Abstract
In this study, we evaluated the role of WNT16 in regulating bone size, an important determinant of bone strength. Mice with targeted disruption of the Wnt16 gene exhibited a 24% reduction in tibia cross-sectional area at 12 weeks of age compared with that of littermate wild-type (WT) mice. Histomorphometric studies revealed that the periosteal bone formation rate and mineral apposition rate were reduced (P < .05) by 55% and 32%, respectively, in Wnt16 knockout (KO) vs WT mice at 12 weeks of age. In contrast, the periosteal tartrate resistant acid phosphatase-labeled surface was increased by 20% in the KO mice. Because mechanical strain is an important physiological regulator of periosteal bone formation (BF), we determined whether mechanical loading–induced periosteal BF is compromised in Wnt16 KO mice. Application of 4800-μe strain to the right tibia using a 4-point bending loading method for 2 weeks (2-Hz frequency, 36 cycles per day, 6 days/wk) produced a significant increase in cross-sectional area (11% above that of the unloaded left tibia, P < .05, n = 6) in the WT but not in the KO mice (−0.2% change). Histomorphometric analyses revealed increases in the periosteal bone formation rate and mineral apposition rate in the loaded bones of WT but not KO mice. Wnt16 KO mice showed significant (20%–70%) reductions in the expression levels of markers of canonical (β-catenin and Axin2) but not noncanonical (Nfatc1 and Tnnt2) WNT signaling in the periosteum at 5 weeks of age. Our findings suggest that WNT16 acting via canonical WNT signaling regulates mechanical strain-induced periosteal BF and bone size.
Osteoporosis is a disease of decreased bone strength leading to increased risk of bone fracture in humans. Loss of bone strength in osteoporotic individuals is primarily determined by bone size (cross-sectional area [CSA]), mineral density, and quality. Although considerable efforts have been focused on identifying the genetic determinants of bone mineral density (BMD), little is known on the identities and mechanisms of action of genes that contribute to bone size variation. Therefore, identification of genes regulating bone size and understanding of their mechanisms of action are of critical importance in developing strategies to strengthen bones in conditions were bone strength is compromised.
In terms of genes regulating bone size, a recent study (1) reported that Wnt16 knockout (KO) mice (24 weeks old) had 40% to 60% reductions in tibia and femur bone strength (stiffness, maximal force to breakage, and work to failure), which was a consequence of decreased CSA (−33%) and cortical thickness (−27%). Furthermore, several independent human genome-wide association studies have identified the WNT16 locus as an important genetic determinant contributing to variations in BMD in humans (2–6). For example, Zheng et al (1) found significant associations between single nucleotide polymorphisms in the WNT16 locus and cortical thickness, in a genome-wide association study of 5 cohorts comprising 5672 men and women. In addition, 2 missense single nucleotide polymorphisms in the WNT16 gene had suggestive associations with forearm fractures. Medina-Gomez et al (7) identified variants mapping to the 7q31 locus, where the WNT16 gene resides, which were associated with total body BMD in 2660 children from a multiethnic cohort. In this study, bone expression of WNT16 correlated with the BMD of 78 women who had iliac crest biopsies. These studies are consistent with the prediction that genetic variations in WNT16 are associated with bone mass phenotypes and are a risk factor for fractures in humans.
Our goal was to further characterize the skeletal phenotype of Wnt16 KO mice and determine the cellular processes influenced by the lack of Wnt16 gene function. We also evaluated whether WNT16 participates in the mechanical strain response in the skeleton, because mechanical strain is a physiological regulator of bone strength, and previous studies have indicated a key role for WNT/β-catenin signaling in mediating the mechanical strain response in bone cells (8–12). In terms of the signaling pathways for WNT16 actions, limited studies thus far indicate involvement of both canonical and noncanonical WNT pathways in different tissues (4, 13–15). However, the molecular mechanism of WNT16 action in bone cells has not been investigated. We, therefore, evaluated the expression levels of components of the canonical and noncanonical WNT signaling pathways in the bones of Wnt16 KO and wild-type (WT) mice.
Materials and Methods
Animals
Wnt16 KO and littermate WT mice were generated at Lexicon Pharmaceuticals as part of a comprehensive KO mouse phenotyping campaign (16), with disruption of the first 3 exons in a C57BL6/J-129SvEv hybrid genetic background as described earlier (1). Breeding pairs of heterozygous Wnt16 mice were provided by Lexicon, and a breeding colony was established to generate Wnt16 KO mice and littermate control WT mice for comprehensive characterization of the skeletal phenotype. This study was confined to female mice because a greater proportion of women than men sustain fragility fractures and are most in need of periosteal expansion to compensate for the loss of endosteal and trabecular bone that occurs as a consequence of estrogen deficiency and advancing age (www.nof.org/articles/4). Animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee of the Jerry L. Pettis Memorial VA Medical Center. Isoflurane was used for anesthesia, and CO2 exposure for euthanasia of animals.
Genotyping
At 3 weeks of age, DNA was extracted from ear or tail tissue using a Puregene DNA purification Kit (Gentra Systems, Inc) according to the manufacturer's protocol. To identify the Wnt16 KO mice, PCR was performed using the forward primer 5′-TCAGACTCCAGGCGCGGTCAG-3′ and the reverse primer 5′-GCTCTGTCCATGGCCCACGCA-3′. To identify WT mice, the forward primer 5′-GCAGCGCATCGCCTTCTATC-3′ and reverse primer 5′-CAGGAAGCTGTTGAAGTTTGGC-3′ were used. The mutant mice were identified by the presence of a 541-bp PCR product, and the WT mice were identified by the presence of a 361-bp PCR product. Heterozygous mice contained both of the products. The PCR products were run on a 1.5% agarose gel, and the images were taken with a ChemiImager 4400 (Alpha Innotech Corp).
Phenotype analysis
To determine when the bone phenotype developed, mice were analyzed at 4 and 8 weeks of age by in vivo dual x-ray absorptiometry (DXA) analysis and at 12 weeks by micro-computed tomography (CT) analysis of isolated tibias. For analysis of the effect of mechanical loading on bone anabolism, the experiment was started when mice were 10 weeks old so that the animals were approximately 12 weeks old when euthanized. Vertebral data were obtained from 16- and 24-week-old mice examined at Lexicon Pharmaceuticals.
Bone densitometry by DXA
Bone mineral content (BMC), areal BMD, and bone area were measured in vivo by DXA, using a PIXImus scanner (Lunar). The precision for BMC and BMD was ±1% as determined for several repeat measurements of the same bones (17). Animals were anesthetized by using isoflurane during the DXA scan. Mouse body weight was measured by a metric balance and body length (head to anus) measured by a ruler. A micro-CT scout view of the entire tibia was used to measure length.
Periosteum RNA isolation and gene expression analysis
Total RNA from the periosteum was isolated using TRIzol from the marrow intact ulna and humerus bones obtained from 5-week-old female Wnt16 KO and WT mice, followed by reagents from an total RNA isolation kit (Zymo Research). In brief, the soft tissues were gently removed from marrow intact bone without disrupting the periosteum. The bones were immersed in 500 μL of TRIzol immediately after the surgical procedure and kept on ice for 1 hour. The tubes were vortexed on ice for 1 minute for every 15 minutes of extraction time to break down cell components from the bone surface. After 1 hour of incubation, the bones were removed, and RNA was extracted from the TRIzol solution according to the manufacturer's instructions. The quality and quantity of RNA were analyzed using the 2100 Bio-analyzer (Agilent) and NanoDrop instruments as per the manufacturer's instructions. Purified total RNA (200 μg/μL) was used to synthesize the first-strand cDNA by reverse transcription according to the manufacturer's instructions (Bio-Rad). Real-time PCR was used to determine the expression levels of genes as described previously (18). The gene-specific primers were designed by using Vector NTI software and ordered from Integrated DNA Technologies. The data were analyzed using SDS software, version 2.0, and the results were exported to Microsoft Excel for further analysis. Data normalization was accomplished using the endogenous control (β-actin), Wnt4 and Fzd6 were used to correct for variations in RNA quality among samples. The normalized cycle threshold (Ct) values were subjected to a 2−ΔΔCt formula to calculate the fold change between the externally loaded and nonloaded groups. The formula and its derivations were obtained from the instrument user guide.
Fluid flow shear stress
In vitro mechanical stress was applied to MC3T3-E1 cells as described earlier (19). Four groups (n = 5 replicates for each group) of MC3T3-E1 cells were plated on glass slides (75 × 38 mm) at 5 × 104 cells/slide in DMEM supplemented with 10% bovine calf serum. When the cells reached ∼80% confluence, they were serum deprived for 24 hours and then subjected to a steady fluid flow shear stress of 20 dynes/cm2 for 30 minutes in Cytodyne flow chambers as described previously (19). The study was terminated 12 hours after fluid flow shear stress, followed by isolation of total RNA using the TRIzol reagent for RT-PCR analysis.
Strain calculation
Mechanical strains produced by loads at the cortical site (mid-diaphysis) in tibias of 10-week-old female Wnt16 KO and WT mice were calculated by using a mathematical approach (20, 21).
Four-point bending and axial loading
To evaluate whether lack of the Wnt16 gene influences the periosteal bone response to mechanical loading, we used a 4-point bending method of loading. At 10 weeks of age, a 9-N load was applied to female WT mice and a 6-N load was applied to female Wnt16 KO mice. The loading was performed at 2-Hz frequency, for 36 cycles, once per day, 6 days/wk, with 1-day rest for 2 weeks on both sets of mice (18, 20). The right tibia was externally loaded and the left tibia served as a contralateral unloaded control. On day 15, mice were euthanized, and tibias were collected and fixed in 10% formalin for further analyses. Axial loading on the right tibia of 10-week-old female C57BL/6J mice was performed (10-N load, 3 alternate days per week for 2 weeks) as described previously (22).
Micro-CT
To measure cortical bone parameters and the periosteal bone response to mechanical loading, we used micro-CT, a high-resolution tomography image system (vivaCT40; Scanco) as described earlier (22). Vertebral bodies for the Wnt16 KO and WT mice were scanned at 8-μm voxel dimensions. The entire vertebral body and the cancellous compartment (for the secondary spongiosa region between the growth plates) were contoured and analyzed separately. Vertebral body cortical bone was determined as the difference between total and trabecular bone volume (BV)/tissue volume (TV). The parameters such as TV (cubic millimeters), CSA (square millimeters), BV (cubic millimeters), BV/TV (percentage), apparent BMD (milligrams hydroxyapatite per cubic centimeter), and cortical thickness (millimeters) were evaluated in the externally loaded right and nonexternally loaded left tibia of Wnt16 KO and WT mice. In this article, total area (long bones) or total volume (vertebral body) will be stated as bone CSA.
Histomorphometric analyses
To detect the bone formation differences due to Wnt16 KO, as well as loading induced changes, histomorphometric analyses were performed in calcein-labeled female WT and Wnt16 KO mice (23). For the loading study, calcein was administered on days 3 and 7 to label newly formed bone. On day 9, mice were euthanized, and both tibias were collected and fixed overnight with 10% cold neutral buffered formalin. Twenty-four hours later, bones were rinsed with PBS to remove formalin and embedded in methyl methacrylate. Thick cross-sections (0.5 mm) were cut from a sampling site 0.3-mm proximal to the fibular junction with a wire saw (Delaware Diamond Knives), and this cross-section was ground lightly. Calcein labels were measured around the entire perimeter to determine periosteal bone formation rate (BFR) and mineral apposition rate (MAR). Femurs of both Wnt16 KO and WT mice were fixed, demineralized, processed for paraffin embedding, and sectioned longitudinally. Mid-diaphyseal sections were stained to measure for tartrate resistant acid phosphatase (TRAP)-labeled surface. Sections stained for hematoxylin and eosin were used to measure the trabecular bone parameters (22). Four to 6 fields were measured for the trabecular data using a ×10 objective.
Statistical analyses
Data were presented as mean ± SE. A standard t test was used to evaluate the skeletal differences and loading-induced differences between KO and WT mice. Mechanical loading–induced changes in skeletal parameters are expressed as percent change in the loaded bone compared with those in unloaded, contralateral control bones. We used SPSS software 21 for our analyses, and the results were considered statistically significant at P < .05.
Results
Skeletal phenotype of Wnt16 KO mice
To evaluate the changes in the skeleton due to loss of Wnt16 gene function, we performed DXA analysis in female Wnt16 KO and WT mice at 4 and 8 weeks of age. Mice with targeted disruption of the Wnt16 gene showed no significant differences in body length at 4 or 8 weeks of age and only minor decreases in body weight (Figure 1, A and B). Furthermore, Wnt16 KO mice showed no significant differences in tissue weights for kidney, liver, and heart compared with those for WT mice (data not shown).
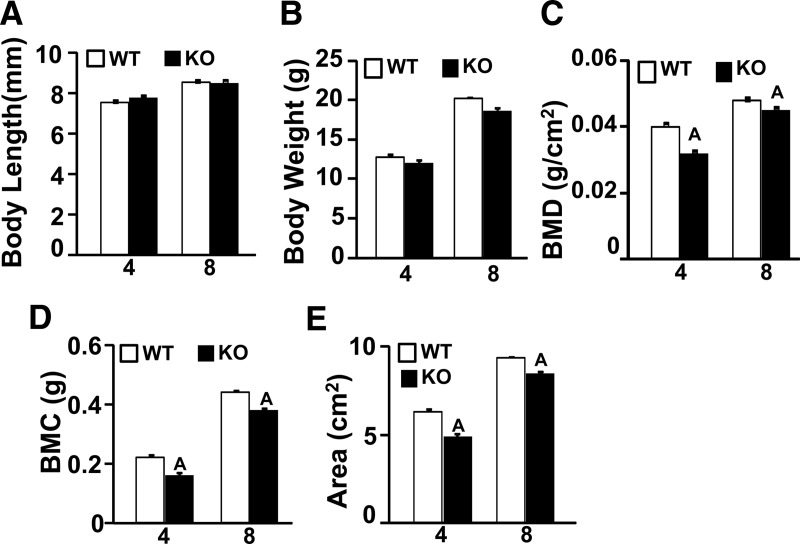
Figure 1.
Phenotype characterization by DXA at 4 and 8 weeks in female WT and Wnt16 KO mice. The y-axis corresponds to skeletal parameters and the x-axis corresponds to age (weeks). A, Body length. B, Body weight. C, BMD. D, BMC, E, Area. Values are means ± SE. (n = 7). A, P < .05 vs WT mice.
Total body BMC, total body bone area, and total body BMD were reduced by 33%, 25%, and 10%, respectively, in the female Wnt16 KO mice compared with those in the WT mice at 4 weeks (Figure 1). Similarly, at 8 weeks, the total body BMC, bone area, and BMD were reduced by 16%, 10%, and 6%, respectively, in the Wnt16 KO mice (Figure 1). BMD values for heterozygous mice lacking 1 allele of Wnt16 were not different from those in the WT mice (data not shown).
Bone size is reduced in female Wnt16 KO mice
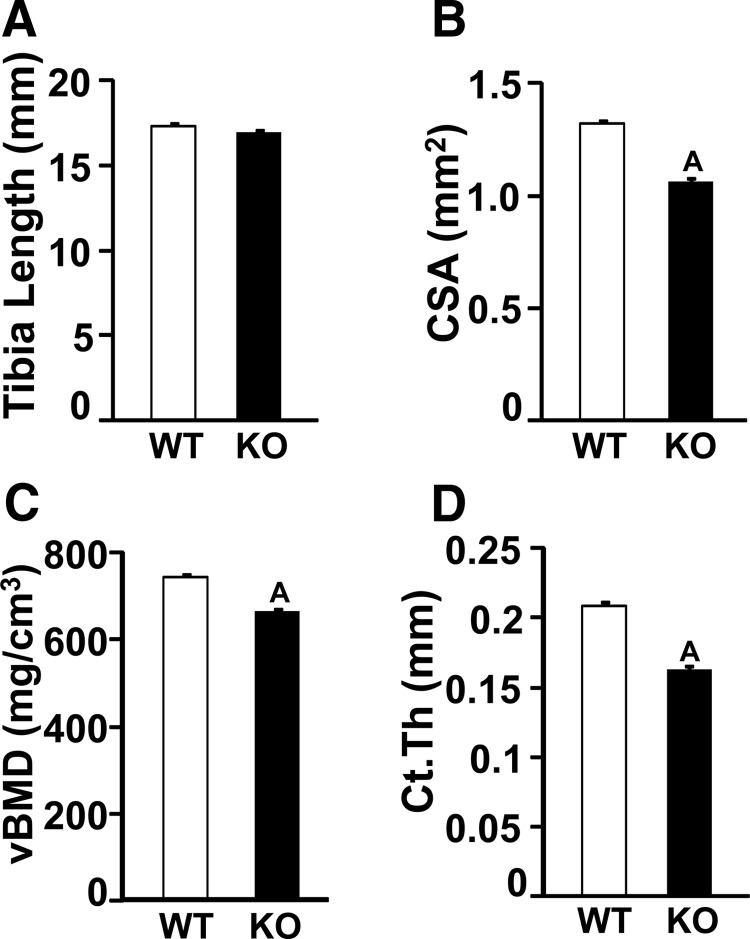
Because DXA analyses revealed a skeletal phenotype in the female Wnt16 KO mice, further 3-dimensional evaluation of the skeleton was done using micro-CT analysis on the tibias of 12-week-old female Wnt16 KO and WT mice. Although tibia length was not significantly different between the 2 genotypes (Figure 2A), tibia CSA was decreased by 24% in the KO mice compared with that in the WT mice at the mid-diaphysis (Figure 2B). Furthermore, cortical thickness (Figure 2D) and volumetric BMD (Figure 2C) were also reduced by 25% and 11%, respectively, in the KO mice.
Figure 2.
Micro-CT measurement of tibia cortical bone parameters in a 12 week-old female WT and Wnt16 KO mice. A, Tibia length. B, CSA. C, Volumetric BMD (vBMD). D, Cortical thickness (Ct.Th). Values are means ± SE (n = 6). A, P < .05 vs WT mice.
To determine whether Wnt16 KO affects lumbar vertebrae, we evaluated LV5 by micro-CT. The female Wnt16 KO mice showed 19% to 25% decreases in cortical BV/TV (P < .05 vs WT) at 16 and 24 weeks of age. In contrast, the trabecular BV/TV was either slightly increased (16 weeks) or unchanged (20 weeks) in the Wnt16 KO mice compared with that in the WT mice (Table 1).
Table 1.
KO of Wnt16 Decreases Cortical but Not Trabecular Bone Volume
| Parameter | Age, wk | WT | KO | Change, % | P Value |
|---|---|---|---|---|---|
| LV5 Tb.BV/TV (%) | 16 | 14.8 ± 0.7 | 19.4 ± 1.0 | 31 ↑ | .05 |
| LV5 Ct.BV/TV (%) | 16 | 18.9 ± 0.5 | 14.3 ± 0.6 | 24 ↓ | <.001 |
| LV5 Tb.BV/TV (%) | 24 | 15.6 ± 0.9 | 16.9 ± 0.8 | 8 ↑ | .35 |
| LV5 Ct.BV/TV (%) | 24 | 14.8 ± 0.4 | 12.1 ± 0.4 | 19 ↓ | .001 |
LV5 vertebral bodies of female mice were examined. BV is normalized to combined cortical (Ct.) plus trabecular (Tb.) TV. Data are means ± SEM for a minimum of 7 bones per group.
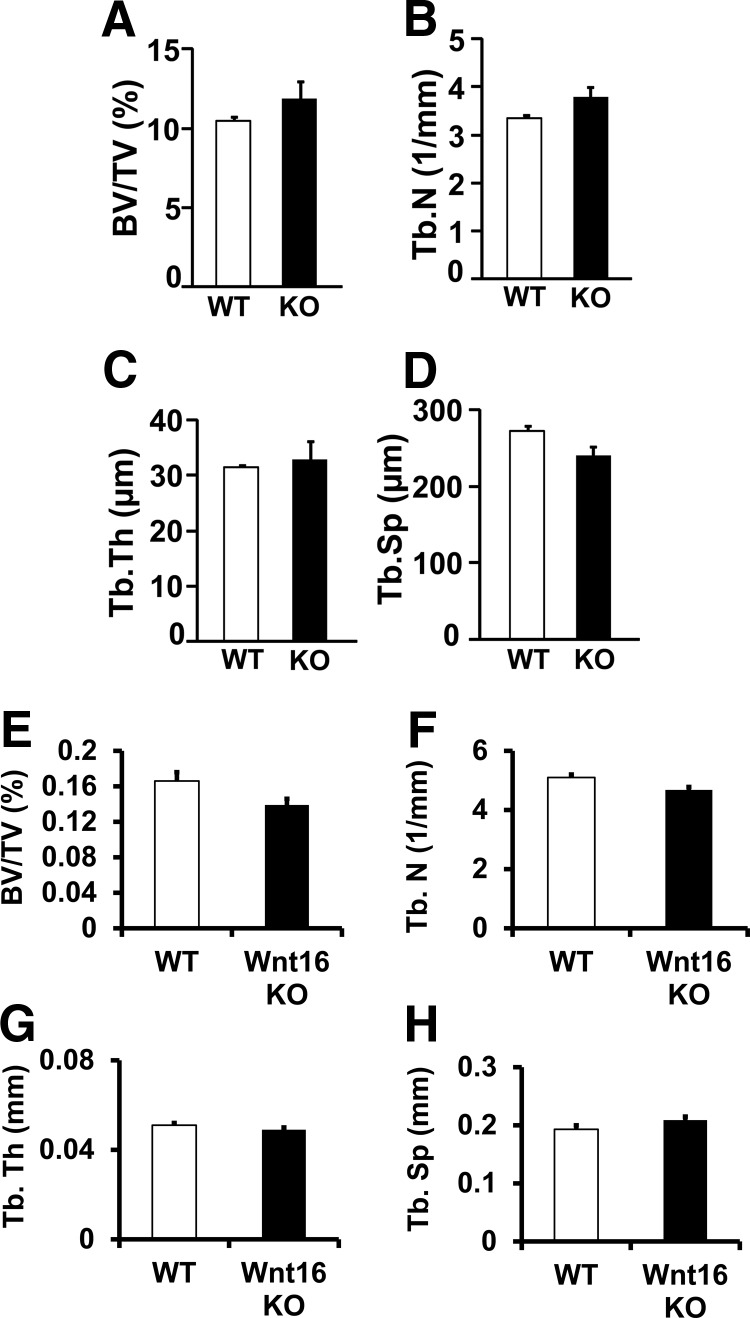
Trabecular bone parameters were also measured by histomorphometric and micro-CT analyses in the secondary spongiosa of femurs of the female Wnt16 KO and WT mice at 12 weeks of age. None of the trabecular bone parameters measured was significantly different between the 2 genotypes (Figure 3).
Figure 3.
Histomorphometric (A–D) and micro-CT (E–H) analysis of trabecular bone at the femur metaphysis site (secondary spongiosa) in 12-week-old female WT and Wnt16 KO mice. A and E, BV/TV. B and F, trabecular number (Tb.N). C and G, Trabecular thickness (Tb.Th). D and H, trabecular spacing (Tb.Sp). Values are means ± SE (n = 5–7).
Periosteal bone formation and resorption are affected in female Wnt16 KO mice
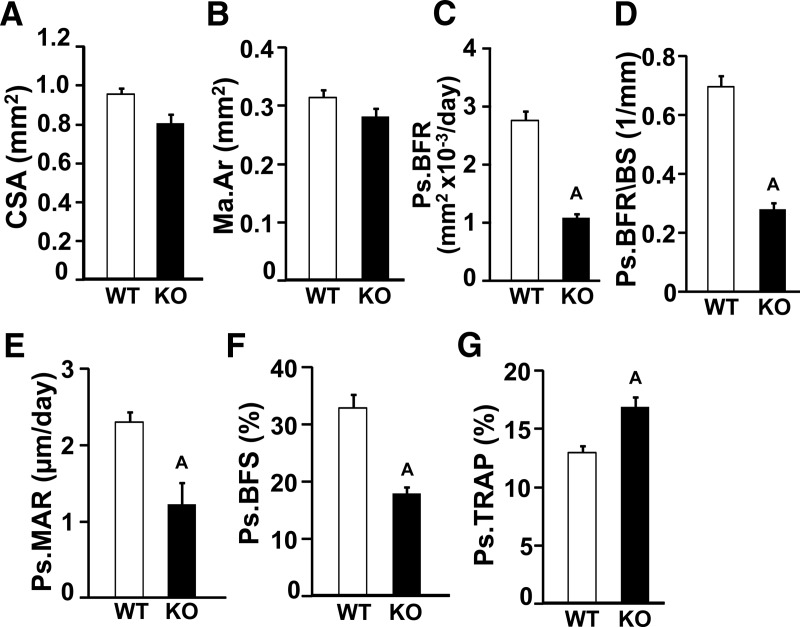
To determine the cellular processes contributing to the decreased bone size in the female Wnt16 KO mice, we performed histomorphometric analyses at the tibia mid-diaphysis of the female mice. Consistent with the micro-CT analysis, histological examination showed a 15% reduction in tibia CSA in the Wnt16 KO mice compared with that in the WT mice (Figure 4A) with little or no difference in marrow area (Figure 4B). The periosteal BFRs adjusted for bone surface (Figure 4, C and D) and MAR (Figure 4E) were reduced by 60% and 55%, respectively, in the Wnt16 KO mice. The Wnt16 KO mice also exhibited a significant reduction in bone-forming surface, which reflects a reduction in osteoblast number (Figure. 4F). In addition, the periosteal TRAP-labeled surface was increased in the Wnt16 KO mice at the mid-diaphysis site (Figure 4G). Although the endocortical TRAP-labeled surface was also increased (31 ± 19%) in bones from Wnt16 KO mice, this change was not statistically significant.
Figure 4.
Histomorphometric analysis of cortical bone parameters in femurs from 12-week-old female WT and Wnt16 KO mice. A, CSA. B, Marrow area (Ma.Ar). C, Periosteal BFR (Ps.BRF). D, Periosteal BFR/bone surface (Ps.BFR/BS). E, Periosteal MAR (Ps.MAR). F, Periosteal bone-forming surface (Ps.BFS). G, Periosteal TRAP (Ps.TRAP). Values are means ± SE. (n = 4–6). A, P < .05 vs WT mice.
Expression levels of bone formation and canonical WNT signaling genes are reduced in the periosteum of Wnt16 KO mice
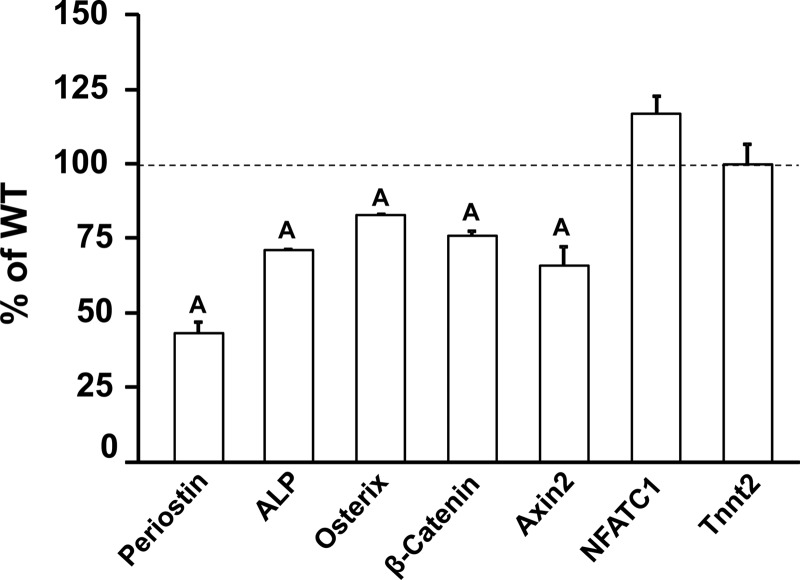
Consistent with the reduced periosteal BFR observed in the histomorphometric analyses, the expression level of bone alkaline phosphatase, a marker of osteoblast differentiation, was reduced significantly in the periosteum of 5-week-old Wnt16 KO mice. We found that the mRNA levels of both osterix and periostin were decreased significantly in the periosteum of 5-week-old Wnt16 KO mice compared with those in WT mice. To determine whether the WNT16 actions on periosteal bone formation are mediated via activation of canonical and/or noncanonical WNT signaling, we measured the expression of component genes of both of these pathways in the periosteum of Wnt16 KO and WT mice. We found that whereas expression of the canonical WNT signaling genes (β-catenin and Axin2) was reduced in the Wnt16 KO mice, expression of noncanonical signaling genes (Nfatc1 and Tnnt2) was not different between the 2 genotypes (Figure 5).
Figure 5.
Quantitation, by real-time PCR, mRNA levels of genes from the periosteum of 5-week-old WT and Wnt16 KO mice. Values are means ± SE (n = 7–8). A, P < .05 vs WT mice. ALP, alkaline phosphatase.
Expression of Wnt ligands and Frizzled receptors
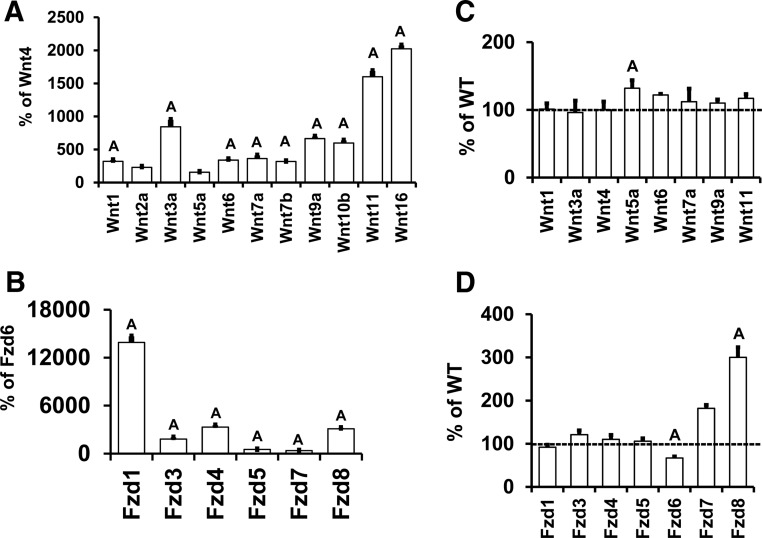
To determine the relative contribution of WNT ligands in regulating periosteal bone formation, we evaluated, by real-time PCR, the expression of 12 (including Wnt4) Wnts in the RNA samples from the periosteum of WT and Wnt16 KO mice. Quantitative analysis revealed that only Wnt10a of those measured did not have measurable mRNA levels. Of these Wnts, Wnt11 and Wnt16 expression levels were higher than those of all other Wnts (Figure 6A). Because WNTs bind to FZD receptors in executing their biological actions, we also evaluated Fzd levels in the periosteum of WT mice. All 6 Fzds tested were expressed, and Fzd1, Fzd4, and Fzd8 expression levels were higher than those of the other Fzds (Figure 6B).
Figure 6.
Expression levels of Wnt ligands and Frizzled receptors in the periosteum of 5-week-old WT (A and B) and Wnt16 KO mice (C and D). Wnt10a was not detected in the periosteum. Values are means ± SE. A, P < .05 vs Wnt4 or Fzd6 or WT.
To determine whether loss of WNT16 affected Wnt or Fzd levels, we measured the periosteal expression of various Wnts and Fzds in bones from Wnt16 KO mice. Bone Wnt5a expression was increased significantly with Wnt16 disruption, whereas no differences were seen in other Wnts (Figure 6C). Fzd6 expression was decreased, whereas Fzd8 expression was increased in the Wnt16 KO mice (Figure 6D).
Mechanical strain influences Wnt16 expression
Mechanical loading is an important physiological regulator of periosteal bone formation. Because bone size is reduced in the Wnt16 KO female mice, we determined whether WNT16 is involved in mediating mechanical strain effects on periosteal bone formation. We first evaluated whether Wnt16 expression is under the influence of mechanical strain in cultured bone cells. Wnt16 expression was increased 2-fold in MC3T3 cells subjected to fluid flow shear stress compared with that in controls (Figure 7A). Consistent with these in vitro data, Wnt16 expression in vivo was increased 2- to 4-fold (P < .05) in the loaded tibia compared with that in the unloaded tibia after 2 weeks of 4-point bending and also by axial compression loading in good responder C57BL/6J mice (Figure 7B). Thus, Wnt16 expression was increased in response to mechanical loading in 3 different loading models.
Figure 7.
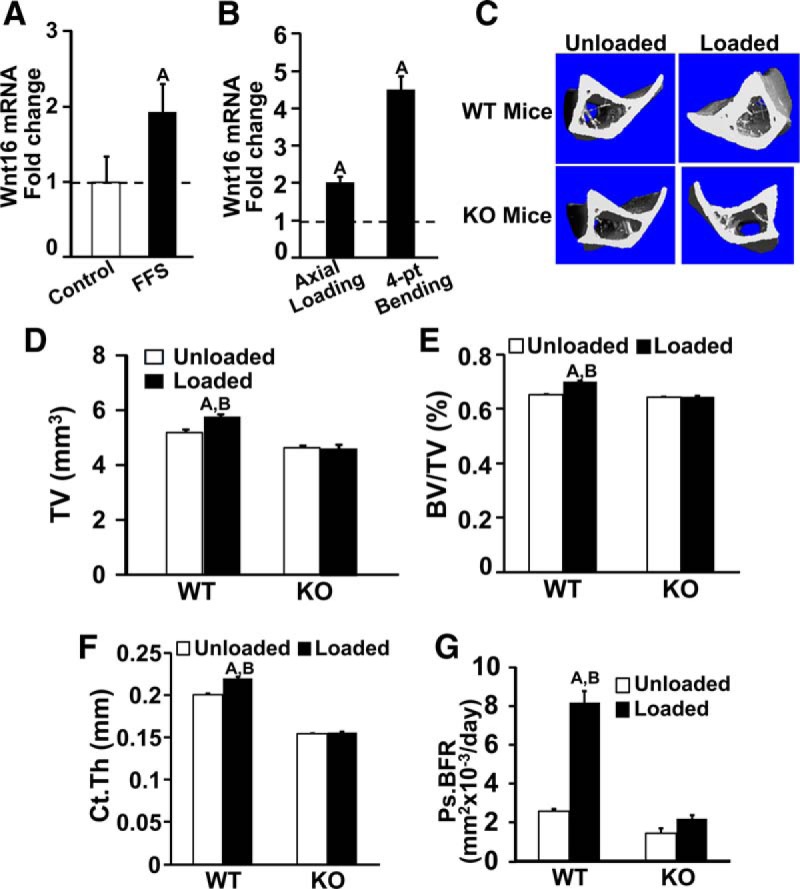
Quantitative analysis of the increase in Wnt16 expression in osteoblast cells after fluid flow shear (FFS) stress in vitro (A) and the increase in Wnt16 expression after 2 weeks of mechanical loading by 4-point bending and by the axial method of loading in 12-week-old female good responder C57BL/6J mice (B). C–G, data from 4-point bending experiments. C, Micro-CT image of loaded and unloaded tibia after 2 weeks of mechanical loading in the WT and Wnt16 KO mice. TV (D), BV/TV (E), cortical thickness (Ct.Th) (F), and histomorphometric analysis of periosteal BFR (Ps.BFR) (G) in the tibias of WT and Wnt16 KO mice after 9 days of mechanical loading. Values are means ± SE (n = 6–7 per group). A, P < .05 vs corresponding unloaded tibiae; B, P < .05 vs Wnt16 KO mice.
Periosteal bone formation response to loading is decreased in Wnt16 KO mice
The effects of mechanical loading–induced periosteal bone formation were compared in the Wnt16 KO and WT mice. Because the magnitude of strains induced by loading depends on bone size and the tibias of the Wnt16 KO mice show a 24% (P < .05) reduction in CSA, applied loading forces were adjusted to produce equivalent tibial strains for the KO and WT mice. We calculated the mechanical strains produced by varying loads in the bones of both mouse genotypes using a mathematical approach (21, 22). These calculations showed that 6- and 9-N loads produced equivalent tibial strains in the Wnt16 KO mice (4804 ± 607 μm, n = 4 mice) and WT mice (4860 ± 395 μm, n = 4 mice), respectively. These adjusted loads were applied to the tibias of the Wnt16 KO and WT mice using a 4-point bending device.
In response to 2 weeks of 4-point bending, parameters related to periosteal bone size were significantly increased in the loaded bones of the WT mice compared with those in the unloaded control bones (Figure 7, C–F). Micro-CT measurements revealed significant increases in tissue volume and CSA reflective of the increased periosteal expansion in response to loading. In contrast, none of these parameters were significantly increased in the loaded bones compared with those in the nonexternally unloaded control bones of Wnt16 KO mice (Figure 7). Consistent with the micro-CT data, histomorphometric studies revealed a significant increase in periosteal BFR in response to mechanical loading in the WT mice but not in the Wnt16 KO mice (Figure 7G).
Discussion
This study provides compelling evidence that WNT16 is an important positive regulator of periosteal bone expansion during postnatal growth in mice. Our female Wnt16 KO mice have reduced cortical CSA and thickness, but spontaneous fractures were not observed. However, 3 other groups have reported fractures in Wnt16 KO mice. The groups of Ohlsson et al. (24) and Baron et al. (25) studied Wnt16 KO mice having a C57BL/6J genetic background and also found reduced cortical bone thickness. Tibial and fibular fractures were also observed by the Wellcome Trust Sanger Institute (as part of the IKMC campaign) in Wnt16 KO mice on a C57BL/6N background (Lelliott, C., personal communication). In our study, Wnt16 KO mice were generated on a C57BL6/J-129SvEv hybrid genetic background. Because the cortical thickness of C57BL/6J mice is reduced considerably compared with that of other inbred strains of mice (26), further reduction in cortical thickness caused by loss of the Wnt16 gene in the C57BL/6J genetic background may make these mice susceptible to spontaneous fractures.
Our longitudinal studies involving DXA measurements revealed that the total bone area was reduced as early as 4 weeks of age in Wnt16 KO female mice despite a minor decrease in body weight and no difference in bone length (Figure 1). The reduction in bone size in female Wnt16 KO mice was confirmed by whole-body DXA measurements at 8 weeks of age and by micro-CT measurements of the tibia at 12 weeks of age. These data suggest that the lack of WNT16 manifests its effects on periosteal bone expansion during early postnatal growth in mice. In terms of mechanisms for WNT16 actions, the published data provide evidence for interactions between estrogen and WNT signaling pathways (27). For example, β-catenin has been shown to physically interact with estrogen receptor α (28, 29), thus raising the possibility that this interaction may play a role in modifying WNT16 actions on periosteal expansion in female mice. Further studies evaluating the possible effects of estrogen on WNT16 signaling are required. To determine the cellular processes contributing to the reduced bone size in female Wnt16 KO mice, we performed histomorphometric studies of bone formation and bone resorption at 10 weeks of age. We found that the BFR at the periosteal surface of the tibial diaphysis was reduced considerably in the Wnt16 KO female mice. This reduction in periosteal BFR is caused by both reduced osteoblast activity, as reflected by decreased MAR, and reduced osteoblast number, as reflected by the decreased bone-forming surface. The reduced expression of alkaline phosphatase mRNA in the periosteum of the Wnt16 KO female mice is consistent with reduced osteoblast activity, supporting the hypothesis that WNT16 stimulates bone formation. In terms of molecular mechanisms for the WNT16 effects on osteoblasts, we found that expression levels of periostin and osterix were reduced in the periosteum of Wnt16 KO mice. Periostin is highly expressed in periosteal cells and has been used as a marker of periosteal cells (30). Furthermore, mice with a targeted disruption of periostin have reduced bone size (31). Osterix is expressed in osteoprogenitor cells and has been shown to be essential for osteoblast maturation and function (32). Thus, WNT16 effects on periosteal bone formation may in part be mediated via increased expression of osterix and periostin. However, the issue of whether WNT16 exerts direct or indirect effects on these genes remains to be determined.
In addition to a reduced BFR, we found increased TRAP-labeled surface at the periosteum in the Wnt16 KO mice, suggesting that increased bone resorption at the periosteum may also contribute to reduced periosteal expansion. Consistent with this stimulatory effect of Wnt16 KO on bone resorption observed in our study, 2 studies presented at the 2013 American Society for Bone and Mineral Research meeting provided evidence for direct effects of WNT16 on osteoclastogenesis in vitro (24, 25). Together, the increased osteoclast and decreased osteoblast functions appear to be responsible for the reduced periosteal expansion in Wnt16 KO female mice during postnatal growth.
The occurrence of cortical but not trabecular bone phenotypes in Wnt16 KO mice is surprising but not without precedence. High trabecular bone mass with low cortical bone mass occurs with disruptions of klotho, leptin, and Sfrp4 genes (33–35). KO of Wnt7b produces low trabecular bone with normal cortical bone mass (36). Frd9 KO mice have low trabecular bone and cortical bone mass (37). WNT signaling is critical for both trabecular and cortical bone as both compartments are affected by KO of Lrp5 and Sost (38, 39). The gene expression data reported in the present study emphasize the importance of WNT16 and FZD8 for cortical bone development and minimize roles for WNT1, WNT2a, WNT7a, WNT9a, WNT10b, FZD3, FZD4, FZD5, and FZD7. Among the numerous tissues examined, WNT16 protein was detected in only bone, testes, and the vasculature of adult mice (http://www.sanger.ac.uk/mouseportal/phenotyping/MUBC/adult-lac-z-expression/).
Because periosteal bone formation was reduced in the Wnt16 KO mice and because mechanical strain is an important physiological regulator of periosteal bone formation, we hypothesized that WNT16 may be involved in mediating the anabolic effects of increased mechanical strain that occurs during postnatal growth. In support of this hypothesis, we found that the expression levels of Wnt16 were increased 2- to 4-fold in the loaded bones of WT C57BL/6J mice in 2 different in vivo models of mechanical loading as well as in osteoblast cells subjected to in vitro mechanical strain. Furthermore, we also found that the periosteal bone response to 2 weeks of 4-point bending of tibia, as measured by the changes in skeletal parameters related to periosteal expansion, failed to occur in the Wnt16 KO mice in contrast to a strong response in WT mice. Consistent with the micro-CT data, our histomorphometric studies revealed significant increases in the periosteal BFR in response to mechanical strain in WT mice but not in the Wnt16 KO female mice. Taken together, these data provide causal evidence that increased synthesis of WNT16 by bone cells is necessary for the periosteal bone anabolic response to mechanical loading. Further studies are needed to address the mechanism by which mechanical strain increases Wnt16 expression.
Studies on Wnt signaling pathways have provided evidence for involvement of both canonical β-catenin signaling and noncanonical signaling in mediating the WNT effects in various target cell types (4, 13–15). We observed that the expression of canonical genes β-catenin and Axin2 (40, 41) but not noncanonical genes Nfatc1 and Tnnt2 (42) were significantly decreased in the bone periosteum from Wnt16 KO mice. Furthermore, a previous study demonstrated that periosteal bridging during fracture repair is dependent on activation of β-catenin signaling (43) consistent with the importance of the canonical pathway in the periosteum. Further studies examining additional signaling pathway components are needed to confirm whether or not the WNT16 effects on periosteal bone formation are mediated predominantly via activation of canonical WNT signaling.
The limitations of this study are as follows. (1) A 6-N load was applied to the tibia of Wnt16 KO mice because this load was calculated to produce mechanical strains equivalent to a 9-N load in the tibia of WT mice. Administering varying loads in a load-response study would provide a more comprehensive comparison of KO and WT bone responses. (2) We applied mechanical strain to the tibia mid-diaphyseal region containing cortical bone. Axial loading studies should be performed to determine whether WNT16 is also essential for trabecular bone responses to mechanical loading. (3) Our study cannot determine whether the effects of WNT16 on bone resorption is mediated via osteoblast-produced WNT16 or whether osteoclasts also express WNT16 to exert autocrine effects. (4) The effects of Wnt16 KO on tibial bone formation were determined using control contralateral bones in the mechanical loading study. These results may have been influenced by a systemic response to loading.
Acknowledgments
We thank Joe Rung Aroon and Nancy Lowen for providing technical assistance. We thank Donna Strong for proofreading the article.
This research was supported by funds from the Veterans Administration and the National Institutes of Health (Grant R01AR031062). All work was performed in facilities provided by the Department of Veterans Affairs. The information contained in this publication does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BFR
- bone formation rate
- BMC
- bone mineral content
- BMD
- bone mineral density
- BV
- bone volume
- CSA
- cross-sectional area
- CT
- computed tomography
- DXA
- dual x-ray absorptiometry
- KO
- knockout
- MAR
- mineral apposition rate
- TRAP
- tartrate resistant acid phosphatase
- TV
- tissue volume
- WT
- wild type.
References
- 1. Zheng HF, Tobias JH, Duncan E, et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012:8:e1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koller DL, Zheng HF, Karasik D, et al. Meta-analysis of genome-wide studies identifies WNT16 and ESR1 SNPs associated with bone mineral density in premenopausal women. J Bone Miner Res. 2013;28:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. García-Ibarbia C, Pérez-Núñez MI, Olmos JM, et al. Missense polymorphisms of the WNT16 gene are associated with bone mass, hip geometry and fractures. Osteoporos Int. 2013;24:2449–2454. [DOI] [PubMed] [Google Scholar]
- 4. Hendrickx G, Boudin E, Fijałkowski I, et al. Variation in the Kozak sequence of WNT16 results in an increased translation and is associated with osteoporosis related parameters. Bone. 2014;59:57–65. [DOI] [PubMed] [Google Scholar]
- 5. Zhang L, Choi HJ, Estrada K, et al. Multistage genome-wide association meta-analyses identified two new loci for bone mineral density. Hum Mol Genet. 2014;23:1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Medina-Gomez C, Kemp JP, Estrada K, et al. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 locus. PLoS Genet. 2012;8:e1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tu X, Rhee Y, Condon KW, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liedert A, Wagner L, Seefried L, Ebert R, Jakob F, Ignatius A. Estrogen receptor and Wnt signaling interact to regulate early gene expression in response to mechanical strain in osteoblastic cells. Biochem Biophys Res Commun. 2010;394:755–759. [DOI] [PubMed] [Google Scholar]
- 10. Santos A, Bakker AD, Zandieh-Doulabi B, Semeins CM, Klein-Nulend J. Pulsating fluid flow modulates gene expression of proteins involved in Wnt signaling pathways in osteocytes. J Orthop Res. 2009;27:1280–1287. [DOI] [PubMed] [Google Scholar]
- 11. Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. β-Catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armstrong VJ, Muzylak M, Sunters A, et al. Wnt/β-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor α. J Biol Chem. 2007;282:20715–20727. [DOI] [PubMed] [Google Scholar]
- 13. Clements WK, Kim AD, Ong KG, Moore JC, Lawson ND, Traver D. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature. 2011;474:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazieres J, You L, He B, et al. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t(1;19) translocation induces apoptosis. Oncogene. 2005;24:5396–5400. [DOI] [PubMed] [Google Scholar]
- 15. Teh MT, Blaydon D, Ghali LR, et al. Role for WNT16B in human epidermal keratinocyte proliferation and differentiation. J Cell Sci. 2007;120:330–339. [DOI] [PubMed] [Google Scholar]
- 16. Brommage R, Liu J, Hansen GM, et al. High-throughput screening of mouse gene knockouts identifies established and novel skeletal phenotypes. Bone Res. 2014;2:14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1α2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007;148:5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sengul A, Santisuk R, Xing W, Kesavan C. Systemic administration of an antagomir designed to inhibit miR-92, a regulator of angiogenesis, failed to modulate skeletal anabolic response to mechanical loading. Physiol Res. 2013;62:221–226. [DOI] [PubMed] [Google Scholar]
- 19. Kapur S, Mohan S, Baylink DJ, Lau KH. Fluid shear stress synergizes with insulin-like growth factor-I (IGF-I) on osteoblast proliferation through integrin-dependent activation of IGF-I mitogenic signaling pathway. J Biol Chem. 2005;280:20163–20170. [DOI] [PubMed] [Google Scholar]
- 20. Kesavan C, Mohan S, Oberholtzer S, Wergedal JE, Baylink DJ. Mechanical loading-induced gene expression and BMD changes are different in two inbred mouse strains. J Appl Physiol. 2005;99:1951–1957. [DOI] [PubMed] [Google Scholar]
- 21. Lau KH, Baylink DJ, Zhou XD, et al. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am J Physiol Endocrinol Metab. 2013;305:E271–E281. [DOI] [PubMed] [Google Scholar]
- 22. Kesavan C, Wergedal JE, Lau KH, Mohan S. Conditional disruption of IGF-I gene in type 1α collagen-expressing cells shows an essential role of IGF-I in skeletal anabolic response to loading. Am J Physiol Endocrinol Metab. 2011;301:E1191–E1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sheng MH, Baylink DJ, Beamer WG, et al. Histomorphometric studies show that bone formation and bone mineral apposition rates are greater in C3H/HeJ (high-density) than C57BL/6J (low-density) mice during growth. Bone. 1999;25:421–429. [DOI] [PubMed] [Google Scholar]
- 24. Moverare-Skrtic S, Henning P, Rjesson BA, et al. Wnt16 is a novel osteoblast-derived paracrine regulator of osteoclastogenesis, cortical bone mass and fracture susceptibility. J Bone Miner Res. 2013;28(Suppl 1):S39. [Google Scholar]
- 25. Liu X, Nagano K, Saito H, Baron R, Gori F. Wnt16 deletion differentially affects cortical and trabecular bone: increased cortical bone resorption, porosity and fracture in Wnt16 knockout mice. J Bone Miner Res. 2013;28(Suppl 1):S20. [Google Scholar]
- 26. Turner CH, Hsieh YF, Müller R, et al. Genetic regulation of cortical and trabecular bone strength and microstructure in inbred strains of mice. J Bone Miner Res. 2000;15:1126–1131. [DOI] [PubMed] [Google Scholar]
- 27. Gao Y, Huang E, Zhang H, et al. Crosstalk between Wnt/beta-catenin and estrogen receptor signaling synergistically promotes osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2013;8:e82436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta N, Schmitt F, Grebhardt S, Mayer D. β-Catenin is a positive regulator of estrogen receptor-α function in breast cancer cells. Cancers (Basel). 2011;3:2990–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Auld KL, Berasi SP, Liu Y, et al. Estrogen-related receptor α regulates osteoblast differentiation via Wnt/β-catenin signaling. J Mol Endocrinol. 2012;48:177–191. [DOI] [PubMed] [Google Scholar]
- 30. Kawanami A, Matsushita T, Chan YY, Murakami S. Mice expressing GFP and CreER in osteochondro progenitor cells in the periosteum. Biochem Biophys Res Commun. 2009;386:477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonnet N, Conway SJ, Ferrari SL. Regulation of β catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin. Proc Natl Acad Sci USA. 2012;109:15048–15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao Y, Zhou Z, de Crombrugghe B, et al. Osterix, a transcription factor for osteoblast differentiation, mediates antitumor activity in murine osteosarcoma. Cancer Res. 2005;65:1124–1128. [DOI] [PubMed] [Google Scholar]
- 33. Yamashita T, Nabeshima Y, Noda M. High-resolution micro-computed tomography analyses of the abnormal trabecular bone structures in klotho gene mutant mice. J Endocrinol. 2000;164:239–245. [DOI] [PubMed] [Google Scholar]
- 34. Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–383. [DOI] [PubMed] [Google Scholar]
- 35. Brommage R, Liu J, Lee EC. Elevated trabecular bone mass with reduced cortical bone thickness in sFRP4 knockout mice. J Bone Miner Res. 2009;24(Suppl 1):S82. [Google Scholar]
- 36. Chen J, Tu X, Esen E, et al. WNT7B promotes bone formation in part through mTORC1. PLoS Genet. 2014;10:e1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Albers J, Schulze J, Beil FT, et al. Control of bone formation by the serpentine receptor Frizzled-9. J Cell Biol. 2011;192:1057–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holmen SL, Giambernardi TA, Zylstra CR, et al. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19:2033–2040. [DOI] [PubMed] [Google Scholar]
- 39. Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. [DOI] [PubMed] [Google Scholar]
- 40. Rossini M, Gatti D, Adami S. Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int. 2013;93:121–132. [DOI] [PubMed] [Google Scholar]
- 41. McGee-Lawrence ME, Li X, Bledsoe KL, et al. Runx2 protein represses Axin2 expression in osteoblasts and is required for craniosynostosis in Axin2-deficient mice. J Biol Chem. 2013;288:5291–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. [DOI] [PubMed] [Google Scholar]
- 43. Chen Y, Whetstone HC, Lin AC, et al. β-Catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4:e249. [DOI] [PMC free article] [PubMed] [Google Scholar]