Abstract
The sensory and motor control of human extraocular muscles (EOMs) have been subjected to considerable speculation in ophthalmic literature, often related to infranuclear structures such as the unique complement of muscle fibres and their associated sensory organs. The intrafusal fibres do not resemble their somatic counterparts and their peculiar morphology has raised questions about their proprioceptive capacity. No Golgi tendon organs have so far been observed and the myotendinous nerve endings, previously assumed to convey sensory information, have recently been argued to merely represent constituents of the efferent innervation serving the multiply innervated muscles fibres. These observations raise questions about the overall capacity to monitor the activity created by the generous efferent nerve supply observed in these muscles. Furthermore, the argued independent activity of muscular layers and compartments suggest that the required feedback must be highly structured and more specific than previously assumed. Yet, uncertainty about the source of such information remains. The purpose of this paper is to provide a short review of neuromuscular properties of human extraocular muscles. Their functional implications and the most reputable sources of proprioception will also be discussed. The promoted views are based on pertinent literature and previous research undertaken by the authors.
Introduction
Detailed knowledge of the characteristics of extraocular muscles (EOMs) is vital for understanding the sensorimotor control of eye movements, yet uncertainty associated with both afferent and efferent modalities persists. The distribution and complement of sensory organs is unique compared with conventional somatic muscles,1 and large interspecies variations have been observed.2 This has created controversy about the role of proprioception and has challenged the notion that it participates in the development of binocular vision, ocular alignment, and adaptive processes.3 Furthermore, recent observations suggest that EOMs are compartmentalised, not only into global and orbital layers,4, 5 but also into superior and inferior compartments of horizontal rectus muscles.6, 7, 8, 9 This implies a broader division of labour between muscle segments than previously assumed, which in turn should require a highly structured and specific neural feedback. Whether this feedback is provided by proprioception or other mechanisms is still unresolved. This paper aims to review current concepts of motor and sensory innervation of human EOMs, and comment on their functional implications. References will be made to pertinent literature and previous research undertaken by the authors.
Efferent innervation
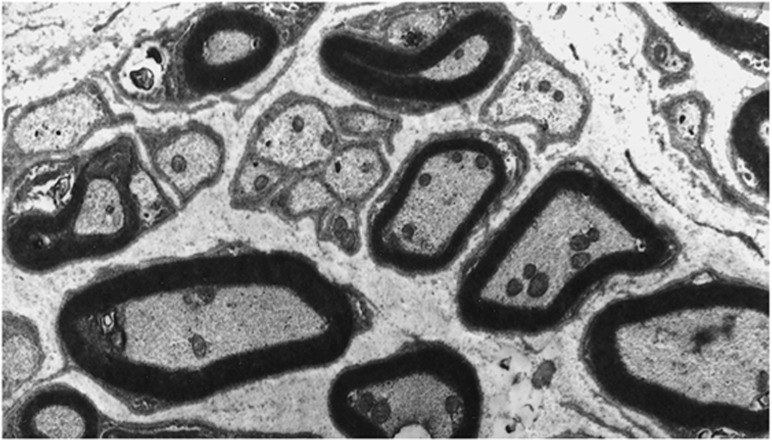
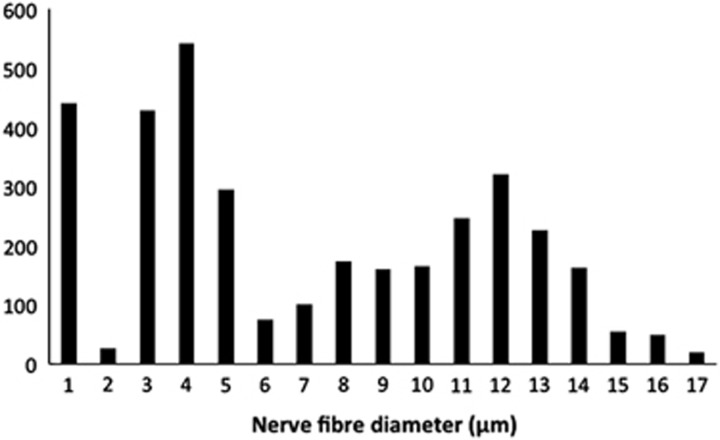
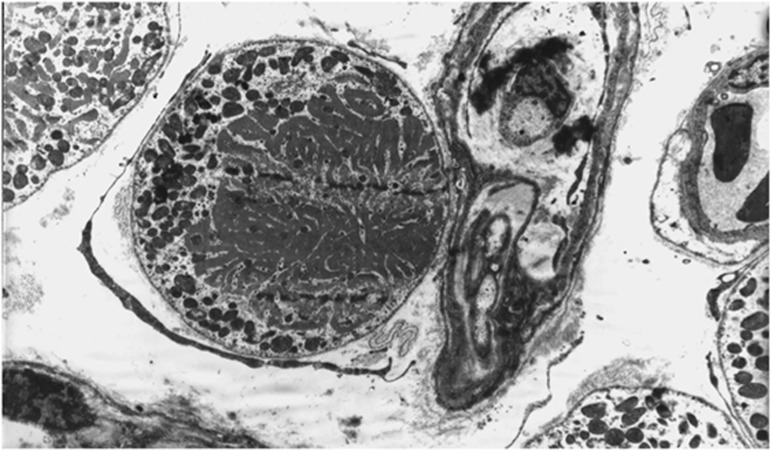
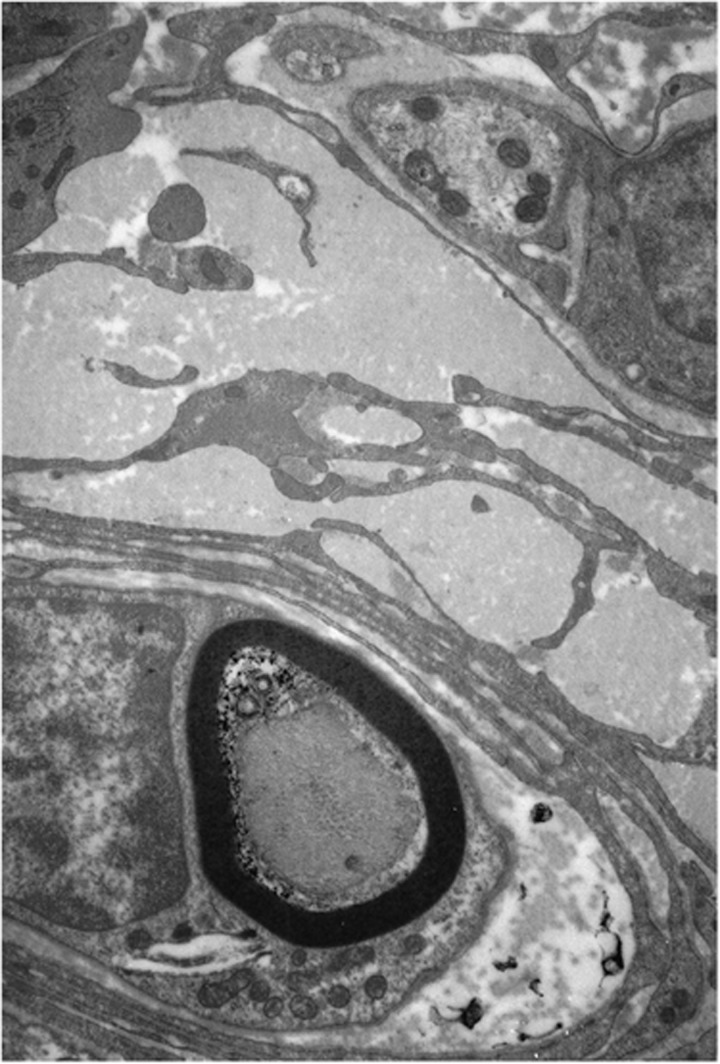
Analyses of the innervation of human EOMs have revealed that there is a clear correlation between nerve fibre diameters and the distinct morphology of the muscle fibres they serve.10 However, many of the small nerve fibres seem to have been missed in earlier studies using low-resolution techniques.11, 12 In a more recent study13 unmyelinated nerve fibres were found to be numerous (Figure 1), constituting 10.2–19.3% (mean 16.1%) of the nerve fibre population in cranial nerves obtained from seven human subjects (Figure 2). The ratio between myelinated and unmyelinated fibres was fairly uniform in all examined nerve branches, with exception of the branch serving the levator palpebrae muscle, known to lack multiply innervated fibres (MIFs). Adjustments were made for autonomic nerve fibres, based on results from preceding observations of superior cervical ganglionectomy in monkey.14 Quantifications of the corresponding muscle fibre population indicated that MIFs, which constituted 18–23% of all fibres, have a more generous efferent supply than previously assumed. The numerical data indicated motor units as small as 1 : 1. This was supported by the absence of bifurcations, when selected nerve fibres were traced in serial sections down to their distal ‘en grappe' terminations. This neural arrangement, the modest amounts of sarcoplasm among myofibrils (Figure 3) and the absence of MIFs in the levator palpebrae muscle, supports the consensus that they provide the fine-tuned tonic activity needed in ocular alignment and eye fixation.15, 16 The consent has been strengthened through more recent studies using tracer injections in non-human primates, indicating that peripheral motor neurones innervating non-twitch MIFs receive inputs from premotor neurones involved in smooth pursuit, convergence, and gaze holding.17, 18 It is therefore of special interest that many of the MIFs, which seem to have a one-to-one relationship with their efferent unmyelinated nerve fibres, also have nerve terminals at their distal end (described below).
Figure 1.
Electron micrograph showing unmyelinated nerve fibres scattered throughout the cross-sectional area of the inferior branch of the third cranial nerve.
Figure 2.
Histogram illustrating the spectrum of nerve fibre diameters in the inferior branch of the third cranial nerve. A trimodal distribution was apparent in all branches. The first peak encompasses the majority of all unmyelinated nerves fibres (≤1 μm).
Figure 3.
Electron micrograph showing a multiply innervated muscle fibre with small amounts of sarcoplasmic reticulum, surrounded by singly innervated muscle fibres. An unmyelinated, pre-terminal nerve fibre is in close vicinity of the muscle fibre.
Afferent innervation
Myotendinous nerve endings
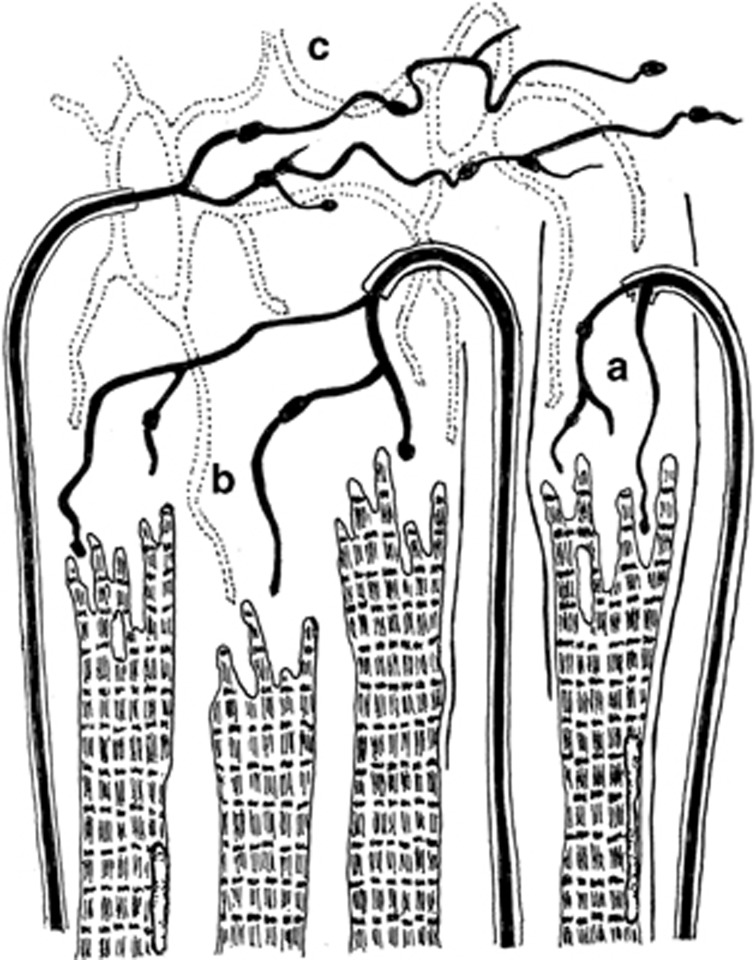
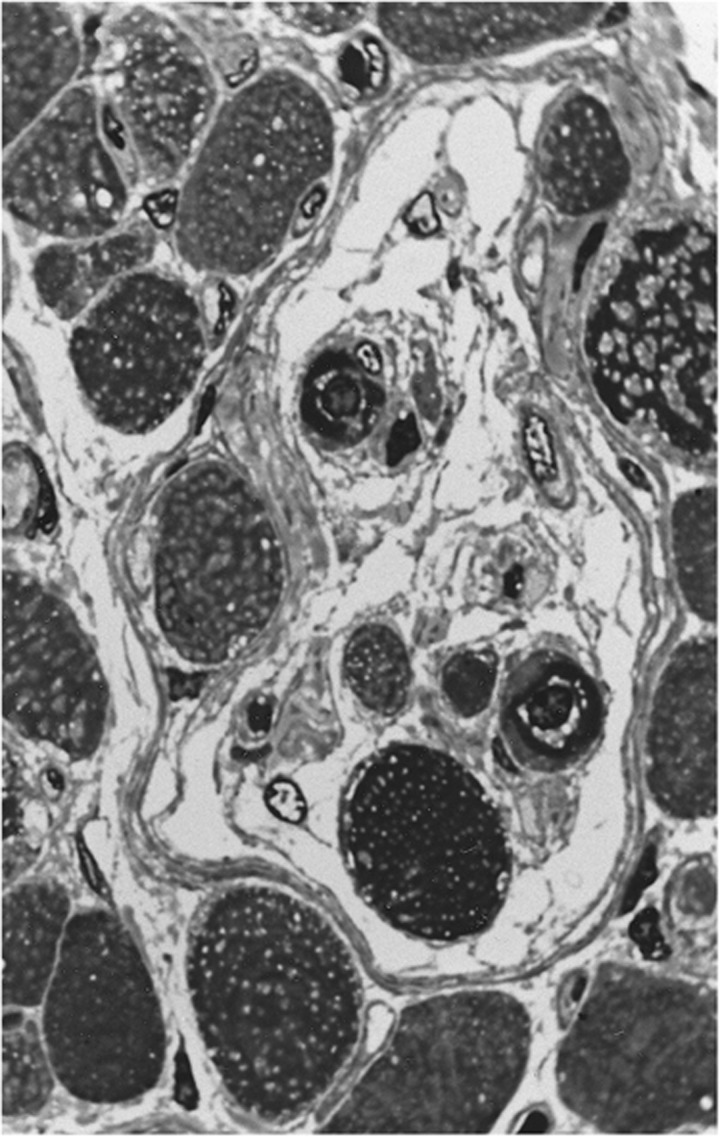
Myotendinous nerve terminals, first reported by Dogiel19 and later described in detail by Richmond et al,20 were regarded as the principal proprioceptors in human EOMs for many years. The notion that these unique, so-called palisade endings have a sensory function was supported through clinical studies, where transient disruption of the assumed proprioceptive pathway caused binocular vision anomalies.21 Structural irregularities observed in nerve endings from strabismus patients provided additional support and indicated that these receptors were implicated in the pathogenesis of oculomotor disorders.22 In a study by Bruenech and Ruskell,23 three varieties of nerve endings were described in the EOMs of six adult and five infant humans. The most frequently occurring structure consisted of a single recurrent myelinated nerve fibre that branched and terminated in between the muscular processes at the distal end of the muscle fibres (Figure 4a). Varicosities containing vesicles and mitochondria occurred at regular intervals. The capsule, enveloping the myotendinous compartments, was thin and composed of fibroblast-like cells rather than extensions of perineurium of the nerve fibre as seen in muscle spindles. These features were collectively referred to as myotendinous cylinders (MTCs) because of their resemblance to distal nerve terminals previously observed in monkey,24 later also found in proximal tendons.25 In some cases the capsule was incomplete or absent, allowing nerve endings to be dispersed in tendon and occasionally terminate on more than one muscle fibre (Figure 4b). In other cases, the nerve fibre terminated in pure tendon in a more distal location (Figures 4c and 5). All three varieties were found in both global and orbital muscle layers, but their distribution was patchy and scarce. It is of interest that the lowest number of these structures, assumed to be sensory, was found in muscle samples from strabismus patients (unpublished). No Golgi tendon organs or other putative receptors were observed, suggesting that MTCs represent the only proprioceptive source in the myotendinous region of human EOMs.23 However, Lukas et al26 observed some myoneural contacts in addition to terminals contacting tendon fibrils in a mature human cadaver. They hence suggested these structures to be regarded as propriocept effectors, with both sensory and motor properties. In later studies of other species, the same authors ascertained that the structures in question are pure cholinergic motor organs arising from axons that also supply the associated MIF.27, 28, 29, 30 They hence argued, in contrast to previous studies,31 that surgical strabismus procedures should have no side effects with respect to eye-position signals.30 Other studies, using central tracer injections in non-human primates, also support a motor function, but suggest that tendon endings and ‘en grappe' terminals arise from separate neurons located around the periphery of the motor nuclei of EOMs.32, 33 Even though it cannot be precluded that myotendinous nerve endings have a sensory function,2, 34 the notion that muscle spindles represents the only structures with proprioceptive potency seem to gain growing support.35
Figure 4.
Illustration showing the three varieties of nerve termination observed in distal tendon. (a) The most frequently occurring terminal, the myotendinous cylinder. (b) Terminals unconfined or partially confined by a capsule. (c) Dispersed terminals from a non-recurrent nerve fibre (redrawn from Bruenech and Ruskell23).
Figure 5.
Electron micrograph showing both a myelinated nerve fibre and neural elements terminating in tendon, distal to the contractile material.
Muscle spindles
Muscle spindles in human EOMs differ morphologically from conventional somatic spindles in several ways.1, 35, 36, 37 They also differ from spindles in EOMs of other mammals.3 Their numbers may have been overestimated in previous studies because encapsulated spindle-like structures, with unmodified intrafusal fibres lacking sensory neuronal contacts, are also present in human EOMs.37, 38, 39 These anomalous fibres found in so-called false spindles, are also observed in true spindles. Bruenech and Ruskell37 reported that 25.7% of the intrafusal fibres in 40 spindles were anomalous, lacking equatorial modifications and sensory endings, some of which were found embedded into the capsule wall (Figure 6). Furthermore, 38.8% of the intrafusal fibres with nuclear chain modifications were fragmented or failed to run the full length of the spindle, clearly unable to create the required deformation of the primary sensory ending during stretching of the muscle. All of the remaining fibres had nuclear chain modifications, while no nuclear bag fibres were observed (Table 1). These and other noted peculiarities, which have not been observed in muscle spindles in the EOMs of other species,3 give reason to question the spindles' proprioceptive capacity in man.37 However, studies based on myosin heavy chain investigations have reached alternative conclusions and promote the view that the anomalous fibres might reflect dynamic structures that support modulating and adapting processes in advancing age and throughout life.35 This hypothesis is attractive in view of the labile neuromuscular organisation and numerous age-related changes that have been observed in human EOMs (described below).
Figure 6.
Micrograph showing a transverse section through the equatorial region of a muscle spindle. The spindle holds both intrafusal chain fibres and anomalous fibres with extrafusal features. The latter type is present both within the lumen and embedded in the capsule wall.
Table 1. The table shows the content of intrafusal fibres in 40 muscle spindles obtained from human extraocular muscles.
| No. of muscle fibres | Fragmented fibres | Intact nuclear chain fibres | |
|---|---|---|---|
| Intrafusal fibres | 130 (74.3%) | 68 (38.8%) | 62 (35.4%) |
| Anomalous fibres | 45 (25.7%) | ||
| Total no. | 175 |
Functional considerations
The appearance of a stable visual world is believed to rely on extraretinal feedback regarding the extent and velocity of executed eye rotations. This feedback is also crucial for other aspects of visual perception since it enables us to make judgments about the position and motion of other objects. An established view is that this can be obtained if visual centres receive a copy of the neural efference to the EOMs.40 However, efference copy would only inform the brain of where the eyes should be, rather than where they actually are.41 This type of feedback alone would therefore not be able to detect the developmental changes, neuromuscular conversions, and age-related alterations, which have been documented in MIFs and to a larger extent in singly innervated muscle fibres.42, 43
The latter fibres are generally innervated by large myelinated nerve fibres with ‘en plaque' terminals confined to the middle third region of the muscle. However, they have also been observed in the distal region,23 frequently with more than one ending on the same muscle fibre.42, 44 In most cases, neural processes extended from the original endplate and formed a second endplate in a more distal location, resembling multiply innervated muscle fibres. Occasionally, the second endplate derived from another nerve fibre, resembling polyneural innervation. These have been argued to represent redundant nerve fibres seeking new targets.42
All these neuromuscular changes have exclusively been observed in mature muscles, and are often associated with Ringbinden fibres, lipofuscin, loss of myofilaments, and other signs of degeneration. Such age-related changes, known to have implications for conduction velocity and muscular force,45 were found throughout the cross-sectional area of both global and orbital layers.46 These layers insert onto the globe and into fibromuscular structures, respectively, and hence subserve different functions in oculomotor control.4, 5 Although the facility for separate adjustments of these layers were argued to be limited in a previous histological study,5 the global layer seems to provide the force for ocular rotation, while the orbital layer influences the muscle's direction of pull, as suggested in the pulley hypothesis.47 This view has been elaborated in more recent studies, where it is promoted that the superior and inferior compartments of horizontal rectus muscles also can be controlled differently.9 The distal insertions would therefore represent an ideal location for monitoring tendon dynamics, and potential changes in the differential muscle activity caused by transient neuromuscular inconsistencies or age-related changes, as described above.44, 48
Discussion
Accepting the notion that a muscle's ability to perform minute and fine-tuned contractions is determined by the size of its motor units, then MIFs must have an important role in all eye movements where accuracy is required.13 A congenital scarcity of these fibres may therefore be implicated in the aetiology of oculomotor anomalies,49 regardless of their potential association with proprioceptors.
The potential role of an additional efferent innervation at their distal myotendon is not easily reconciled. While a small percentage of the nerve terminals make contact with contractile material and putative postsynaptic acetylcholine receptors in EOMs of some species,30 attempts to confirm these findings in other species have failed.50 Animal studies may hence not always be directly applicable as models for human oculomotor control.2 The majority of the human nerve terminals are located in tendon, in some distance from muscular processes. These features are more characteristic of mechanoreceptors than motor terminals. Furthermore, the majority of the nerve endings derive from myelinated fibres.23 The notion that the efferent innervation of MIFs would increase in diameter after bifurcation and even become myelinated is unlikely. By accepting the premise that MTCs are sensory, then any potential branches extending from the efferent innervation of MIFs could serve to tune the sensitivity of the receptor, consistent with the neuromuscular arrangement of intrafusal fibres. The topographical location of these nerve terminals would enable them to monitor and tune the contraction in all four muscle quadrants, and thus support the proposed compartmental neuromuscular activity.
The complement and distribution of muscle spindles could represent a valuable additional source of proprioception, especially for activity in the orbital layer where many of them reside. The large number of fragmented and foreshortened intrafusal fibres, however, raises question about their proprioceptive capacity. This suggests that the tendon terminals remain the most reputable sensory source and that the promoted concern for the postoperative function31 of these receptors should be retained. However, proprioception and efference copy may not necessarily be mutually exclusive, and the possibility that they coexist should not be precluded. A copy of the velocity-to-position command from neural integrators could serve to maintain perceptual stability and other short-term visual functions during eye rotations. Feedback from sensory organs could initiate the adaptation processes needed to compensate for development, neural lability, and senescence.48
Acknowledgments
We greatly acknowledge financial support from the Norwegian Research Council.
The authors declare no conflict of interest.
Footnotes
The work was presented at the Cambridge Ophthalmological Symposium
References
- Lienbacher K, Horn AK. Palisade endings and proprioception in extraocular muscles: a comparison with skeletal muscles. Biol Cybern. 2012;106:643–655. doi: 10.1007/s00422-012-0519-1. [DOI] [PubMed] [Google Scholar]
- Haugen IBK, Bruenech JR. The potential role of sensory receptors in ocular movements. Acta Ophthalmol Scand. 2007;85 (s240 [Google Scholar]
- Ruskell GL. Extraocular muscle proprioceptors and proprioception. Prog Retin Eye Res. 1999;18 (3:269–291. doi: 10.1016/s1350-9462(98)00029-9. [DOI] [PubMed] [Google Scholar]
- Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41 (6:1280–1290. [PubMed] [Google Scholar]
- Ruskell GL, Kjellevold Haugen IB, Bruenech JR, van der Werf F. Double insertions of extraocular muscles in humans and the pulley theory. J Anat. 2005;206 (3:295–306. doi: 10.1111/j.1469-7580.2005.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demer JL, Clark RA, da Silva Costa RM, Kung J, Yoo L. Expanding repertoire in the oculomotor periphery: selective compartmental function in rectus extraocular muscles. Ann NY Acad Sci. 2011;1233:8–16. doi: 10.1111/j.1749-6632.2011.06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Demer JL. Differential lateral rectus compartmental contraction during ocular counter-rolling. Invest Ophthalmol Vis Sci. 2012;53 (6:2887–2896. doi: 10.1167/iovs.11-7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin A, Yoo L, Chaudhuri Z, Demer JL. Independent passive mechanical behavior of bovine extraocular muscle compartments. Invest Ophthalmol Vis Sci. 2012;53 (13:8414–8423. doi: 10.1167/iovs.12-10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demer JL, Clark RA. Magnetic resonance imaging of differential compartmental function of horizontal rectus extraocular muscles during conjugate and converged ocular adduction. J Neurophysiol. 2014;112 (4:845–855. doi: 10.1152/jn.00649.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt M. Beitrag zur anatomie des musculus rectus externus und des musculus rectus internus bei hund und mensch. Ophthalmologica. 1969;157:381–390. doi: 10.1159/000305689. [DOI] [PubMed] [Google Scholar]
- Bors E. Über das zahlenverhältnis zwischen nerven- und muskelfasern. Anat Anz. 1926;60:415–416. [Google Scholar]
- Torre M. Nombre et dimensions des unités motrices dans les muscles extrinsèques de l'œil et, en général, dans les muscles squélettiques reliés à des organes de sens. Schweiz Arch Neurol Psychiatr. 1953;72:362–376. [PubMed] [Google Scholar]
- Kjellevold Haugen IB, Bruenech JR.Histological analysis of the efferent innervation of human extraocular musclesIn: De Faber JT, (ed). 29th European Strabismological Association Meeting: Transactions Taylor & Francis, CRC Press: London, UK; 200591–94. [Google Scholar]
- Ruskell GL. Fibre analysis of the nerve to the inferior oblique muscle in monkeys. J Anat. 1983;137:445–455. [PMC free article] [PubMed] [Google Scholar]
- Scott AB. Active force tests in lateral rectus paralysis. Arch Ophthalmol. 1971;85 (4:397–404. doi: 10.1001/archopht.1971.00990050399001. [DOI] [PubMed] [Google Scholar]
- Scott AB, Collins CC. Division of labor in human extraocular muscle. Arch Ophthalmol. 1973;90 (4:319–322. doi: 10.1001/archopht.1973.01000050321017. [DOI] [PubMed] [Google Scholar]
- Ugolini G, Klam F, Doldan Dans M, Dubayle D, Brandi AM, Büttner-Ennever J, et al. Horizontal eye movement networks in primates as revealed by retrograde transneuronal transfer of rabies virus: differences in monosynaptic input to “slow” and “fast” abducens motoneurons. J Comp Neurol. 2006;498 (6:762–785. doi: 10.1002/cne.21092. [DOI] [PubMed] [Google Scholar]
- Erichsen JT, Wright NF, May PJ. Morphology and ultrastructure of medial rectus subgroup motoneurons in the macaque monkey. J Comp Neurol. 2014;522 (3:626–641. doi: 10.1002/cne.23437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogiel AS. Die endigungen der sensiblen nerven in den augenmuskeln und deren sehnen beim menschen und den saugetieren. Arch f mikr Anat. 1906;68:501–526. [Google Scholar]
- Richmond FJ, Johnston WS, Baker RS, Steinbach MJ. Palisade endings in human extraocular muscles. Invest Ophthalmol Vis Sci. 1984;25 (4:471–476. [PubMed] [Google Scholar]
- Campos EC, Chiesi C, Bolzani R. Abnormal spatial localization in patients with herpes zoster ophthalmicus. Evidence for the presence of proprioceptive information. Arch Ophthalmol. 1986;104 (8:1176–1177. doi: 10.1001/archopht.1986.01050200082055. [DOI] [PubMed] [Google Scholar]
- Park SE, Sa HS, Oh SY. Innervated myotendinous cylinders alterations in human extraocular muscles in patients with strabismus. Korean J Ophthalmol. 2009;23 (2:93–99. doi: 10.3341/kjo.2009.23.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenech JR, Ruskell GL. Myotendinous nerve endings in human infant and adult extraocular muscles. Anat Rec. 2000;260 (2:132–140. doi: 10.1002/1097-0185(20001001)260:2<132::AID-AR30>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. The fine structure of innervated myotendinous cylinders in extraocular muscles in rhesus monkey. J Neurocytol. 1978;7:693–708. doi: 10.1007/BF01205145. [DOI] [PubMed] [Google Scholar]
- Haugen IBK, Bruenech JR. Sensory receptors in extraocular muscles (EOM) and their potential role in oculomotor control. Ophthalmic Res. 2005;37 (s1:98. [Google Scholar]
- Lukas JR, Blumer R, Denk M, Baumgartner I, Neuhuber W, Mayr R. Innervated myotendinous cylinders in human extraocular muscles. Invest Ophthalmol Vis Sci. 2000;41 (9:2422–2431. [PubMed] [Google Scholar]
- Konakci KZ, Streicher J, Hoetzenecker W, Blumer MJF, Lukas J-R, Blumer R. Molecular characteristics suggest an effector function of palisade endings in extraocular muscles. Invest Ophthalmol Vis Sci. 2005;46:155–165. doi: 10.1167/iovs.04-1087. [DOI] [PubMed] [Google Scholar]
- Konakci KZ, Streicher J, Hoetzenecker W, Haberl I, Blumer MJF, Wieczorek G, et al. Palisade endings in extraocular muscles of the monkey are immunoreactive for choline acetyltransferase and vesicular acetylcholine transporter. Invest Ophthalmol Vis Sci. 2005;46 (12:4548–4554. doi: 10.1167/iovs.05-0726. [DOI] [PubMed] [Google Scholar]
- Blumer R, Konakci KZ, Streicher J. Proprioception in the extraocular muscles of mammals and man. Strabismus. 2006;14:101–106. doi: 10.1080/09273970600701192. [DOI] [PubMed] [Google Scholar]
- Blumer R, Konakci KZ, Pomikal C, Wieczorek G, Lucas JR, Streicher J. Palisade Endings: cholinergic sensory organs or effector organs. Invest Ophthalmol Vis Sci. 2009;50 (3:1176–1186. doi: 10.1167/iovs.08-2748. [DOI] [PubMed] [Google Scholar]
- Steinbach MJ, Smith DR. Spatial localization after strabismus surgery: evidence for inflow. Science. 1981;213 (4514:1407–1409. doi: 10.1126/science.7268444. [DOI] [PubMed] [Google Scholar]
- Zimmermann L, Morado-Díaz CJ. Davis-López de Carrizosa MA, de la Cruz RR, May PJ, Streicher J et al. Axons giving rise to the palisade endings of feline extraocular muscles display motor features. J Neurosci. 2013;33 (7:2784–2793. doi: 10.1523/JNEUROSCI.4116-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienbacher K, Mustari M, Ying HS,3 Büttner-Ennever JA, Horn AKE. Do palisade endings in extraocular muscles arise from neurons in the motor nuclei. Invest Ophthalmol Vis Sci. 2011;52 (5:2510–2519. doi: 10.1167/iovs.10-6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienbacher K, Mustari M, Hess B, Büttner-Ennever J, Horn AKE. Is there any sense in the palisade endings of eye muscles. Ann NY Acad Sci. 2011;1233:1–7. doi: 10.1111/j.1749-6632.2011.06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicke W, Wasicky R, Brugger PC, Kaminski S, Lukas JR. Histochemical and immunohistochemical study on muscle fibers in human extraocular muscle spindles. Exp Eye Res. 2007;84 (4:670–679. doi: 10.1016/j.exer.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Blumer R, Lukas JR, Aigner M, Bittner R, Baumgartner I, Mayr R. Fine structural analysis of extraocular muscle spindles of a two-year-old human infant. Invest Ophthalmol Vis Sci. 1999;40 (1:55–64. [PubMed] [Google Scholar]
- Bruenech JR, Ruskell GL. Muscle spindles in extraocular muscles of human infants. Cells Tissues Organs. 2001;169 (4:388–394. doi: 10.1159/000047906. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. The fine structure of human extraocular muscle spindles and their potential proprioceptive capacity. J Anat. 1989;167:199–214. [PMC free article] [PubMed] [Google Scholar]
- Lukas JR, Aigner M, Blumer R, Heinzl H, Mayr R. Number and distribution of neuromuscular spindles in human extraocular muscles. Invest Ophthalmol Vis Sci. 1994;35 (13:4317–4327. [PubMed] [Google Scholar]
- Helmholtz HV. Handbuch der Physiologishen Optik. Voss: Leipzig; 1866. [Google Scholar]
- Bridgeman B. How the brain makes the world appear stable. Iperception. 2010;1 (2:69–72. doi: 10.1068/i0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellevold Haugen IB, Bruenech JR.Age-related neuromuscular changes in human extraocular musclesIn: Gomez de Liano R, (ed). 30th European Strabismological Association Meeting: Transactions Madrid, Spain; 2006141–144. [Google Scholar]
- Bruenech JR, Kjellevold Haugen IB. Age-related changes in the orbital layer of human extraocular muscles (EOMs) Acta Ophthalmol Scand. 2006;84 (s239:109. [Google Scholar]
- Bruenech JR.Age-related changes in the human oculomotor systemIn: C. Cavallotti CAP, Cerulli L, (eds). Age-related Changes of the Human Eye Springer, Humana Press: New York, NY, USA; 2008343–373. [Google Scholar]
- Mühlendyck H, Ali SS. Histological and ultrastructural studies on the ringbands in human extraocular muscles. Graefes Arch Clin Exp Ophthalmol. 1978;208 (1-3:177–191. doi: 10.1007/BF00406992. [DOI] [PubMed] [Google Scholar]
- Bruenech JR, Kjellevold Haugen IB. Structural organization of the distal insertion of human extraocular muscles (EOM) Ophthalmic Res. 2005;37 (s1:98. [Google Scholar]
- Demer JL. Current concepts of mechanical and neural factors in ocular motility. Curr Opin Neurol. 2006;19 (1:4–13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenech JR, Kjellevold Haugen IB, Bak U, Maagaard M, VanderWerf F. The oculomotor system's ability to adapt to structural changes caused by the process of senescence: a review. Scand J. Optom Vis Sci. 2012;5 (1:1–14. [Google Scholar]
- Bruenech JR, Kjellevold Haugen IB. Morphological variations found in human extraocular muscles and their functional implications. Acta Ophthalmol Scand. 2007;85 (s240 [Google Scholar]
- Eberhorn AC, Horn AK, Eberhorn N, Fischer P, Boergen KP, Büttner-Ennever JA. Palisade endings in extraocular eye muscles revealed by SNAP-25 immunoreactivity. J Anat. 2005;206 (3:307–315. doi: 10.1111/j.1469-7580.2005.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]