Abstract
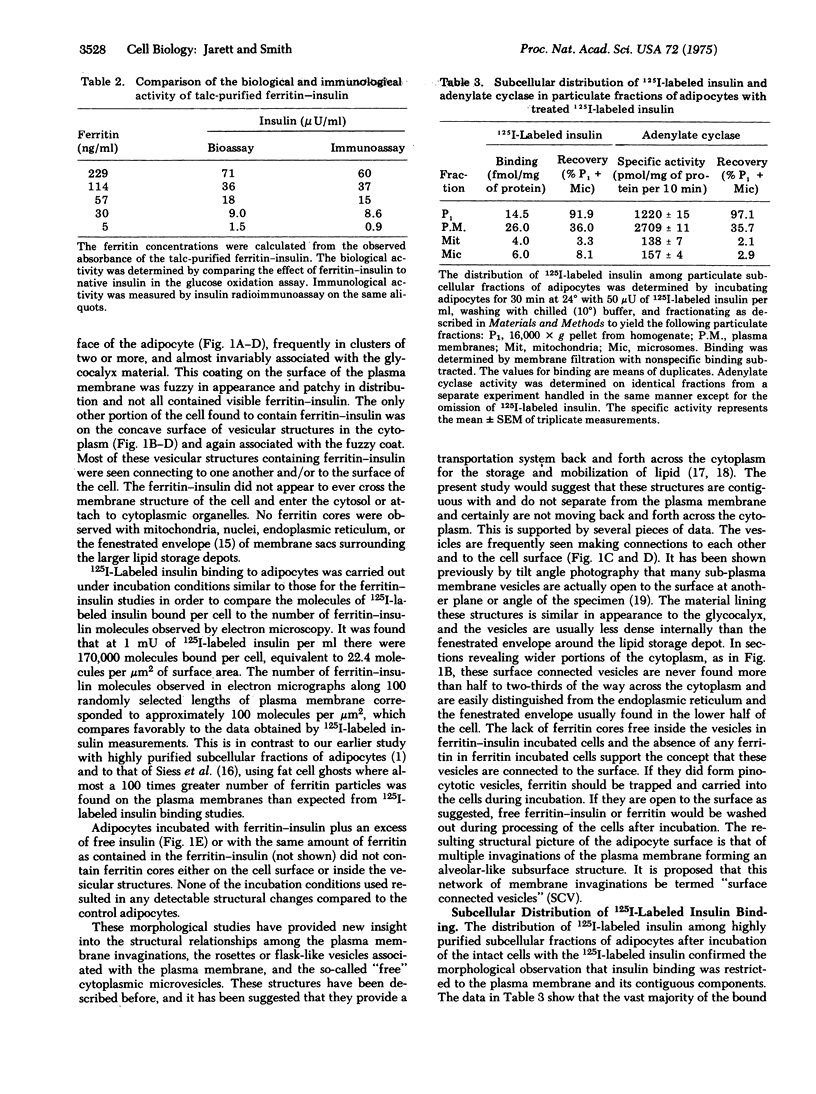
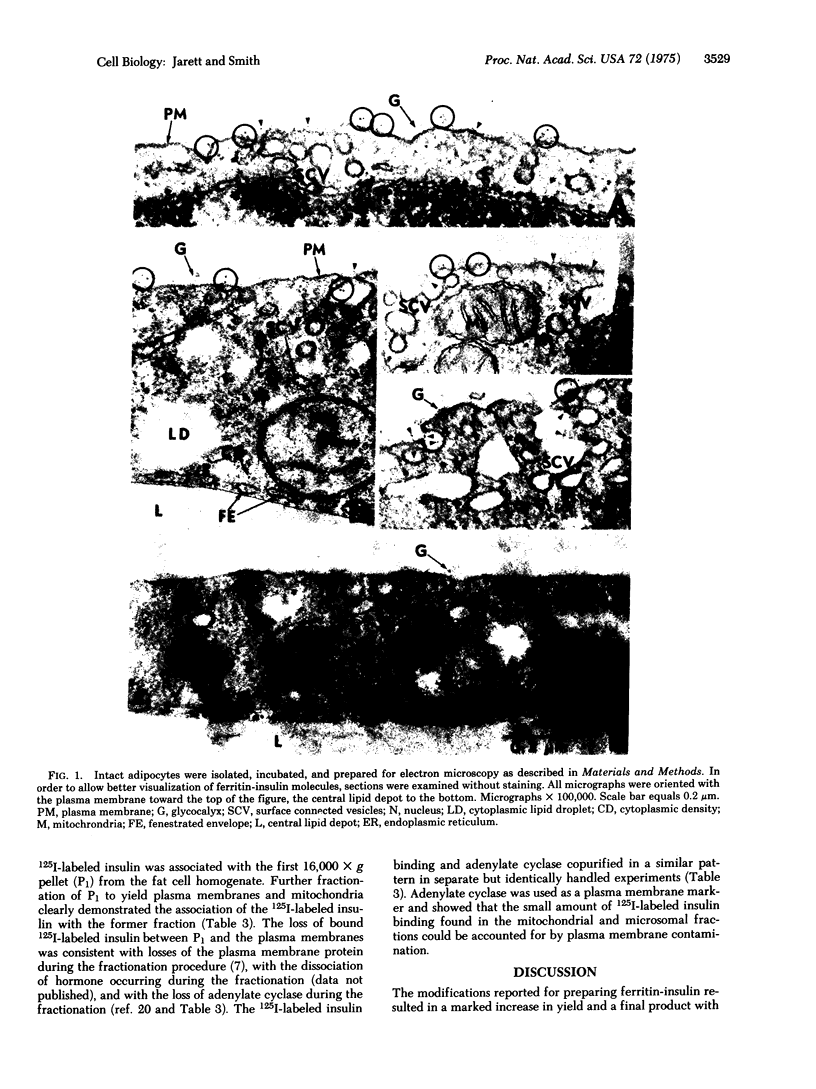
The method for preparing a stable, biologically active, covalently linked ferritin--insulin complex has been modified to provide a 25-fold increase in yield compared to the original procedure while reducing the molar fatio of ferritin to insulin to 1:1 from 40:1. Ultrastructural studies of isolated adipocytes revealed specific binding of ferritin--insulin to the cell surface in irregular clusters associated with the glycocalyx coating. The number of ferritin--insulin molecules observed was consistent with the number of sulin molecules observed was consistent with the number of receptors calculated from 125I-labeled insulin binding studies. The ferritin--insulin was not observed in the cytoplasm of the cell but was found on the convave side of surface connected vesicles. These surface connected vesicles were part of an alveolar-like system of plasma membrane invaginations which project in various directions in the cytoplasm and by thin sectioning can appear as pinocytotic-like microvesicles. The morphological observations on ferritin--insulin binding were supported by the finding that 125I-labeled insulin binding was almost exclusively localized to highly purified plasma membranes isolated by fractionation of adipocytes after incubation with 125I-labeled insulin. These data supported the theory that insulin did not need to enter a cell to cause biological effects and was consistent with the negative cooperativity concept of insulin binding to cell receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRNETT R. J., BALL E. G. Metabolic and ultrastructural changes induced in adipose tissue by insulin. J Biophys Biochem Cytol. 1960 Sep;8:83–101. doi: 10.1083/jcb.8.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard W., Avrameas S. Ultrastructural visualization of cellular carbohydrate components by means of concanavalin A. Exp Cell Res. 1971 Jan;64(1):232–236. doi: 10.1016/0014-4827(71)90217-5. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin--receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of concanavalin A and wheat germ agglutinin with the insulin receptor of fat cells and liver. J Biol Chem. 1973 May 25;248(10):3528–3534. [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor of isolated fat cell membranes. J Biol Chem. 1971 Dec 10;246(23):7265–7274. [PubMed] [Google Scholar]
- Cuatrecasas P., Tell G. P. Insulin-like activity of concanavalin A and wheat germ agglutinin--direct interactions with insulin receptors. Proc Natl Acad Sci U S A. 1973 Feb;70(2):485–489. doi: 10.1073/pnas.70.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W. Structure-function relationships in the adipose cell. I. Ultrastructure of the isolated adipose cell. J Cell Biol. 1970 Aug;46(2):326–341. doi: 10.1083/jcb.46.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrecasas P. Perturbation of the insulin receptor of isolated fat cells with proteolytic enzymes. Direct measurement of insulin-receptor interactions. J Biol Chem. 1971 Nov;246(21):6522–6531. [PubMed] [Google Scholar]
- Czech M. P., Lawrence J. C., Jr, Lynn W. S. Activation of hexose transport by concanavalin A in isolated brown fat cells. Effects of cell surface modification with neuraminidase and trypsin on lectin and insulin action. J Biol Chem. 1974 Dec 10;249(23):7499–7505. [PubMed] [Google Scholar]
- Czech M. P., Lynn W. S. Stimulation of glucose metabolism by lectins in isolated white fat cells. Biochim Biophys Acta. 1973 Feb 28;297(2):368–377. doi: 10.1016/0304-4165(73)90084-6. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- Hales C. N., Luzio J. P., Chandler J. A., Herman L. Localization of calcium in the smooth endoplasmic reticulum of rat isolated fat cells. J Cell Sci. 1974 Jun;15(1):1–15. doi: 10.1242/jcs.15.1.1. [DOI] [PubMed] [Google Scholar]
- Hammond J. M., Jarett L., Mariz I. K., Daughaday W. H. Heterogeneity of insulin receptors on fat cell membranes. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1122–1128. doi: 10.1016/0006-291x(72)90329-4. [DOI] [PubMed] [Google Scholar]
- Jarett L., McKeel D. W. The distribution and characterization of ATPase activity of isolated fat cells. Arch Biochem Biophys. 1970 Oct;140(2):362–370. doi: 10.1016/0003-9861(70)90077-9. [DOI] [PubMed] [Google Scholar]
- Jarett L., Reuter M., McKeel D. W., Smith R. M. Loss of adenyl cyclase hormone receptors during purification of fat cell plasma membranes. Endocrinology. 1971 Nov;89(5):1186–1190. doi: 10.1210/endo-89-5-1186. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. Electron microscopic demonstration of insulin receptors on adipocyte plasma membranes utilizing a ferritin-insulin conjugate. J Biol Chem. 1974 Nov 10;249(21):7024–7031. [PubMed] [Google Scholar]
- Jarett L., Smith R. M. The stimulation of adipocyte plasma membrane magnesium ion-stimulated adenosine triphosphatase by insulin and concanavalin A. J Biol Chem. 1974 Aug 25;249(16):5195–5199. [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A. Negative co-operativity in clustered receptors as a possible basis for membrane action. J Theor Biol. 1974 Apr;44(2):367–372. doi: 10.1016/0022-5193(74)90167-2. [DOI] [PubMed] [Google Scholar]
- Oseroff A. R., Robbins P. W., Burger M. M. The cell surface membrane: biochemical aspects and biophysical probes. Annu Rev Biochem. 1973;42:647–682. doi: 10.1146/annurev.bi.42.070173.003243. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Nestorescu M. L., Wieland O. H. Localization of insulin on fat cell ghosts by ferritin labelled insulin antibodies. Diabetologia. 1974 Oct;10(5):439–447. doi: 10.1007/BF01221635. [DOI] [PubMed] [Google Scholar]
- Slavin B. G. The cytophysiology of mammalian adipose cells. Int Rev Cytol. 1972;33:297–334. doi: 10.1016/s0074-7696(08)61453-9. [DOI] [PubMed] [Google Scholar]
- Soifer D., Braun T., Hechter O. Insulin and microtubules in rat adipocytes. Science. 1971 Apr 16;172(3980):269–271. doi: 10.1126/science.172.3980.269. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON J. R. ADIPOSE TISSUE. MORPHOLOGICAL CHANGES ASSOCIATED WITH LIPID MOBILIZATION. J Cell Biol. 1964 Jan;20:57–74. doi: 10.1083/jcb.20.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]



