Abstract
Binocular stereopsis, or stereo vision, is the ability to derive information about how far away objects are, based solely on the relative positions of the object in the two eyes. It depends on both sensory and motor abilities. In this review, I briefly outline some of the neuronal mechanisms supporting stereo vision, and discuss how these are disrupted in strabismus. I explain, in some detail, current methods of assessing stereo vision and their pros and cons. Finally, I review the evidence supporting the clinical importance of such measurements.
Introduction
Stereo vision is the computation of depth based on the binocular disparity between the images of an object in left and right eyes (Figure 1). This requires matching up features in the two eyes, that is, identifying features in the left and right retinas that are both images of the same point in the visual scene. The matching process begins in primary visual cortex of the brain, but requires a series of computations across several distinct areas of visual cortex before it is fully achieved.1
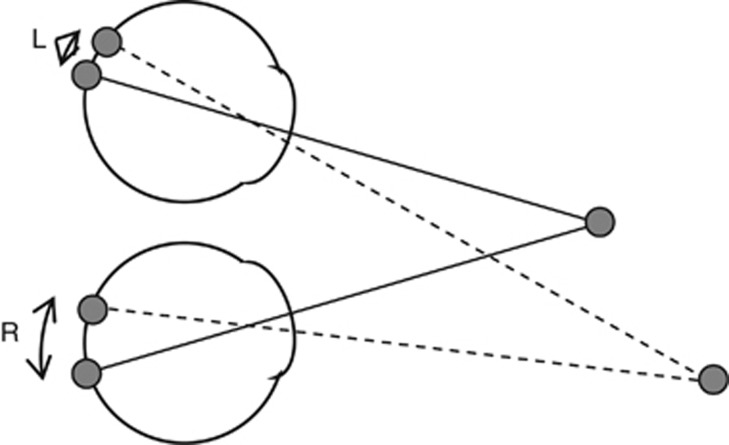
Figure 1.
The optic axes of each eye are shown with solid lines. The fixated object, by definition, projects to the fovea in each eye. The retinal projections of another object are shown with dashed lines. Because this object is not at the fixation distance, its images in the two eyes fall at different locations relative to the fovea, as indicated by the angles L and R. Absolute binocular disparity is defined as the difference between the angles to the fovea in each eye: Δ=R−L. Note that in this figure, angles are exaggerated for clarity. In reality, the second object would be seen double as its disparity is too large to be fused.
In principle, if the eyes were free to move independently, the match for a given feature in the right image could be located anywhere in the left retina (Figure 2). Humans, however, simplify the matching problem by imposing severe constraints on our eye movements. Most notably, our binocular vision requires that the optic axes intersect, that is, both eyes fixate the same point in space. This, together with the other laws governing eye movements,2, 3 means that matches generally lie at essentially the same vertical position in both eyes.4 The visual system simplifies further by considering only potential matches that are at very similar horizontal locations in the two eyes, within a degree or so (Figure 2). The effect of this is to limit the search to objects at or near the fixation distance.
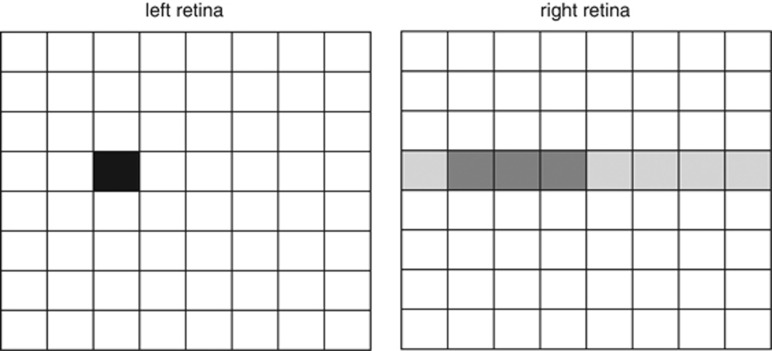
Figure 2.
Cartoon of the two retinas, imagining them tiled by 8 × 8 receptive fields. Consider searching for the location in the right retina viewing the same object as the black-shaded location in the left retina. In principle, there are 64 possible locations. However, the horizontal offset of the eyes together with the laws governing eye movements means that the geometrically possible matches are largely confined to the row at the same vertical position (shaded light grey). The visual system then further restricts its search to matches at a similar horizontal position (shaded dark grey).
Together, these simplifications greatly reduce the number of brain cells required for binocular vision. The primary visual cortex of the brain contains neurons receiving information from both eyes. Each such neuron corresponds to a pair of ‘tiles' in Figure 2, with the size of the tile corresponding to the size of the retinal receptive field (<1° near the fovea). Experimentally, such neurons are found to view very similar visual directions in space, with a wider range of horizontal than vertical disparity.5, 6, 7 That is, the brain contains only those neurons representing the most likely disparities. This represents a great reduction in the number of neurons required (from 64 down to 3 in the cartoon shown in Figure 2).
The cost is that objects that are too far from the fixation distance, such as the second object in Figure 1, are not fused, but are seen double. This physiological diplopia can be noticed in everyday life, but is not generally problematic. Natural scenes tend to vary relatively smoothly in depth, so that points near fixation are generally at similar depth. Where this is not the case, for example where we fixate the edge of a surface, more distant objects will be blurred as we are accommodating on the fixated surface. A more minor side effect is that stereopsis fails for sufficiently extreme eye postures.3 We typically avoid such eye postures by moving our heads to point at what we want to look at. Thus, this strategy works well in practice.
However, this strategy clearly depends critically on the ability of the oculomotor system to direct both foveas at the object of interest—precisely the ability that fails in strabismus. The normal visual system contains several mechanisms to support this. Neuronal crosslinks between accommodation and vergence help the eyes to fixate at the correct distance.8, 9 Images with non-zero disparity trigger vergence reflexes, intended to null out disparity at the fovea and thus ensure that both foveas are directed at the same object in space.10 Many people show phorias, misalignments of the eyes that occur when normal visual input is removed (eg, by occluding one eye's view), demonstrating the importance of sensory feedback in maintaining normal binocular alignment.
Strabismic patients exhibit tropias: binocular misalignments that persist even with normal viewing. Normal stereo vision is not possible when the eyes are misaligned, because an object's images on the two retinas are too far apart; they do not fall within the range of matches that the brain can consider. Some strabismic patients show abnormal retinal correspondence; that is, their brain learns to perceive objects as lying in the same visual direction, even though they fall at what would normally be noncorresponding locations in the retina.11, 12 If a tropia is constant and present from an early age, one might have expected stereopsis to be present but similarly shifted, with the receptive fields of binocular visual neurons offset on the retina by the amount of the deviation. However, apparently the normal retinotopic projection is not plastic enough to allow this to occur, perhaps because this would involve matching up physically very different parts of the retina, for example, the fovea in one eye with peripheral retina of the other. Wong et al11 have suggested that anomalous retinal correspondence may be achieved by using chains of neurons across abnormally large areas of visual cortex. Apparently, this mechanism cannot support the binocular neurons necessary to support stereopsis. In addition, eye position in strabismus may be not only misaligned, but also more variable. As noted, receptive fields in early visual cortex are well under 1°, and respond to disparities typically over a range of under 0.5°. Thus, vergence fluctuations of ±0.25°, although not clinically noticeable, could damage the development of stereopsis.
Because stereo vision depends upon good vision in both eyes, excellent oculomotor control and cortical mechanisms for sensory fusion, it is regarded as the gold standard for binocular visual function.13 Where strabismus therapy succeeds in restoring stereo vision, this proves that the patient has excellent and stable binocular alignment, and that the necessary sensory cortical mechanisms have been preserved.
How to measure stereo vision
Several commercial stereoacuity tests are available for use in the clinic.14, 15 Some of these are listed in Table 1 along with some of their properties. At its most basic, a stereotest can aim simply to determine whether a patient has any stereo vision at all, without quantifying their stereoacuity. The ‘gross stereo' component of the Randot test is one example. For many clinical applications it is also desirable to quantify a patient's stereo vision. This requires a measurement of stereoacuity. Stereoacuity is defined as the reciprocal of stereo threshold, the smallest binocular disparity that can reliably be distinguished by a patient.
Table 1. Some stereotests available for use in the clinic.
| Name | Viewing distance | Available disparities (arcsec) | Task | Number of choices | Physical depth? | Monoc contours? | Monoc cue? | Technology | 3D glasses needed? | Some relevant citations |
|---|---|---|---|---|---|---|---|---|---|---|
| Infant Randot Stereoacuity Cards, Stereo Optical Company | Near (70 cm) | 45–1736 | Preferential looking to stereotarget | 2 | No | No | No | Printed polarised vectograph | Yes | 98 |
| Randot Preschool, Stereo Optical Company | Near (40 cm) | 20–800 | Identify disparity-defined shapes | 4 | No | No | No | Printed polarised vectograph | Yes | 22, 28, 99, 100 |
| Stereo Smile, Stereo Optical Company | Near (40 cm) | 120–480 | Identify disparity-defined object | 2 | No | No | No | Printed polarised vectograph | Yes | 21, 22, 100, 101, 102 |
| Randot Stereo Butterfly (gross component), Stereo Optical Company | Near (40 cm) | 1200–2500 | Report seeing disparity-defined object | — | No | No | No | Printed polarised vectograph | Yes | 103 |
| TNO (Alfred Poll Inc.) | Near (40 cm) | 15–480 | Identify disparity-defined object | 4 | No | No | No | Printed anaglyph cards | Yes | 57, 104 |
| Frisby Near, Clement-Clarke Ltd | Near (30–150 cm) | 5–600 | Identify disparity-defined object | 4 | Yes | No | Yes | Printed pattern on front/back of transparent tiles | No | 28, 105, 106 |
| Lang I, Lang Stereotests AG/Forch | Near (40 cm) | 550–1200 | Detect disparity-defined object | No | No | Yes | Printed lenticular stereogram | No | 107 | |
| Lang II, Lang Stereotests AG | Near (40 cm) | 200–600 | Detect disparity-defined object | No | No | Yes | Printed lenticular stereogram | No | 100, 108 | |
| Wirt/Titmus/Randot Fly (gross stereo component) Stereo Optical Company | Near (40 cm) | 40–3000 | Report seeing object in depth | — | No | Yes | No | Printed polarised vectograph | Yes | 109, 110 |
| Randot Graded Circles/Animals (fine component of Randot Fly Butterfly test) | Near (40 cm) | 400–800 | Odd-one-out task based on disparity | 3, 5 | No | Yes | No | Printed polarised vectograph | Yes | 109, 110 |
| Test Chart Xpert 3Di, Thomson Software Solutions | Variable | Selected by user | Report seeing disparity-defined object | — | No | No | No | 3D computer monitor using passive polarisation | Yes | |
| Random-Dot E (RDE), Stereo Optical Company | Variable (50–500 cm) | 50–504 arcsec | Identify disparity-defined object | 2 | No | No | No | Printed polarised vectograph | Yes | 22, 102, 111, 112 |
| Distance Randot, Stereo Optical Company | Far (3 m) | 60–800 | Identify disparity-defined object | 4 | No | No | No | Printed polarised vectograph | Yes | 28, 39, 55, 113, 114 |
| Frisby Davis Distance (FD2), Clement-Clarke Ltd | Far (6 m) | 5–50 | Odd-one-out task based on disparity | 4 | Yes | Yes | Yes | Physical objects moved on rods | No | 19, 20, 39, 70, 105, 115, 116, 117 |
‘Available disparities' and ‘number of alternatives' are examples and may vary between plates or versions of the test. By ‘monocular depth cue', I mean that it is theoretically possible for a subject to perform the task using a cue other than binocular disparity. For example, the Lang stereotests consist of a random-dot pattern printed on lenticular cards that are intended to direct different images to the two eyes, and hence the test image contains no monocular cues to fusion. However, by moving the head relative to the card, the subject can present both views successively to the same eye. This results in monocularly visible motion parallax. In theory, any contour stereogram could be solved monocularly by closing one eye at a time and noting the direction of displacement of the contours.
There are two problems with this definition. First, just as in other areas of sensation, there is no abrupt cutoff between visible and nonvisible disparities. If subjects are given some task that depends on the perception of the disparity, performance will rise smoothly from chance to perfect over some finite range. ‘Threshold' must then be defined as a given level of performance, for example, when the subject has a 50–50 chance of detecting the disparity. On clinical tests, the reported threshold is taken to be the smallest tested disparity on which the subject was correct on at least M out of N presentations (where N and M are small integers). Second, the threshold depends on the particular task, on the viewing distance, and on the details of the stimulus. These issues will all be discussed in detail below.
Monocular cues
It is clearly essential that the patient should not be able to pass a stereotest using monocular cues. Where the test images have monocularly visible contours (see below), as in the FD2 or the graded-circles component of the Randot stereotest, large disparities may be visible monocularly as a shift in the contour. Fawcett16 states that this ‘frequently' gives rise to false-positive results in patients. Using a test image with no monocular contours, as in a random-dot pattern, avoids this. The Frisby and Lang stereotests avoid monocular contours, but because of the way in which they introduce disparity, they still offer monocular cues if the patient does not remain perfectly stationary relative to the test image,17 and this is hard to ensure in children. Fawcett et al18 state that steroacuity scores above 160 seconds of arc in such tests should be interpreted with caution, as they may represent a response to monocular cues. To avoid such artefacts, the test should be repeated with monocular viewing. If the same score is obtained as with binocular, the binocular score is not a measure of stereoacuity.
Unfortunately, the only way of avoiding monocular cues in currently available stereotests requires the use of 3D glasses. These are an additional barrier to using a test in young children; the child may be distracted by the glasses or unwilling to wear them, or the glasses may not fit. The TNO test and the anaglyph version of the Test Chart Xpert 3Di test use red/blue glasses, in which each eye sees a different colour. This is undesirable as it tends to promote rivalry and dissociation, and may promote suppression of one eye by the other. The Randot family of tests uses polarised vectographs to present different images to each eye. As humans are not sensitive to the polarisation of light, both images appear the same apart from the disparity.
Number of alternatives
Clinical stereotests typically ask patients to use disparity cues to choose between a number of alternatives. The number of alternatives varies. For example, the FD2 test and the graded components of the Randot test all ask subjects to identify which of several shapes is closer than the others. The Randot ‘circles' task asks subjects to choose between three circles, the FD2 test asks them to choose between four shapes, and the Randot ‘animals' task between five pictures. At first sight, the advantage of offering more alternatives is that patients are less likely to succeed by guessing. Thus, four-alternative tests generally require fewer correct responses to pass a level: two out of three19 or two out of two20 correct responses, as compared with four out of five21 for a two-alternative test. However, the logic behind this seems debatable. If patients are willing to guess, all stereotests are in trouble. On a 2-alternative test, scoring 4 out of 5 is not significantly different from the chance performance of 50%. In fact, if subjects answered at random with both eyes closed, 20% of them would score 4/5 or better. On a 4-alternative test, answering correctly on 2 consecutive trials does not enable us to reject the null hypothesis of stereoblindness at the conventional 5% significance level, as a sightless subject stands a 6% chance of obtaining this score by guessing. Thus, stereotests depend on patients not guessing; for this reason, test protocols often stress that patients should be instructed not to guess but only to report clear depth percepts. If we dismiss the possibility of guessing, the two-alternative tests may actually be preferable, as they reduce task complexity and are more accessible to small children.22
Precision and reliability of stereoacuity measurements
Very few clinical stereo tests are capable of measuring the stereo threshold of healthy controls. Subjects with good binocular vision can have stereoacuity thresholds as low as 2 seconds of arc, and 80% have thresholds 30 arcsec (sec 19.3.1 of Howard and Rogers23). As Table 1 shows, most commercial tests are not designed to measure such low thresholds, with a threshold of 20 arcsec often being considered ‘normal'. In principle, commercial tests can be used to present arbitrarily low disparities by increasing the viewing distance. This is not an ideal solution, as it also changes the size and spatial frequency content of the image.24 Clinical stereotests are designed to quantify the degree of impairment rather than measuring the abilities of healthy subjects.
Several studies have examined the test–retest variability of stereotests. These have generally quantified agreement by the 95% limits of agreement, that is, ±2σ, where σ is the SD of the difference between two measurements in the same subject using the same test.25 The ‘measurement' here refers to log10 (threshold in arcsec), as the uncertainty on stereo threshold is roughly constant when expressed as a fractional error. In clinical practice, one is often interested in the change in the measured value: if a stereo threshold is lower now than on the last visit, does this represent a real improvement? In order to be confident that a stereo threshold has really changed, the log10-thresholds must differ by 2σ. That is, the two thresholds must differ by a factor of at least F=102σ.
Fawcett and Birch26 found F=2.1 for the Randot Preschool stereotest. Adler et al,27 using the Randot graded circles test, report σ as 1.57 Randot plates rather than in log10 arcsec, but on average each Randot plate increases disparity by a factor of 1.4. Thus, this corresponds to F=2.8. Adams et al28 examined reliability for several stereotests and found F=3.9 for preschool Randot, F=1.7 for the near Frisby, F=4.8 for the FD2, and F=2.9 for the distance Randot. They concluded that changes in stereoacuity of less than a factor of four are not clinically meaningful, as they cannot be distinguished from measurement error. This is fairly poor, especially as stereotests only offer a few possible scores, often differing by a factor of two, which would tend to increase the reported reliability compared with the same test with a finer range of possible scores. The poor reliability makes it hard for clinicians to monitor the effect of therapy on stereoacuity.
One reason for this poor reliability must be the low number of trials required by protocols: as we have seen, if the ‘pass' level is set on just three trials of a four-alternative test, it is possible to pass by chance. It is difficult to obtain many trials in small children, but current protocols ‘waste' trials, for example by conducting three repeats of the early, easy levels. It would be more efficient to use a staircase procedure, where one or two correct answers moves the subject onto the next level, but a wrong answer sends them back. These are statistically a more efficient way of obtaining information about the patient's abilities, but are hard for the tester to implement. In addition, current tests offer a limited range of trials; for example, the Randot graded-circles test offers only one trial at each level. Disparities can be presented only at a limited number of preset levels.
Many of these problems could be avoided by using computerised tests. These are routine in vision science laboratories, but are not usual in the clinic because of the cost and space required and the lack of suitable software. A few recent papers have explored the use of computerised stereotests in clinical populations,29, 30, 31, 32 but none of these are currently commercially available. The only commercial computerised stereotest of which I am aware is the Test Chart Xpert 3Di from Thomson Software Solutions, available with either anaglyph (red/blue) or polarising 3D display. It enables the user to present unlimited trials at a range of available disparities, but does not implement the mathematical techniques used to improve the precision of threshold estimates in the lab. As yet, no published studies have used the stereotest aspect of the Test Chart Xpert.
Effect of viewing distance
In healthy controls, stereo thresholds in arcsec are independent of viewing distances over a very wide range (30 cm–10 m).33, 34, 35, 36 That is, the threshold depends only on the retinal disparity, and not on the vergence angle. (Bradshaw and Glennerster33 found a small increase in stereo threshold when the viewing distance was halved from 60 to 30 cm, but at <2 arcsec this is not clinically measurable.) This would suggest that the viewing distance of a clinical stereotest should be immaterial. Accordingly, most stereotests are designed for arm's length viewing. This is convenient in the clinic, as it requires less space and makes it easier to maintain the attention of young patients.
However, this independence on viewing distance is true only for the sensory component of stereopsis. The binocular neurons in visual cortex that detect disparity are sensitive almost exclusively to retinal information, regardless of how this is presented.37 In normal subjects, the oculomotor system ensures that subjects fixate correctly upon stimuli of all viewing distances, ensuring that the fixated object has zero retinal disparity regardless of its distance. In strabismus, this system is impaired. Even a misalignment of just 0.25° (900 arcsec, 0.44 prism dioptres) would have a profoundly damaging on stereoacuity, as it would add a disparity of 900 arcsec to all parts of the stimulus. A subject who can easily see the disparity boundary between 0 and 20 arcsec might well be completely blind to the difference between 900 and 920 arcsec.38 This effect probably explains why distance stereotests such as the FD2 or Distance Randot have been found to be more sensitive than near tests in intermittent exotropia, and to be a more valuable tool in management and predicting surgery.39, 40, 41, 42, 43 Patients with intermittent exotropia generally find it easier to maintain correct fixation at near distances. When tested at distance, even if they do not show a measurable deviation, they may fixate less accurately or precisely (meaning that there may be a mean misalignment, or simply greater fluctuations). This will reduce their measured stereoacuity. Thus, near stereotests are more practical for assessing sensory mechanisms of stereopsis, but distance stereotests are probably more useful in assessing oculomotor function.
Contour vs cyclopean stereograms
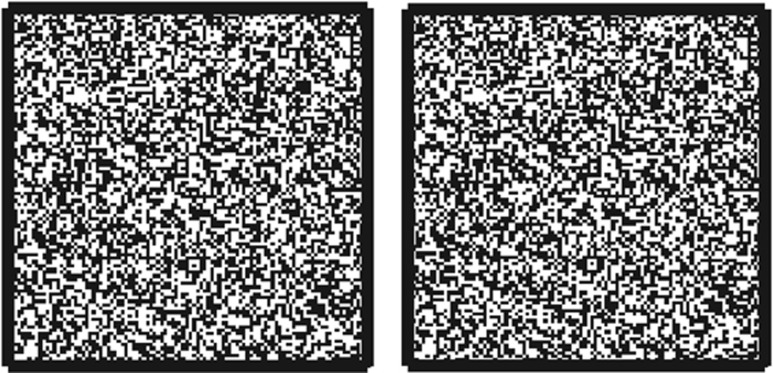
Stereotest stimuli can be divided into two classes. In the first, disparity is applied to monocularly visible contours, as in a line drawing of a circle or fly, that are defined by their luminance in the usual way. In the second, the object to be detected is defined only by its disparity. Monocularly, the image appears as a pattern of dots or the ‘static' of an untuned television, without any contours that define the edges of the object (Figure 3). The object is revealed only when the two eyes' images are compared. These are sometimes known as ‘cyclopean' stimuli, a term introduced by Julesz.44 In Table 1, the column headed ‘Monocular contours?' distinguishes these two types of test image. Examples of ‘cyclopean' stereotests include the Lang and Randot Preschool tests; examples of ‘contour' stereotests include the Randot Circles and FD2. Several lines of evidence suggest that neuronal processing may be different for contour and cyclopean stereograms. Disparity-tuned neurons in primary visual cortex respond to disparity in both types of image, but additional mechanisms may be available to extract disparity in contour images. Contour and cyclopean stereograms are affected differently when one eye's image is replaced with its photographic negative, as in Figure 4. In dense random-dot patterns like those in Figure 3, this manipulation either destroys depth perception completely or leaves a weak perception of reversed depth.44, 45, 46 However, when the image is sufficiently sparse, for example, the line drawing shown in Figure 4, depth is seen in the direction consistent with the disparity of the contours.45, 47 As a second example, sparse line stimuli presented with large disparities, outside Panum's fusional range, are seen double, but subjects nevertheless show appropriate vergence movements and can report the sign of the disparity.48, 49 For random-dot stereograms with similarly large disparities, subjects are at chance.50 Several workers have suggested that human stereo vision consists of at least two distinct components, sometimes dubbed ‘coarse' and ‘fine' stereopsis, supported by different neural mechanisms.51, 52, 53 Contour and cyclopean test images probably activate these components to differing amounts.
Figure 3.
An example of a cyclopean stereogram. If the eyes are crossed or diverged such that each eye fixates the centre of one of the patterns, a square region will be seen standing out in depth. This square is not defined in either of the monocular images.
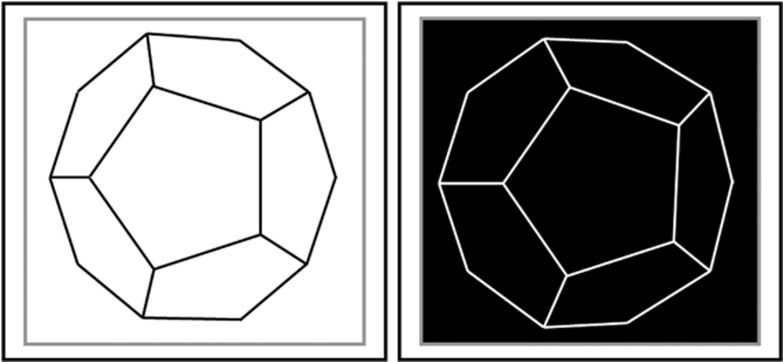
Figure 4.
A stereogram where one eye's image is replaced with its photographic negative, redrawn from Helmholtz47 (Plate IV, Figure Q). When these images are fused divergently, the central pentagon should appear in front, in accordance with the disparity of the lines, whereas the contrast mismatch produces the impression of a ‘crystal … of some dark shining substance like graphite'.47
Surprisingly, little seems to have been done in comparing contour vs cyclopean stereoacuity in healthy controls. Fawcett16 reports that, in 54 controls, there were no significant differences in stereoacuity measured with the Titmus circles, Randot circles, or Preschool Randot, but this may reflect the floor effect noted above, that is, all available disparities were above threshold for subjects with good stereoacuity. Wong et al36 report that, in 12 controls, stereoacuity was better with Contour Circles than with a Random Dot E stimulus; the median stereo threshold was ∼40 arcsec lower with the Circles than with the E.
Conversely, in strabismus patients, it is well established that patients will generally show better stereoacuity (ie, lower thresholds) when measured on a monocular-contour test such as the Randot graded-circles, as compared with a cyclopean pattern such as the Randot Preschool test.15, 16, 54, 55, 56, 57, 58, 59 Fu et al55 suggest that the better performance on contour stereograms may be because these ‘provide cues to fusion that allow some patients with strabismus to better control their deviations than random dot targets'. This motor explanation may well contribute,58 but sensory mechanisms probably contribute as well. Giaschi et al56 have reported that amblyopic children with poor or no stereopsis on the Randot Preschool test nevertheless perform as well as controls when the stimulus was a monocularly visible cartoon character with a large disparity. They conclude that although the ‘fine stereo' sensory system is impaired in these children, ‘coarse stereo' is spared.
The clinical importance of stereoacuity measurements
As we have seen, stereopsis in humans requires good vision in each eye individually, precise oculomotor control in order to direct the two eyes at a common target, and a population of binocular sensory neurons in visual cortex in order to detect the disparity between the two eyes' images. Stereopsis with cyclopean stimuli probably requires additional neuronal mechanisms over and above contour stimuli. Thus, good stereoacuity with cyclopean stimuli is the most demanding achievement of binocular vision. For this reason, as the Cochrane review on ‘Interventions for infantile esotropia' states,13 a measurement of stereoacuity is regarded as the gold standard for diagnosing the presence and quality of binocular vision. It is a key component of outcome measures in most studies of interventions for strabismus and amblyopia. For example, the Cochrane review on botulinum treatment for strabismus60 classifies outcomes based on angle of deviation, simultaneous perception, motor vergence, and stereoacuity.
Strabismus in early life prevents the normal development of binocular sensory neurons in visual cortex.61 Accordingly, early strabismus has a profoundly damaging effect on stereoacuity, particularly on the ‘fine' stereoacuity that works with cyclopean images and depends on these binocular neurons. Studies in non-human primates suggest that the sensitive period for binocular vision may be substantially longer than for other aspects of vision such as spectral sensitivity.62 In healthy controls, although cyclopean stereo vision can be demonstrated in infants at ∼3 months,63, 64 stereoacuity continues to improve up to the age of ∼10 years.65, 66, 67, 68, 69, 70 This long period of plasticity implies that binocular vision remains vulnerable to disruption until later in development, for example, by the onset of accommodative esotropia in toddlerhood.71 Conversely, the window for recovery remains open for longer. Indeed, there are occasional reports of strabismus patients recovering stereopsis as adults, many years after treatment.72 Thus, although early strabismus is extremely damaging to stereo vision, it is also clear that sufficiently early intervention can go some way to restoring it.
Infantile esotropia, defined as a large-angle inwards deviation that becomes constant before 6 months of age, unsurprisingly has a particularly disruptive effect on stereo vision. Among children whose eyes are surgically aligned after the age of 24 months, only 12% achieve any stereo vision,73 although this rises to 74% among children aligned before 6 months of age.73 Birch et al73 suggest that the poorer outcome of surgery after 6 months does not reflect the closure of a sensitive period, but simply the brain's longer exposure to misaligned visual input. However, although early surgery does restore some stereo vision, stereoacuity is by no means normal.73, 74, 75 One possibility is that infantile esotropia reflects a pre-existing sensory deficit. However, Birch et al73, 74, 75 argue that the available evidence does not support this, and argue that the sensory deficit is secondary to the motor deficit. They suggest that stereoacuity remains subnormal because surgery was not carried out soon enough, and did not align the eyes precisely enough. Normal stereoacuity may require alignment within 0.6 prism dioptres, yet ocular alignment can only be measured clinically to around ±3 prism dioptres.76 Birch et al75 suggest that very early (<2–3 months of age) treatment may be necessary, with botulinum treatment preferable to surgery.77 These conclusions are supported in other forms of strabismus such as accommodative esotropia and intermittent exotropia. In all cases, early intervention is associated with better stereo vision,13, 78 and the critical factor is the duration of the misalignment. Fawcett et al78 conclude that fine stereoacuity is likely to be permanently impaired by a constant misalignment that persists for longer than 4 months. Prompt treatment is therefore important if normal stereoacuity is to be achieved.
Achieving good stereo vision is a valuable goal. Binocular disparity cues help us guide our hand movements precisely79, 80, 81, 82 and both children and adults with impaired stereo vision perform worse on a range of visuomotor tasks than their peers with normal stereoacuity.83, 84, 85, 86, 87, 88, 89, 90 Stereoacuity has also been linked to better reading ability,91, 92 perhaps because both stereopsis and reading require precise control of eye movements.93 Untreated children with infantile esotropia lag behind on developmental milestones but catch up following early alignment surgery (in the first year of life),94 an outcome that the authors attribute to better binocular vision and stereopsis. However, it remains unclear whether this motor improvement reflects stereopsis specifically or some other aspect of binocular vision.95
As well as enabling better visual and motor performance, stereoacuity is also linked to long-term stability of alignment.96, 97 Birch et al96, 97 studied children who underwent surgery for infantile esotropia, resulting in stable alignment within 4 prism dioptres by 2 years of age. Children who had no stereo vision postoperatively were 3.6 times more likely to need repeat surgery later in childhood. Out of 60 children with accommodative esotropia who received successful optical correction to within 4 prism dioptres by age 4, those who had no stereo vision following alignment were 17 times more likely to need surgery later. These are very striking differences. Several mechanisms may contribute to these differential risk factors. For example, it may be that perfect orthotropia is more stable than less perfect alignment, and also allows better stereoacuity. The children with stereopsis may simply have been those whose misalignment was corrected most accurately. The low precision of clinical measurement of misalignment makes it hard to test this hypothesis; after treatment, all these children were equally well aligned to within the precision possible with clinical measurements.76 Alternatively, the differences may have been sensory: perhaps the children who were stereoblind after alignment had lost the sensory neurons that normally support stereopsis. As these neurons help maintain correct alignment by triggering vergence reflexes, it is not surprising that children in whom these mechanisms are spared are better able to maintain long-term alignment. What is clear is that stereoacuity is the most sensitive outcome measure currently available; stereoacuity measures immediately after treatment predict the long-term success.
Conclusion
Human stereo vision is capable of remarkably precise judgments, discriminating binocular disparities as small as 2 seconds of arc. Such performance requires good vision in both eyes, very precise oculomotor coordination and specialised sensory neurons in visual cortex. A measurement of stereoacuity is therefore a very sensitive test of binocular function at both the ocular and cortical levels. As well as enabling the clinician to assess the short-term success of surgery, it can also predict long-term outcomes. However, current measures of stereoacuity are plagued by low reliability that limits their usefulness in practice. Making the measurement of stereoacuity more precise and reliable, especially in young children, should improve its value as a tool to manage strabismus.
Acknowledgments
I thank Michael Clarke for reading the manuscript, and to everyone at the Cambridge Ophthalmological Symposium for helpful feedback and interesting discussions.
JCAR is the Principal Investigator on a Health Innovation Challenge Fund award entitled ‘Accurate and patient-friendly measurement of binocular visual function using a 3D mobile device' that aims to develop a new stereotest in game format.
Footnotes
This work was presented at the Cambridge Ophthalmological Symposium, 5 September 2014.
References
- Cumming BG, DeAngelis GC. The physiology of stereopsis. Ann Rev Neurosci. 2001;24:203–238. doi: 10.1146/annurev.neuro.24.1.203. [DOI] [PubMed] [Google Scholar]
- Tweed D. Visual-motor optimization in binocular control. Vision Res. 1997;37 (14:1939–1951. doi: 10.1016/s0042-6989(97)00002-3. [DOI] [PubMed] [Google Scholar]
- Schreiber K, Crawford JD, Fetter M, Tweed D. The motor side of depth vision. Nature. 2001;410 (6830:819–822. doi: 10.1038/35071081. [DOI] [PubMed] [Google Scholar]
- Read JC, Phillipson GP, Glennerster A. Latitude and longitude vertical disparities. J Vis. 2009;9 (13:11.1–37. doi: 10.1167/9.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SJ, Pointon AD, Cumming BG, Parker AJ. Quantitative analysis of the responses of V1 neurons to horizontal disparity in dynamic random-dot stereograms. J Neurophysiol. 2002;87:191–208. doi: 10.1152/jn.00465.2000. [DOI] [PubMed] [Google Scholar]
- Read JCA, Cumming BG. Understanding the cortical specialization for horizontal disparity. Neural Comput. 2004;16:1983–2020. doi: 10.1162/0899766041732440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming BG. An unexpected specialization for horizontal disparity in primate primary visual cortex. Nature. 2002;418 (6898:633–636. doi: 10.1038/nature00909. [DOI] [PubMed] [Google Scholar]
- Eadie AS, Carlin PJ. Evolution of control system models of ocular accommodation, vergence and their interaction. Med Biol Eng Comput. 1995;33 (4:517–524. doi: 10.1007/BF02522508. [DOI] [PubMed] [Google Scholar]
- Schor CM. A dynamic model of cross-coupling between accommodation and convergence: simulations of step and frequency responses. Optom Vis Sci. 1992;69 (4:258–269. doi: 10.1097/00006324-199204000-00002. [DOI] [PubMed] [Google Scholar]
- Howard IP. Seeing in Depth, Volume 1: Basic Mechanisms. I Porteous: Ontario, Canada; 2002. [Google Scholar]
- Wong AM, Lueder GT, Burkhalter A, Tychsen L. Anomalous retinal correspondence: neuroanatomic mechanism in strabismic monkeys and clinical findings in strabismic children. J AAPOS. 2000;4 (3:168–174. [PubMed] [Google Scholar]
- Von Noorden G, Campos EC. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. Mosby-Year Book: St Louis; 2002. [Google Scholar]
- Elliott S, Shafiq A. Interventions for infantile esotropia. Cochrane Database Syst Rev. 2013;7:CD004917. doi: 10.1002/14651858.CD004917.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron S, Lages M. Screening and sampling in studies of binocular vision. Vision Res. 2012;62:228–234. doi: 10.1016/j.visres.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Fricke TR, Siderov J. Stereopsis, stereotests, and their relation to vision screening and clinical practice. Clin Exp Optom. 1997;80 (5:165–172. [Google Scholar]
- Fawcett SL. An evaluation of the agreement between contour-based circles and random dot-based near stereoacuity tests. J AAPOS. 2005;9 (6:572–578. doi: 10.1016/j.jaapos.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Mayhew EW, Frisby JP. The induced effect: arguments against the theory of Arditi, Kaufman and Movshon (1981) Vision Res. 1982;22 (9:1225–1228. doi: 10.1016/0042-6989(82)90090-6. [DOI] [PubMed] [Google Scholar]
- Fawcett SL, Birch EE. Validity of the Titmus and Randot circles tasks in children with known binocular vision disorders. J AAPOS. 2003;7 (5:333–338. doi: 10.1016/s1091-8531(03)00170-8. [DOI] [PubMed] [Google Scholar]
- Adams WE, Hrisos S, Richardson S, Davis H, Frisby JP, Clarke MP. Frisby Davis distance stereoacuity values in visually normal children. Br J Ophthalmol. 2005;89 (11:1438–1441. doi: 10.1136/bjo.2005.071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Fawcett SL. Testing distance stereoacuity with the Frisby-Davis 2 (FD2) test. Am J Ophthalmol. 2005;139 (1:193–195. doi: 10.1016/j.ajo.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Ciner EB, Ying GS, Kulp MT, Maguire MG, Quinn GE, Orel-Bixler D, et al. Stereoacuity of preschool children with and without vision disorders. Optom Vis Sci. 2014;91 (3:351–358. doi: 10.1097/OPX.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PP, Maguire MG, Moore B, Cyert L. Testability of preschoolers on stereotests used to screen vision disorders. Optom Vis Sci. 2003;80 (11:753–757. doi: 10.1097/00006324-200311000-00012. [DOI] [PubMed] [Google Scholar]
- Howard IP, Rogers BJ. Seeing in Depth, Volume 2: Depth Perception. I Porteous: Ontario, Canada; 2002. [Google Scholar]
- Siderov J, Harwerth RS. Stereopsis, spatial frequency and retinal eccentricity. Vision Res. 1995;35 (16:2329–2337. doi: 10.1016/0042-6989(94)00307-8. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1 (8476:307–310. [PubMed] [Google Scholar]
- Fawcett SL, Birch EE. Interobserver test-retest reliability of the Randot preschool stereoacuity test. J AAPOS. 2000;4 (6:354–358. doi: 10.1067/mpa.2000.110340. [DOI] [PubMed] [Google Scholar]
- Adler P, Scally AJ, Barrett BT. Test—retest variability of Randot stereoacuity measures gathered in an unselected sample of UK primary school children. Br J Ophthalmol. 2012;96 (5:656–661. doi: 10.1136/bjophthalmol-2011-300729. [DOI] [PubMed] [Google Scholar]
- Adams WE, Leske DA, Hatt SR, Holmes JM. Defining real change in measures of stereoacuity. Ophthalmology. 2009;116 (2:281–285. doi: 10.1016/j.ophtha.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegbaum-Stehberger B, Jiang X, Mojon DS. Performance of a new, 3D-monitor based random-dot stereotest for children under 4 years of age. Graefes Arch Clin Exp Ophthalmol. 2008;246 (1:1–7. doi: 10.1007/s00417-007-0632-3. [DOI] [PubMed] [Google Scholar]
- Kim J, Yang HK, Kim Y, Lee B, Hwang JM.Distance stereotest using a 3-dimensional monitor for adult subjects Am J Ophthalmol 2011151(61081–1086.e1. [DOI] [PubMed] [Google Scholar]
- Bach M, Schmitt C, Kromeier M, Kommerell G. The Freiburg Stereoacuity Test: automatic measurement of stereo threshold. Graefes Arch Clin Exp Ophthalmol. 2001;239 (8:562–566. doi: 10.1007/s004170100317. [DOI] [PubMed] [Google Scholar]
- Breyer A, Jiang X, Rutsche A, Mojon DS. A new 3D monitor-based random-dot stereotest for children. Invest Ophthalmol Vis Sci. 2006;47 (11:4842–4846. doi: 10.1167/iovs.06-0238. [DOI] [PubMed] [Google Scholar]
- Bradshaw MF, Glennerster A. Stereoscopic acuity and observation distance. Spat Vis. 2006;19 (1:21–36. doi: 10.1163/156856806775009250. [DOI] [PubMed] [Google Scholar]
- Ogle KN. Note on stereoscopic acuity and observation distance. J Opt Soc Am. 1958;48 (11:794–798. doi: 10.1364/josa.48.000794. [DOI] [PubMed] [Google Scholar]
- Jameson D, Hurvich LM. Note on factors influencing the relation between stereoscopic acuity and observation distance. J Opt Soc Am. 1959;49 (6:639. doi: 10.1364/josa.49.000639. [DOI] [PubMed] [Google Scholar]
- Wong BP, Woods RL, Peli E. Stereoacuity at distance and near. Optom Vis Sci. 2002;79 (12:771–778. doi: 10.1097/00006324-200212000-00009. [DOI] [PubMed] [Google Scholar]
- Cumming B, Parker A. Local disparity not perceived depth is signaled by binocular neurons in cortical area V1 of the Macaque. J Neurosci. 2000;20 (12:4758–4767. doi: 10.1523/JNEUROSCI.20-12-04758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. The range and scope of binocular depth discrimination in man. J Physiol. 1970;211 (3:599–622. doi: 10.1113/jphysiol.1970.sp009296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams WE, Leske DA, Hatt SR, Mohney BG, Birch EE, Weakley DR, Jr, et al. Improvement in distance stereoacuity following surgery for intermittent exotropia. J AAPOS. 2008;12 (2:141–144. doi: 10.1016/j.jaapos.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck D, Hatt SR, Haggerty H, Hrisos S, Strong NP, Steen NI, et al. The use of the Newcastle Control Score in the management of intermittent exotropia. Br J Ophthalmol. 2007;91 (2:215–218. doi: 10.1136/bjo.2006.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal TD, Rosenbaum AL, Stathacopoulos RA.Distance stereo acuity improvement in intermittent exotropic patients following strabismus surgery J Pediatr Ophthalmol Strabismus 199532(6353–357.discussion 8. [DOI] [PubMed] [Google Scholar]
- Stathacopoulos RA, Rosenbaum AL, Zanoni D, Stager DR, McCall LC, Ziffer AJ, et al. Distance stereoacuity. Assessing control in intermittent exotropia. Ophthalmology. 1993;100 (4:495–500. doi: 10.1016/s0161-6420(93)31616-7. [DOI] [PubMed] [Google Scholar]
- Hatt SR, Haggerty H, Buck D, Adams W, Strong NP, Clarke MP. Distance stereoacuity in intermittent exotropia. Br J Ophthalmol. 2007;91 (2:219–221. doi: 10.1136/bjo.2006.099465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julesz B. Foundations of Cyclopean Perception. University of Chicago Press: Chicago; 1971. [Google Scholar]
- Cogan AI, Lomakin AJ, Rossi AF. Depth in anticorrelated stereograms: effects of spatial density and interocular delay. Vision Res. 1993;33 (14:1959–1975. doi: 10.1016/0042-6989(93)90021-n. [DOI] [PubMed] [Google Scholar]
- Read JCA, Eagle RA. Reversed stereo depth and motion direction with anti-correlated stimuli. Vision Res. 2000;40 (24:3345–3358. doi: 10.1016/s0042-6989(00)00182-6. [DOI] [PubMed] [Google Scholar]
- Helmholtz HV. Handbuch der Physiologischen Optik. Leopold Voss: Leipzig; 1867. [Google Scholar]
- Ogle KN. On the limits of stereoscopic vision. J Exp Psychol. 1952;44 (4:253–259. doi: 10.1037/h0057643. [DOI] [PubMed] [Google Scholar]
- Mitchell DE. Properties of stimuli eliciting vergence eye movements and stereopsis. Vision Res. 1970;10 (2:145–162. doi: 10.1016/0042-6989(70)90112-4. [DOI] [PubMed] [Google Scholar]
- Glennerster A. dmax for stereopsis and motion in random-dot displays. Vision Res. 1998;38 (6:925–935. doi: 10.1016/s0042-6989(97)00213-7. [DOI] [PubMed] [Google Scholar]
- Tyler CW. A stereoscopic view of visual processing streams. Vision Res. 1990;30 (11:1877–1895. doi: 10.1016/0042-6989(90)90165-h. [DOI] [PubMed] [Google Scholar]
- Norcia AM, Sutter EE, Tyler CW. Electrophysiological evidence for the existence of coarse and fine disparity mechanisms in human. Vision Res. 1985;25 (11:1603–1611. doi: 10.1016/0042-6989(85)90130-0. [DOI] [PubMed] [Google Scholar]
- Poggio GF, Talbot WH. Mechanisms of static and dynamic stereopsis in foveal cortex of the rhesus monkey. J Physiol. 1981;315:469–492. doi: 10.1113/jphysiol.1981.sp013759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske DA, Birch EE, Holmes JM. Real depth vs randot stereotests. Am J Ophthalmol. 2006;142 (4:699–701. doi: 10.1016/j.ajo.2006.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu VL, Birch EE, Holmes JM. Assessment of a new Distance Randot stereoacuity test. J AAPOS. 2006;10 (5:419–423. doi: 10.1016/j.jaapos.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Giaschi D, Lo R, Narasimhan S, Lyons C, Wilcox LM.Sparing of coarse stereopsis in stereodeficient children with a history of amblyopia J Vis 201313(10pii: 17. [DOI] [PubMed] [Google Scholar]
- Simons K. A comparison of the Frisby, Random-Dot E, TNO, and Randot circles stereotests in screening and office use. Arch Ophthalmol. 1981;99 (3:446–452. doi: 10.1001/archopht.1981.03930010448011. [DOI] [PubMed] [Google Scholar]
- Frisby JP, Mein J, Saye A, Stanworth A. Use of random-dot sterograms in the clinical assessment of strabismic patients. Br J Ophthalmol. 1975;59 (10:545–552. doi: 10.1136/bjo.59.10.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke RD, Simons K. A new stereoscopic test for amblyopia screening. Am J Ophthalmol. 1974;78 (4:714–721. doi: 10.1016/s0002-9394(14)76311-1. [DOI] [PubMed] [Google Scholar]
- Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2012;2:CD006499. doi: 10.1002/14651858.CD006499.pub3. [DOI] [PubMed] [Google Scholar]
- Crawford ML, von Noorden GK. The effects of short-term experimental strabismus on the visual system in Macaca mulatta. Invest Ophthalmol Vis Sci. 1979;18 (5:496–505. [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, 3rd, Duncan GC, Crawford ML, von Noorden GK. Multiple sensitive periods in the development of the primate visual system. Science. 1986;232 (4747:235–238. doi: 10.1126/science.3952507. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Julesz B, Kropfl W, Bodis-Wollner I, Raab E. Cortical binocularity in infants. Nature. 1980;288 (5789:363–365. doi: 10.1038/288363a0. [DOI] [PubMed] [Google Scholar]
- Braddick O, Wattam-Bell J, Day J, Atkinson J. The onset of binocular function in human infants. Hum Neurobiol. 1983;2 (2:65–69. [PubMed] [Google Scholar]
- Schmid M, Largo RH. Visual acuity and stereopsis between the ages of 5 and 10 years. A cross-sectional study. Eur J Pediatr. 1986;145 (6:475–479. doi: 10.1007/BF02429046. [DOI] [PubMed] [Google Scholar]
- Simons K. Stereoacuity norms in young children. Arch Ophthalmol. 1981;99 (3:439–445. doi: 10.1001/archopht.1981.03930010441010. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell J. Stereo and motion Dmax in infants. J Vis. 2009;9 (6:9 1–9 9. doi: 10.1167/9.6.9. [DOI] [PubMed] [Google Scholar]
- Oduntan AO, Al-Ghamdi M, Al-Dosari H. Randot stereoacuity norms in a population of Saudi Arabian children. Clin Exp Optom. 1998;81 (5:193–197. doi: 10.1111/j.1444-0938.1998.tb06734.x. [DOI] [PubMed] [Google Scholar]
- Serrano-Pedraza I, Manjunath V, Osunkunle O, Clarke MP, Read JC. Visual suppression in intermittent exotropia during binocular alignment. Invest Ophthalmol Vis Sci. 2011;52 (5:2352–2364. doi: 10.1167/iovs.10-6144. [DOI] [PubMed] [Google Scholar]
- Hong SW, Park SC. Development of distant stereoacuity in visually normal children as measured by the Frisby-Davis distance stereotest. Br J Ophthalmol. 2008;92 (9:1186–1189. doi: 10.1136/bjo.2008.138362. [DOI] [PubMed] [Google Scholar]
- Birch EE. Marshall Parks lecture. Binocular sensory outcomes in accommodative ET. J AAPOS. 2003;7 (6:369–373. doi: 10.1016/j.jaapos.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Sacks O. Stereo Sue: Why two eyes are better than one. New Yorker. 2006;6:64. [Google Scholar]
- Birch EE, Fawcett S, Stager DR. Why does early surgical alignment improve stereoacuity outcomes in infantile esotropia. J AAPOS. 2000;4 (1:10–14. doi: 10.1016/s1091-8531(00)90005-3. [DOI] [PubMed] [Google Scholar]
- Birch EE, Stager DR, Everett ME. Random dot stereoacuity following surgical correction of infantile esotropia. J Pediatr Ophthalmol Strabismus. 1995;32 (4:231–235. doi: 10.3928/0191-3913-19950701-07. [DOI] [PubMed] [Google Scholar]
- Birch EE, Wang J. Stereoacuity outcomes after treatment of infantile and accommodative esotropia. Optom Vis Sci. 2009;86 (6:647–652. doi: 10.1097/OPX.0b013e3181a6168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Leske DA, Hohberger GG. Defining real change in prism-cover test measurements. Am J Ophthalmol. 2008;145 (2:381–385. doi: 10.1016/j.ajo.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeer KW, Tucker MG, Guerry CH, Spencer RF. Incidence of stereopsis after treatment of infantile esotropia with botulinum toxin A. J Pediatr Ophthalmol Strabismus. 2003;40 (5:288–292. doi: 10.3928/0191-3913-20030901-10. [DOI] [PubMed] [Google Scholar]
- Fawcett S, Leffler J, Birch EE. Factors influencing stereoacuity in accommodative esotropia. J AAPOS. 2000;4 (1:15–20. doi: 10.1016/s1091-8531(00)90006-5. [DOI] [PubMed] [Google Scholar]
- Bradshaw MF, Elliott KM, Watt SJ, Hibbard PB, Davies IR, Simpson PJ. Binocular cues and the control of prehension. Spat Vis. 2004;17 (1-2:95–110. doi: 10.1163/156856804322778288. [DOI] [PubMed] [Google Scholar]
- Watt SJ, Bradshaw MF. The visual control of reaching and grasping: binocular disparity and motion parallax. J Exp Psychol Hum Percept Perform. 2003;29 (2:404–415. doi: 10.1037/0096-1523.29.2.404. [DOI] [PubMed] [Google Scholar]
- Melmoth DR, Grant S. Advantages of binocular vision for the control of reaching and grasping. Exp Brain Res. 2006;171 (3:371–388. doi: 10.1007/s00221-005-0273-x. [DOI] [PubMed] [Google Scholar]
- Melmoth DR, Storoni M, Todd G, Finlay AL, Grant S. Dissociation between vergence and binocular disparity cues in the control of prehension. Exp Brain Res. 2007;183 (3:283–298. doi: 10.1007/s00221-007-1041-x. [DOI] [PubMed] [Google Scholar]
- Grant S, Melmoth DR, Morgan MJ, Finlay AL. Prehension deficits in amblyopia. Invest Ophthalmol Vis Sci. 2007;48 (3:1139–1148. doi: 10.1167/iovs.06-0976. [DOI] [PubMed] [Google Scholar]
- O'Connor AR, Birch EE, Anderson S, Draper H. The functional significance of stereopsis. Invest Ophthalmol Vis Sci. 2010;51 (4:2019–2023. doi: 10.1167/iovs.09-4434. [DOI] [PubMed] [Google Scholar]
- Sachdeva R, Traboulsi EI.Performance of patients with deficient stereoacuity on the EYESi microsurgical simulator Am J Ophthalmol 2011151(3427–433,.e1. [DOI] [PubMed] [Google Scholar]
- Suttle CM, Melmoth DR, Finlay AL, Sloper JJ, Grant S. Eye-hand coordination skills in children with and without amblyopia. Invest Ophthalmol Vis Sci. 2011;52 (3:1851–1864. doi: 10.1167/iovs.10-6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Gole GA, Brown B. The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci. 2008;49 (2:594–603. doi: 10.1167/iovs.07-0869. [DOI] [PubMed] [Google Scholar]
- Murdoch JR, McGhee CN, Glover V.The relationship between stereopsis and fine manual dexterity: pilot study of a new instrument Eye 19915(Pt 5):642–643. [DOI] [PubMed] [Google Scholar]
- Hrisos S, Clarke MP, Kelly T, Henderson J, Wright CM. Unilateral visual impairment and neurodevelopmental performance in preschool children. Br J Ophthalmol. 2006;90 (7:836–838. doi: 10.1136/bjo.2006.090910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielder AR, Moseley MJ. Does stereopsis matter in humans. Eye. 1996;10 (Pt 2:233–238. doi: 10.1038/eye.1996.51. [DOI] [PubMed] [Google Scholar]
- Kulp MT, Schmidt PP. Visual predictors of reading performance in kindergarten and first grade children. Optom Vis Sci. 1996;73 (4:255–262. doi: 10.1097/00006324-199604000-00007. [DOI] [PubMed] [Google Scholar]
- Kulp MT, Schmidt PP.A pilot study. Depth perception and near stereoacuity: is it related to academic performance in young children Binocul Vis Strabismus Q 200217(2129–134.discussion 33.. [PubMed] [Google Scholar]
- Palomo-Alvarez C, Puell MC. Binocular function in school children with reading difficulties. Graefes Arch Clin Exp Ophthalmol. 2010;248 (6:885–892. doi: 10.1007/s00417-009-1251-y. [DOI] [PubMed] [Google Scholar]
- Drover JR, Stager DR, Sr, Morale SE, Leffler JN, Birch EE. Improvement in motor development following surgery for infantile esotropia. J AAPOS. 2008;12 (2:136–140. doi: 10.1016/j.jaapos.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RK, Lee DN. Why two eyes are better than one: the two views of binocular vision. J Exp Psychol Hum Percept Perform. 1981;7 (1:30–40. doi: 10.1037//0096-1523.7.1.30. [DOI] [PubMed] [Google Scholar]
- Birch EE, Stager DR, Sr, Berry P, Leffler J. Stereopsis and long-term stability of alignment in esotropia. J AAPOS. 2004;8 (2:146–150. doi: 10.1016/j.jaapos.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Birch EE, Fawcett SL, Stager DR., Sr Risk factors for the development of accommodative esotropia following treatment for infantile esotropia. J AAPOS. 2002;6 (3:174–181. doi: 10.1067/mpa.2002.122962. [DOI] [PubMed] [Google Scholar]
- Birch EE, Morale SE, Jeffrey BG, O'Connor AR, Fawcett SL. Measurement of stereoacuity outcomes at ages 1 to 24 months: Randot Stereocards. J AAPOS. 2005;9 (1:31–36. doi: 10.1016/j.jaapos.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Birch E, Williams C, Drover J, Fu V, Cheng C, Northstone K, et al. Randot Preschool Stereoacuity Test: normative data and validity. J AAPOS. 2008;12 (1:23–26. doi: 10.1016/j.jaapos.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsari S, Rose KA, Pai AS, Gole GA, Leone JF, Burlutsky G, et al. Diagnostic reliability and normative values of stereoacuity tests in preschool-aged children. Br J Ophthalmol. 2013;97 (3:308–313. doi: 10.1136/bjophthalmol-2012-302192. [DOI] [PubMed] [Google Scholar]
- Ciner EB, Schanel-Klitsch E, Herzberg C. Stereoacuity development: 6 months to 5 years. A new tool for testing and screening. Optom Vis Sci. 1996;73 (1:43–48. doi: 10.1097/00006324-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Maguire M, Dobson V, Quinn G, Ciner E, Cyert L, et al. Comparison of preschool vision screening tests as administered by licensed eye care professionals in the Vision In Preschoolers Study. Ophthalmology. 2004;111 (4:637–650. doi: 10.1016/j.ophtha.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Moll AM, Rao RC, Rotberg LB, Roarty JD, Bohra LI, Baker JD. The role of the random dot Stereo Butterfly test as an adjunct test for the detection of constant strabismus in vision screening. J AAPOS. 2009;13 (4:354–356. doi: 10.1016/j.jaapos.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Cooper J, Feldman J, Medlin D. Comparing stereoscopic performance of children using the Titmus, TNO, and Randot stereo tests. J Am Optom Assoc. 1979;50 (7:821–825. [PubMed] [Google Scholar]
- Bohr I, Read JC. Stereoacuity with Frisby and Revised FD2 Stereo Tests. PLoS One. 2013;8 (12:e82999. doi: 10.1371/journal.pone.0082999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J, Clift GD. The validity of the Frisby stereotest as a measure of precise stereoacuity. J Am Optom Assoc. 1984;55 (7:505–506. [PubMed] [Google Scholar]
- Brown S, Weih L, Mukesh N, McCarty C, Taylor H. Assessment of adult stereopsis using the Lang 1 Stereotest: a pilot study. Binocul Vis Strabismus Q. 2001;16 (2:91–98. [PubMed] [Google Scholar]
- Huynh SC, Ojaimi E, Robaei D, Rose K, Mitchell P. Accuracy of the Lang II stereotest in screening for binocular disorders in 6-year-old children. Am J Ophthalmol. 2005;140 (6:1130–1132. doi: 10.1016/j.ajo.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Lindstrom A, Davis H, Frisby JP. Does binocularly perceived depth correlate with reduced stereoacuity. Ophthalmic Physiol Opt. 2009;29 (1:92–98. doi: 10.1111/j.1475-1313.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- Costa MF, Moreira SM, Hamer RD, Ventura DF. Effects of age and optical blur on real depth stereoacuity. Ophthalmic Physiol Opt. 2010;30 (5:660–666. doi: 10.1111/j.1475-1313.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- Schmidt PP. Vision screening with the RDE stereotest in pediatric populations. Optom Vis Sci. 1994;71 (4:273–281. doi: 10.1097/00006324-199404000-00008. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Maguire M, Kulp MT, Dobson V, Quinn G. Random Dot E stereotest: testability and reliability in 3- to 5-year-old children. J AAPOS. 2006;10 (6:507–514. doi: 10.1016/j.jaapos.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hatt SR, O'Connor AR, Drover JR, Adams R, Birch EE, et al. Final version of the Distance Randot Stereotest: normative data, reliability, and validity. J AAPOS. 2010;14 (2:142–146. doi: 10.1016/j.jaapos.2009.12.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Birch EE, Leske DA, Fu VL, Mohney BG. New tests of distance stereoacuity and their role in evaluating intermittent exotropia. Ophthalmology. 2007;114 (6:1215–1220. doi: 10.1016/j.ophtha.2006.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Sharma P, Singh D, Saxena R, Sharma A, Menon V. Evaluation of FD2 (Frisby Davis distance) stereotest in surgical management of intermittent exotropia. Br J Ophthalmol. 2013;97 (10:1318–1321. doi: 10.1136/bjophthalmol-2012-302321. [DOI] [PubMed] [Google Scholar]
- Saxena R, Kakkar A, Menon V, Sharma P, Phuljhele S. Evaluation of factors influencing distance stereoacuity on Frisby-Davis Distance Test (FD2) in intermittent exotropia. Br J Ophthalmol. 2011;95 (8:1098–1101. doi: 10.1136/bjo.2010.182139. [DOI] [PubMed] [Google Scholar]
- Young BJ, Sueke H, Wylie JM, Kaye SB. Contribution of a real depth distance stereoacuity test to clinical management. J Ophthalmol. 2009;2009:343827. doi: 10.1155/2009/343827. [DOI] [PMC free article] [PubMed] [Google Scholar]