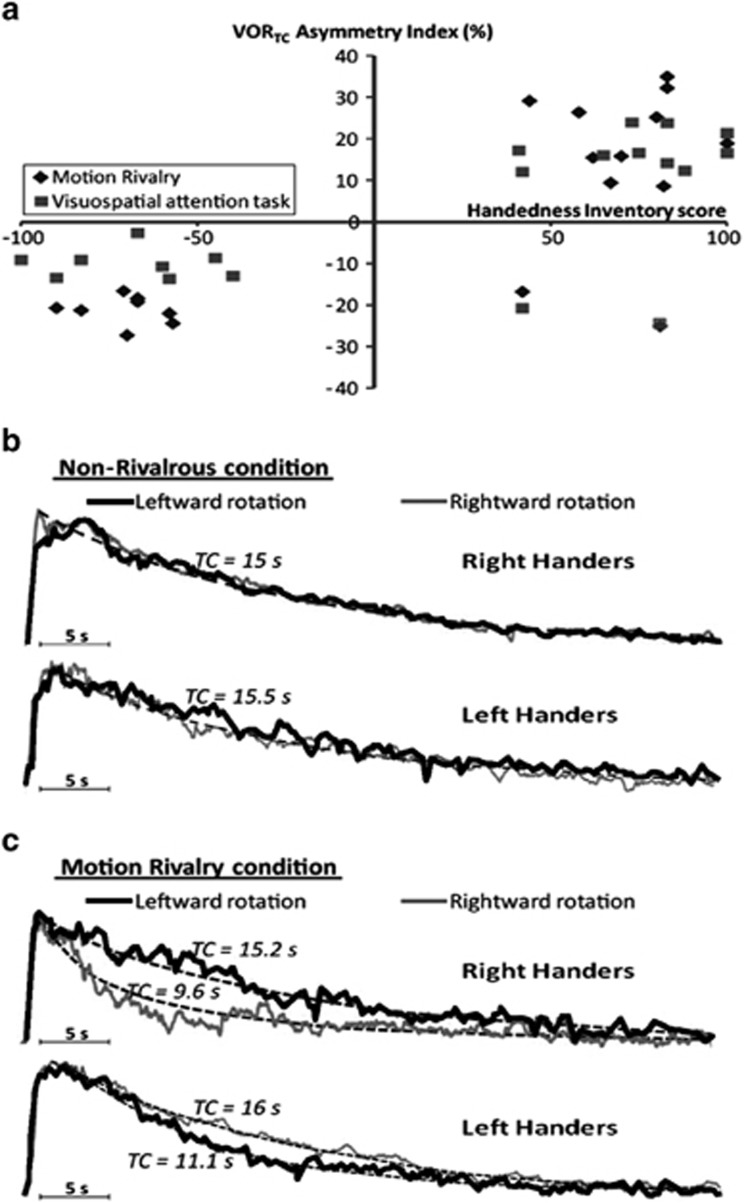
Furthermore, based on the premise that overlapping neural networks subserve spatial attention and vestibular processing, we developed a novel paradigm in normal subjects to see if this induced modulation of the VOR.24 During velocity step rotations, subjects viewed bi-stable percepts (ie, binocular rivalry or motion induced blindness), or performed complex attention tasks. Upon stopping, the VOR was measured in darkness. It was found that following concurrent vestibular activation and either viewing bi-stable percepts that contained a spatial component (ie, motion rivalry but not colour rivalry) or performance of a visuospatial working memory task, the VOR was asymmetrically supressed. Intriguingly, the direction of the modulation was determined by the subject's handedness, strongly implicating the role of higher-order mechanisms for the modulation.24 As shown in Figure 6, in right-handed subjects the VOR was supressed following rightward rotations and conversely following leftward rotations in left-handed subjects.
Figure 6.
Shows a summary for results for the effects of binocular rivalry and the visuospatial working memory tasks upon the VOR.24 (a) Plots of each subject's individual asymmetry index (ie, degree of nystagmus suppression) and handedness inventory score. Right handers showed a positive asymmetry index except for two subjects who were retrained left handers in both the motion binocular rivalry condition (diamonds) and visuospatial task (squares). In contrast, all left-handed subjects and the two retrained right-handed subjects had a negative VORTC asymmetry index. (b and c) Plots of the exponential grand averages for the eye velocity for right- and left-handed subjects. (b) When a control visual stimulus was presented the VOR was symmetrical following either rightward or leftward rotations (overlapping black and grey curves). (c) The VOR following the viewing of motion rivalry is shown. Note that in right-handed subjects the VOR was supressed following rightward rotation (grey curve), whereas in left-handed subjects the VOR was supressed following leftward rotations (black curve).
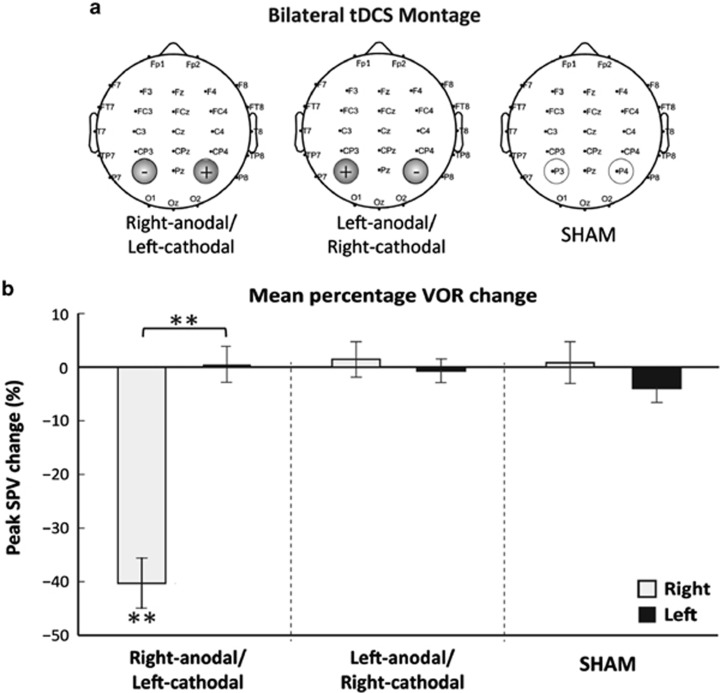
To understand the neural mechanisms of the above VOR modulation, we applied non-invasive trans-cranial direct current stimulation (tDCS) over the posterior parietal cortex to directly modulate cortical excitability. The VOR was assessed using caloric stimulation before and after tDCS. Excitation of the right hemisphere (ie, anodal stimulation) and concurrent inhibition of the left hemisphere (ie, cathodal stimulation) induced an asymmetrical VOR response with suppression of left beating nystagmus in response to right cold (30 °C) irrigations. No effect of tDCS was found for the processing of right-beating nystagmus elicited via left cold irrigations25 as shown in Figure 7. To elucidate the ‘active' electrode we applied unipolar tDCS and found that the modulation was specifically attributable to the suppression of the left hemisphere.25 Further, to ascertain whether the tDCS was impacting upon pursuit and/or VOR suppression mechanisms, that in turn might mediate VOR modulation, we performed a follow-up study and ruled out such an explanation.26 Hence, we tentatively conclude that cortical modulations of the VOR that we have reported both in behavioural and electrical stimulation studies are subject to dynamic inter-hemispheric competition. Moreover, such a mechanism can also explain the asymmetrical VOR observed following parietal lesions. Anatomically, such top-down modulation is proposed to be mediated by descending projections identified in primates connecting the parietal cortex and the vestibular nuclei.27
Figure 7.
(a) tDCS montage used during bilateral stimulation. The electrodes were placed over the right (P4) and left (P3) parietal hemispheres respectively according to the 10–20 EEG international positioning system. Three types of bilateral parietal tDCS stimulation were applied: anodal stimulation over right hemisphere, cathodal stimulation over right hemisphere, and SHAM a control condition. (b) Right-anodal stimulation resulted in asymmetrical modulation of the VOR with a significant decrease in peak slow-phase velocity (SPV) during the right caloric with no change observed during the left caloric. Neither right-cathodal stimulation nor SHAM resulted in any VOR modulation of peak SPV. Data marked ** significant at P<0.001.
Taken together, our findings in neurologically intact individuals and reports in the literature from brain damaged patients imply that the vestibular cortex is able to down-regulate the VOR and specifically influence the ‘low frequency' component of the horizontal velocity storage mechanism.25 However, much remains unknown; for example, what are the exact anatomical pathways in man, what functionality does such top-down modulation serve, and why is there such a close relationship with spatial attention mechanisms? To conclude, in contrast to the extensive and well-defined knowledge regarding low-level control of the VOR, much is unknown and remains to be elucidated regarding the higher-order mechanisms that govern central modulation of the VOR.
Acknowledgments
We thank Otometrics, UK, for the Video Head Impulse Test equipment.
The authors declare no conflict of interest.
Footnotes
Contributed at the Cambridge Ophthalmological symposium
References
- Leigh JR, Zee DS.The Neurology of Eye Movements5th ednOxford University Press: New York; 2015 [Google Scholar]
- Bronstein AM. Vision and vertigo: some visual aspects of vestibular disorders. J Neurol. 2004;251 (4:381–387. doi: 10.1007/s00415-004-0410-7. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45 (7:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- Weber KP, MacDougall HG, Halmagyi GM, Curthoys IS. Impulsive testing of semicircular-canal function using video-oculography. Ann N Y Acad Sci. 2009;1164:486–491. doi: 10.1111/j.1749-6632.2008.03730.x. [DOI] [PubMed] [Google Scholar]
- Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40 (11:3504–3510. doi: 10.1161/STROKEAHA.109.551234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber Tehrani AS, Kattah JC, Mantokoudis G, Pula JH, Nair D, Blitz A, et al. Small strokes causing severe vertigo: frequency of false-negative MRIs and nonlacunar mechanisms. Neurology. 2014;83 (2:169–173. doi: 10.1212/WNL.0000000000000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AM, Miller DH, Rudge P, Kendall BE. Down beating nystagmus: magnetic resonance imaging and neuro-otological findings. J Neurol Sci. 1987;81 (2-3:173–184. doi: 10.1016/0022-510x(87)90094-3. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Milea D. Vertical nystagmus: clinical facts and hypotheses. Brain. 2005;128 (Pt 6:1237–1246. doi: 10.1093/brain/awh532. [DOI] [PubMed] [Google Scholar]
- Okada T, Grunfeld E, Shallo-Hoffmann J, Bronstein AM. Vestibular perception of angular velocity in normal subjects and in patients with congenital nystagmus. Brain. 1999;122 (Pt 7:1293–1303. doi: 10.1093/brain/122.7.1293. [DOI] [PubMed] [Google Scholar]
- Dai M, Klein A, Cohen B, Raphan T. Model-based study of the human cupular time constant. J Vestib Res. 1999;9 (4:293–301. [PubMed] [Google Scholar]
- Grunfeld EA, Shallo-Hoffmann JA, Cassidy L, Okada T, Faldon M, Acheson JF, et al. Vestibular perception in patients with acquired ophthalmoplegia. Neurology. 2003;60 (12:1993–1995. doi: 10.1212/01.wnl.0000067992.17185.60. [DOI] [PubMed] [Google Scholar]
- Seemungal BM, Glasauer S, Gresty MA, Bronstein AM. Vestibular perception and navigation in the congenitally blind. J Neurophysiol. 2007;97 (6:4341–4356. doi: 10.1152/jn.01321.2006. [DOI] [PubMed] [Google Scholar]
- Cawthorne TE, Fitzgerald G, Hallpike CS. Studies in human vestibular function: II. observations on the directional preponderance of caloric nystagmus (‘Nystagmusbereitschaft‘) resulting from unilateral labyrinthectomy. Brain. 1942;65 (2:138–160. [Google Scholar]
- Bottini G, Karnath HO, Vallar G, Sterzi R, Frith CD, Frackowiak RS, et al. Cerebral representations for egocentric space: Functional-anatomical evidence from caloric vestibular stimulation and neck vibration. Brain. 2001;124 (Pt 6:1182–1196. doi: 10.1093/brain/124.6.1182. [DOI] [PubMed] [Google Scholar]
- Bottini G, Sterzi R, Paulesu E, Vallar G, Cappa SF, Erminio F, et al. Identification of the central vestibular projections in man: a positron emission tomography activation study. Exp Brain Res. 1994;99 (1:164–169. doi: 10.1007/BF00241421. [DOI] [PubMed] [Google Scholar]
- Brandt T, Glasauer S, Stephan T, Bense S, Yousry TA, Deutschlander A, et al. Visual-vestibular and visuovisual cortical interaction: new insights from fMRI and pet. Ann N Y Acad Sci. 2002;956:230–241. doi: 10.1111/j.1749-6632.2002.tb02822.x. [DOI] [PubMed] [Google Scholar]
- Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, et al. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. NeuroImage. 2002;17 (3:1384–1393. doi: 10.1006/nimg.2002.1241. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Dieterich M. Spatial neglect—a vestibular disorder. Brain. 2006;129 (Pt 2:293–305. doi: 10.1093/brain/awh698. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, et al. Dominance for vestibular cortical function in the non-dominant hemisphere. Cerebral Cortex. 2003;13 (9:994–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Spatial neglect and attention networks. Ann Rev Neurosci. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventre-Dominey J, Nighoghossian N, Denise P. Evidence for interacting cortical control of vestibular function and spatial representation in man. Neuropsychologia. 2003;41 (14:1884–1898. doi: 10.1016/s0028-3932(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Malhotra P, Coulthard EJ, Husain M. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain. 2009;132 (Pt 3:645–660. doi: 10.1093/brain/awn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens AB. Caloric stimulation and unilateral visual neglect. Neurology. 1985;35 (7:1019–1024. doi: 10.1212/wnl.35.7.1019. [DOI] [PubMed] [Google Scholar]
- Arshad Q, Nigmatullina Y, Bronstein AM. Handedness-related cortical modulation of the vestibular-ocular reflex. J Neurosci. 2013;33 (7:3221–3227. doi: 10.1523/JNEUROSCI.2054-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad Q, Nigmatullina Y, Roberts RE, Bhrugubanda V, Asavarut P, Bronstein AM. Left cathodal trans-cranial direct current stimulation of the parietal cortex leads to an asymmetrical modulation of the vestibular-ocular reflex. Brain Stimul. 2014;7 (1:85–91. doi: 10.1016/j.brs.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad H, Arshad Q, Siddiqui S, Nigmatullina Y, Patel M, Bronstein AM, et al. Applications of neuromodulation to explore vestibular cortical processing; new insights into the effects of direct current cortical modulation upon pursuit, VOR and VOR suppression J Vestib Res 2014. accepted. [DOI] [PubMed]
- Ventre-Dominey J. Vestibular function in the temporal and parietal cortex: distinct velocity and inertial processing pathways. Front Integr Neurosci. 2014;8:53. doi: 10.3389/fnint.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AM, Lempert T. Dizziness: A Practical Approach to Diagnosis and Management. Cambridge University Press: Cambridge; 2007. [Google Scholar]