Abstract
Distinct male and female patterns of pituitary GH secretion produce sexually differentiated hepatic gene expression profiles, thereby influencing steroid and xenobiotic metabolism. We used a fully automated system to obtain serial nocturnal blood samples every 15 minutes from cannulated wild-type (WT) and somatostatin knockout (Sst-KO) mice to determine the role of SST, the principal inhibitor of GH release, in the generation of sexually dimorphic GH pulsatility. WT males had lower mean and median GH values, less random GH secretory bursts, and longer trough periods between GH pulses than WT females. Each of these parameters was feminized in male Sst-KO mice, whereas female Sst-KO mice had higher GH levels than all other groups, but GH pulsatility was unaffected. We next performed hepatic mRNA profiling with high-density microarrays. Male Sst-KO mice exhibited a globally feminized pattern of GH-dependent mRNA levels, but female Sst-KO mice were largely unaffected. Among the differentially expressed female-predominant genes was Serpina6, which encodes corticosteroid-binding globulin (CBG). Increased CBG was associated with elevated diurnal peak plasma corticosterone in unstressed WT females and both sexes of Sst-KO mice compared with WT males. Sst-KO mice also had exaggerated ACTH and corticosterone responses to acute restraint stress. However, consistent with their lack of phenotypic signs of excess glucocorticoids, cerebrospinal fluid concentrations of free corticosterone in Sst-KO mice were not elevated. In summary, SST is necessary for the prolonged interpulse troughs that define masculinized pituitary GH secretion. SST also contributes to sexual dimorphism of the hypothalamic-pituitary-adrenal axis via GH-dependent regulation of hepatic CBG production.
GH is released in a pulsatile manner from somatotrophs in all species studied (1), is positively regulated by GHRH, and negatively regulated by somatostatin (SST) inputs from the hypothalamus. Ghrelin, produced primarily by the stomach, has an additional role in stimulating GH secretion (2, 3) and acts synergistically with GHRH. The specific properties of GH secretion differ between the sexes in rodents and humans, measured by frequent repeated blood sampling from indwelling venous cannulae. In male rats, low basal GH levels are interrupted by large episodic bursts every 3–4 hours (4); on the other hand, female rats exhibit higher basal GH levels and more frequent but smaller bursts of GH secretion (5). Humans exhibit a similar but less pronounced sex difference, with females secreting more GH due to more pulses (6). Additionally, in humans and rats, GH secretory patterns are more regular in males than females (7). In the only report of sex differences in GH pulsatility in mice, males and females exhibited similar baseline and peak GH levels, but males had longer interpulse troughs than females (8).
An important consequence of the sex difference in GH secretion is the resulting sexual dimorphism of the liver. First shown in rats (9) and later confirmed in mice (10), specific features of ultradian GH secretion dictate sex differences in the hepatic mRNA levels of many genes, including cytochrome p450 and sulfotransferase enzymes. Sex-specific hepatic gene expression is also evident in humans, although the magnitude of expression difference is much less than in rodents, and the sex predominance of many orthologous genes is reversed (11). Sexually dimorphic gene expression patterns are the result of a complex interplay of many transcription factors, the most well studied being signal transducer and activator of transcription 5b (Stat5b), which is phosphorylated in response to GH receptor activation (for review, see Ref. 12). Extensive studies in rats have determined that it is the relatively long interpulse interval of very low or undetectable plasma GH levels, rather than any change in pulse amplitude, duration, or frequency, that is critical to produce the typical masculine hepatic gene expression profile (13).
The mechanism driving GH secretory dynamics and pulsatility in male vs female mammals is not fully understood. The principal driver of plasma GH pulsatility is likely GHRH, because neutralization with anti-GHRH antibodies or treatment with GHRH receptor-specific antagonists completely ablates GH pulses in rats (14, 15). On the other hand, immune-neutralization of SST does not affect GH peak amplitude, pulse frequency, or average plasma GH levels but elevates the mean trough levels (16, 17), which is critical to sexual differentiation of hepatic gene expression. Indeed, Sst mRNA levels are greater in the hypothalamus of male rats compared with female throughout development and adulthood (18) and Sst expression is regulated by testosterone (19), suggesting that SST could play a role in sexually dimorphic GH secretion. However, confirmation of this role has been challenging, because there are no available SST receptor antagonists and serial blood sampling has not been done previously in strains of Sst-knockout (Sst-KO) mice (20, 21).
Therefore, the current study was designed to define the role of SST in sex-specific GH pulsatility and the subsequent downstream effects on hepatic gene expression in C57BL/6 mice. In a previous study of Sst-KO mice, we inferred the alterations in GH secretion from analysis of multiple, randomly timed GH measurements together with hepatic mRNA levels of 5 sexually dimorphic genes (20). Here, we used an automated blood sampling system in freely behaving Sst-KO and control wild-type (WT) mice of both sexes to directly measure plasma GH pulsatility patterns and correlated those results with global changes in hepatic mRNA levels measured by microarrays and specific changes in corticosteroid-binding globulin (CBG) and circulating levels of corticosterone.
Materials and Methods
Animals
Sst-KO mice were generated as described previously (20) and backcrossed onto the C57BL/6J (The Jackson Laboratory) genetic background for more than or equal to 10 generations. Sst-KO and WT littermates were generated from the cross of Sst-heterozygote breeders. Experimental female mice were not controlled for estrous cycle. All mice were group housed in ventilated cages on a 12-hour light, 12-hour dark cycle (lights on from 6 am to 6 pm) in a temperature-controlled environment (22 ± 1°C) with free access to standard rodent chow and water. All animal studies were approved by either the Institutional Animal Care and Use Committee at Oregon Health and Science University or the University Committee on Use and Care of Animals at University of Michigan and followed Public Health Service guidelines.
Carotid artery cannulations
Five- to eight-month-old mice were anesthetized with sodium pentobarbital (50–60 mg/kg, ip). Under aseptic conditions, the right carotid artery was isolated. A 2-part cannula constructed from microrenathane tubing (0.635 mm outer diameter, stretched) and SILASTIC tubing (0.635 mm outer diameter) was inserted to the level of the aortic arch. The free end was tunneled sc and exteriorized at the back of the neck via a stainless steel tubing connector that was fixed sc during closure of the skin incision. Cannulae were flushed with saline and sealed with heparin-saline (200 U/mL) daily after surgery. Cannulated mice were housed individually and weighed daily. A total of 30 of 33 mice survived the procedure, and of these, cannulae remained patent in 27 (90%; n = 8 male WT, 5 female WT, 5 male Sst-KO, 6 female Sst-KO).
Automated blood sampling
After surgery, mice appeared healthy with normal activity and body weight regain to more than or equal to 90% of their presurgery level. They were acclimatized for 3–4 days to Ratturn cages as part of the Culex automated blood sampling system (Basi, Inc), and then, 44 serial blood samples were obtained from the carotid cannulae at 15-minute intervals during the 12-hour nocturnal period in complete darkness starting at 7 pm. Because of the dead volume in cannulae and tubing, 55 μL of whole blood was withdrawn at each time point, 10 μL was diluted with 50 μL of heparinized saline into refrigerated glass tubes, and the remaining 45 μL blood with 10 μL of heparinized saline was infused back into the mouse to maintain blood volume. The next morning, all mice appeared healthy. Collection tubes were centrifuged, and 50 μL of clear, diluted plasma was frozen at −20°C before assay. Samples were considered to be adequate if they contained the expected pellet of red blood cells (∼5 μL). Only occasional samples were excluded from mice with patent cannulae.
Manual collection of blood and cerebrospinal fluid (CSF)
For basal daytime corticosterone analysis, adult mice (n = 8 per sex, genotype, and time point) were euthanized by decapitation immediately upon removal from their cages at 9 am or 5 pm and trunk blood was collected into tubes containing 10 μL of 75 mg/mL EDTA. Plasma was separated via centrifugation and stored at −20°C before assay. For the initial restraint stress study, adult mice (n = 6 per sex, genotype, and time point) were either decapitated between 8 and 10 am (time = 0) or subjected to restraint stress in 50-mL Falcon tubes for 5, 10, or 20 minutes and then decapitated. An additional group recovered for 40 minutes after the stress. Trunk blood was processed as above. For the CSF study, adult mice were either anesthetized with tribromoethanol immediately upon removal from their cages (nonstressed controls) between 4:30 and 6 pm or subjected to restraint stress for 20 minutes as described above, then anesthetized and placed in a stereotaxic frame. Under a dissecting microscope, the neck muscles were bluntly dissected through a sagital incision, and the dura mater overlying the spinal medulla and cisterna magna was visualized. The dura was punctured with a tapered glass micropipette, and 4- to 10-μL CSF was collected within 10 minutes as previously described (22). Samples that were red were considered to be contaminated with blood and discarded. Trunk blood was processed as above, and matching plasma and CSF samples were stored at −20°C. For plasma corticosteroid-binding capacity (CBC), adult mice (n = 7–8 per sex, genotype, and time point) were euthanized by decapitation immediately upon removal from their cage at 9 am or 5 pm, and trunk blood was collected and processed as above. A subset of the samples collected at 9 am (n = 4–5 per sex and genotype) were also analyzed for CBG immunoreactivity via Western blotting as described below.
Plasma hormone and CBG assays
Prediluted plasma samples from the Culex study were assayed in singlet using ELISA kits for rat/mouse GH (Millipore Corp) and corticosterone (Arbor Assays). Sample concentrations were calculated from the standard curves using a 4PLC sigmoidal fit in Prism 6.0 (GraphPad). Inter- and intraassay coefficients of variability (CVs) were 13% and 8%, respectively, for the GH assay. We also assayed plasma samples in 10 replicates from 2 Snell dwarf (dw/dw) mice that lack circulating GH, and the optical density of each sample was below the absorption of the blank controls. For our experimental samples, results less than the lowest point of the standard curve were assigned that value (0.4 ng/mL after correcting for dilution). The intraassay CV for the corticosterone assay was 8%, and the interassay CV was 8% as reported by the manufacturer. Occasional measurements less than the lowest point of the standard curve were assigned that value (7.8 ng/mL). Basal daytime corticosterone measurements were measured by an in-house assay at the Oregon National Primate Research Center hormone assay core as previously described (23). Plasma ACTH was measured by immunoradiometric assay (Nichols Institute). For the restraint stress and CSF collection experiment, corticosterone was measured by a double antibody RIA kit (MP Biomedicals) with both inter- and intraassay CVs of 7% as reported by the manufacturer. Measurements lower than the lowest point on the standard curve were assigned that value (6.3 ng/mL). A CBC assay was performed as described using 3H-corticosterone and 10μM corticosterone (competitor) for nonspecific binding determination (24). For Western blotting, plasma samples were diluted 1:500 in 100mM Tris (pH 7.5), boiled for 3 minutes, and 10 μL of each sample were assayed using antiserum specific for mouse CBG, as described previously (25).
Deconvolution analysis
Plasma GH concentration time series (total 10.75 h) were analyzed by a variable-waveform deconvolution method (26). The MatLab (MathWorks, Inc) program first detrended and normalized concentrations to the unit interval [0, 1] (27), then successive potential pulse-time sets were created by an incremental smoothing process, which deleted the least significant nadir one at a time. Maximum-likelihood expectation parameter estimation was used to calculate secretion and elimination rates simultaneously for each candidate pulse-time set. The model specified basal secretion, a slow-phase half-life, secretory-burst mass, random effects on burst mass, procedural/measurement error, and a 3-parameter flexible γ probability distribution to embody secretory-burst waveform. The rapid phase half-life was assumed to be 1.0 minutes, and the slow half-life was calculated to be 8.0 minutes. Finally, the Akaike information criterion was applied to distinguish objectively among candidate pulse-time sets (28). Observed interpulse intervals are described by a 2-parameter Weibull renewal process. Units of parameters are burst frequency (number per 10.75 h), regularity of interpulse intervals (unitless γ of Weibull), basal and pulsatile secretion rates (ng/mL per 10.75h), mass secreted per burst (ng), and waveform mode (time delay to maximal secretion after burst onset, min).
Microarrays
Three mice per genotype and sex were euthanized between 9 am and 12 pm by cervical dislocation at age 4 months. Liver RNA was extracted using TRIzol reagent (Invitrogen) and its quality determined by Agilent Bioanalyzer. Total RNA samples with RNA integrity numbers between 7.4 and 7.9 were submitted for cDNA/amplified RNA synthesis using 3′ IVT Express kits and hybridization to GeneChip Mouse Genome 430 2.0 Arrays (Affymetrix). After quality control checks, expression values were calculated using a robust multiarray average (29), weighted (30), 3 specific contrasts computed (31), and P values adjusted using false discovery rates (FDRs) (32). Analyses were performed using the affy (33), affyPLM, and limma (34) packages of Bioconductor implemented in the R statistical environment. Expression values were entered into Cluster 3.0, and both genes and samples were hierarchically clustered using average linkage and visualized on a heatmap using Java TreeView. The complete microarray dataset has been deposited to Gene Expression Omnibus under the accession number GSE56520.
Semiquantitative real-time PCR
Between 9 am and 3 pm, 4–10 hours after serial blood sampling, mice were euthanized and samples of liver collected and stored at −80°C. Total RNA was extracted with RNeasy spin columns (QIAGEN), quantified, and quality checked by NanoDrop (Thermo Scientific), and 400 ng were used to synthesize cDNA with random hexamers (GoScript Reverse Transcription System; Promega). Real-time PCR was performed on a StepOne Real-Time PCR System (Applied Biosystems) using SYBR Green Master mix (Life Technologies) and the relative quantification method. Expression of all genes was normalized to peptidylprolyl isomerase A (Ppia) as the internal control. Primers were designed using Primer3 to span at least one intron when possible (35) and are listed in Supplemental Table 1. Primers were used at a final concentration of 300nM and had calculated efficiencies of 85%–100% over a 1000-fold change standard curve. Relative values below the lowest point on the standard curve were assigned that value, and occasional outliers were identified using Grubbs' test and removed.
Northern blottings
Total RNA was prepared from 100 mg of liver tissue per animal (n = 8 per sex/genotype) using TRIzol reagent (Invitrogen). A total of 30-μg RNA samples were resuspended in 50% formamide, 17% vol/vol formaldehyde and 1× 3-(N-morpholino)propansulfonic acid, loaded in a 1.5% denaturing agarose gel, run 5 hours at 80 V, and transferred to Nylon membranes (Duralon-UV; Stratagene) overnight using 20× SSC. A random priming protocol (Invitrogen) was used to label a rat CBG cDNA with 32P-dCTP (36). Hepatic CBG mRNA was detected after overnight hybridization of Northern blottings with the radiolabeled cDNA in a 50% formamide solution at 42°C, followed by washing protocol, as previously described (36, 37). The Northern blottings were then exposed in a Phosphor Imager, and the relative intensity of the 1.2-kb bands was quantified using IPGel Lab software. Sample RNA loading was controlled by hybridization of a 32P-labeled oligonucleotide (5′-ACGGTATCTGATCGTCTTCGAACC-3′) to ribosomal 18s RNA on the same membranes as previously described (37).
Statistical analyses
Repeated measures ANOVA (RMANOVA) followed by Tukey's multiple comparisons test was used for analyses of serial corticosterone and ACTH measurements. Raw plasma GH values were normalized by log transformation before calculation of the means and then analyzed by two-factor ANOVA. Median GH levels and lengths of interpulse troughs were compared by the nonparametric Kruskal-Wallis test followed by Dunn's post hoc test. Student's unpaired t test was used for all other comparisons. For all tests, P < .05 was considered significant. All statistical analyses were performed with Prism 6.0.
Results
WT C57BL/6 mice exhibit sexually dimorphic ultradian GH secretion
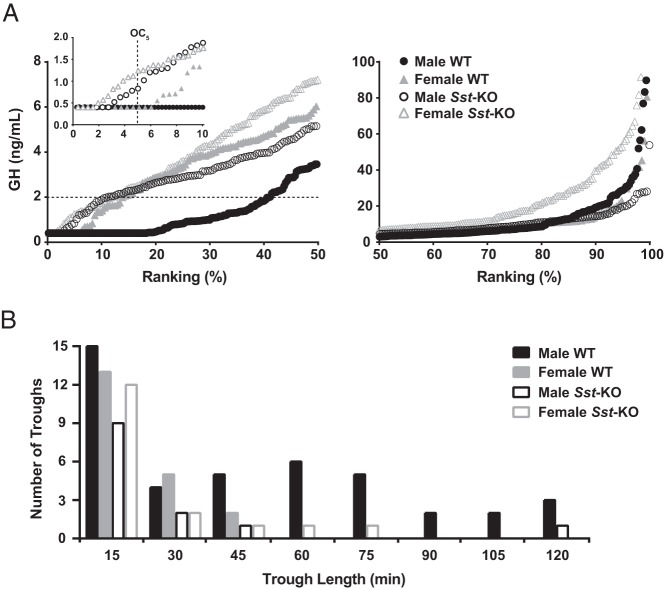
Representative plasma GH profiles for 2 WT mice of each sex are shown in Figure 1, A and B. There were significant individual variations in peak GH levels but no systematic differences between sexes for the average maximum plasma GH value per mouse or the range of values. However, both the mean and median GH levels were significantly higher in WT female than male mice (Table 1). To further visualize these results, we organized the data for every GH measurement in rank plots (Figure 2A) (38). The dissociation constant for human GH binding to its receptor is 2 ng/mL (39), and this concentration is likely close to the lower physiological limit for cellular effects of GH. A much higher percentage of GH values were less than 2 ng/mL in male WT (41%) than in female WT (13%) mice, indicating that females were more likely to have a physiologically relevant level of plasma GH than males at any given time point. Additionally, male WT mice had significantly more prolonged trough periods with consecutive GH values of less than 2 ng/mL compared with females (Figure 2B).
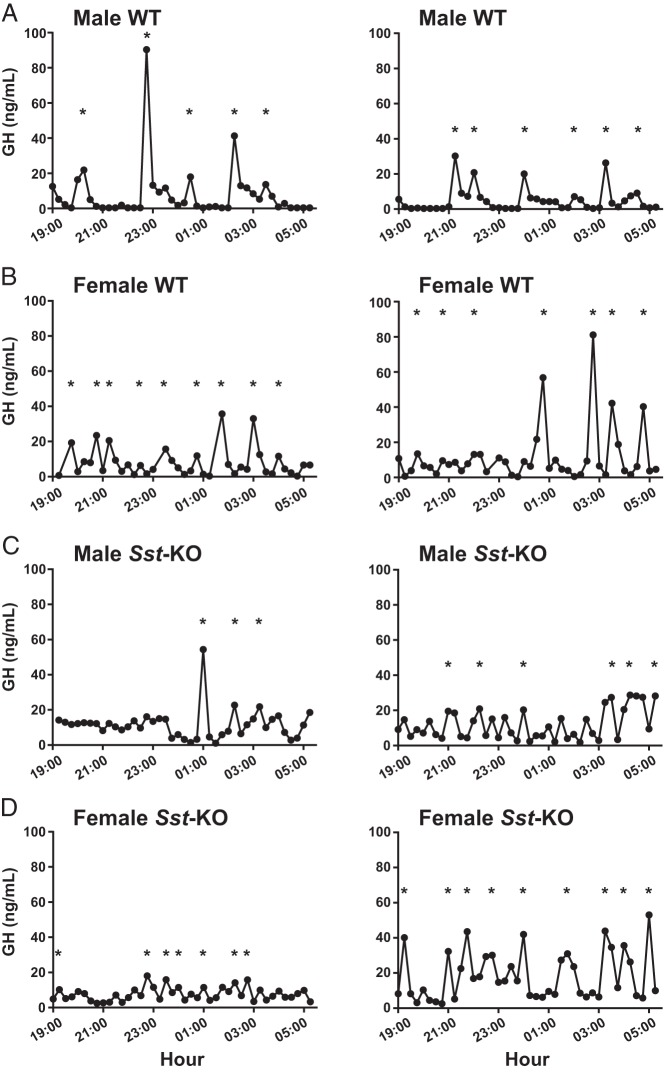
Figure 1.
Serial plasma GH levels in C57BL/6J mice. Raw traces of plasma GH collected every 15 minutes during the nighttime in 2 representative animals of each sex (A and C, male; B and D, female) and genotype (A and B, WT; C and D, Sst-KO). Asterisks indicate the corresponding GH secretory bursts identified by deconvolution analysis.
Table 1.
Descriptive Statistics and Deconvolution Analysis of Serial GH Measurements
| Male WT | Female WT | Male Sst-KO | Female Sst-KO | |
|---|---|---|---|---|
| Raw GH (ng/mL) measurements | n = 349 | n = 215 | n = 217 | n = 258 |
| Mean | 2.9 | 5.3 | 5.0 | 7.8 |
| Median | 3.6 | 6.0a | 5.2a | 7.3a,b |
| Range | <0.4–262 | <0.4–201 | <0.4–54 | <0.4–198 |
| Mean maximum per group (n = 5–8) | 71 ± 80 | 74 ± 76 | 26 ± 17 | 82 ± 71 |
| Deconvolution analysis | n = 8 | n = 5 | n = 5 | n = 6 |
| Total GH secretion rate (ng/mL per 10.75 h) | 696 ± 520 | 782 ± 331 | 591 ± 317 | 1248 ± 890 |
| Pulsatile GH secretion rate (ng/mL per 10.75 h) | 562 ± 516 | 500 ± 337 | 180 ± 75 | 879 ± 814 |
| Mass of GH secreted/burst (ng/mL) | 99 ± 108 | 68 ± 42 | 36 ± 22 | 108 ± 89 |
| Basal secretion rate (ng/mL per 10.75 h) | 134 ± 74 | 282 ± 203 | 412 ± 250c | 369 ± 171c |
| Number of bursts/10.75 h | 6.3 ± 1.7 | 6.8 ± 1.9 | 5.8 ± 2.6 | 7.5 ± 1.4 |
| Mode of secretory bursts (min) | 14.3 ± 2.1 | 16.1 ± 3.5 | 14.7 ± 2.7 | 13.3 ± 1.5 |
| ApEn | 0.84 ± 0.18 | 1.11 ± 0.25c | 1.08 ± 0.15c | 1.06 ± 0.15c |
For mean GH values, data were normalized by log transformation and a two-way ANOVA showed a significant effect of sex (F1,1037 = 44.14, P < .0001) and of genotype (F1,1037 = 36.99, P < .0001) but no significant interaction; values shown are back transformed. The nonparametric Kruskal-Wallis ANOVA was used to compare medians of the untransformed data. For mean maximum per group, values are mean ± SD.
P < .001 compared with WT male, Dunn's post hoc comparison test.
P < .05 compared with WT female, Dunn's post hoc comparison test.
P < .05 compared with WT male, Student's unpaired t test.
Figure 2.
Distributions of individual GH levels and lengths of GH troughs. A, Rank plots of all GH values from all mice grouped by sex and genotype, divided into lower (left) and upper (right) halves to accommodate the difference in scale of the y-axes. The horizontal dotted line in the left panel indicates the probable physiologically relevant lower limit of GH at 2 ng/mL. The inset shows the lowest range of GH values and the vertical dashed line indicates the OC5. A Kruskal-Wallis nonparametric ANOVA of the complete datasets revealed a highly significant (P < .0001) difference between the groups, and Dunn's multiple comparison post hoc tests showed significant differences for every pairwise comparison except between WT females and Sst-KO males. To dissect these differences further, we performed the same tests on the 3 subsets of data shown on the graphs and found a significant difference between groups (P < .0001) for each subset. In the lowest 10% of the rankings (inset), WT male and females were not different from each other but Sst-KO mice of both sexes were significantly different from their WT controls (P < .01). In the lower 50% rankings of the data (left panel), WT males were significantly different (P < .0001) from all other groups, which were not different from each other. In the upper 50% of the rankings (right panel), Sst-KO females had significantly different (P < .0001) GH values from all other groups, which were not different from each other. Seven GH values of more than 100 ng/mL are not shown on the graph but were included in the statistical analyses. B, A distribution histogram of trough lengths, where trough length is defined by consecutive GH values of less than 2 ng/mL at 15-minute intervals. Kruskal-Wallis nonparametric ANOVA followed by Dunn's pairwise post hoc comparisons showed Male WT different from each other group, P < .05.
Deconvolution analysis revealed no significant differences between male and female WT mice in total GH secretion, pulsatile GH secretion rate, GH mass per secretory burst, mode of secretory bursts, or number of secretory bursts in the 10.75-hour time frame (Table 1). There was a trend toward increased basal secretion rate in females (Table 1). Indeed, the only sex difference found for WT mice was in the approximate entropy (ApEn) parameter, which is a measure of pulse regularity, and the lower value in male mice is consistent with more reproducible (nonrandom) pulsing in males than females (Table 1). Notably, although the deconvolution algorithm identified similar numbers of secretory bursts with a range of 6–7 for both sexes, it is obvious from inspection of the raw GH profiles (Figure 1, A and B) that in male mice, the bursts tend to occur frequently in volleys lasting up to 2 hours separated by relatively long trough periods or valleys, whereas in female mice, they are more sharply defined and isolated with short intervening troughs. Taken together, the physiologically relevant period of complex GH secretory events separated by prolonged troughs in WT male mice appears to be in the range of 3–3.5 hours, consistent with data from other rodent studies.
SST deficiency alters GH secretory patterns in a sex-specific manner
Representative plasma GH profiles from Sst-KO mice are shown in Figure 1, C and D. Both male and female Sst-KO mice had significantly higher mean and median GH levels than WT mice of the respective sexes (Table 1), although the range of values was similar in all groups. The percentage of GH values of less than 2 ng/mL was significantly reduced in the male Sst-KO mice (11%) compared with male WT mice (41%) but similar to both WT (13%) and Sst-KO (12%) females (Figure 2A). In addition, the distribution of trough lengths was skewed to the right in the male Sst-KO mice and was indistinguishable from that of female WT and Sst-KO mice (Figure 2B). Further parsing of the GH rank plots revealed that Sst-KO mice had higher GH values than WT mice at the observed concentration for 5%, or OC5, which is one approximation of baseline values (Figure 2A, inset) (40). In the lower 10%–40% ranks of the data distribution, Sst-KO mice of both sexes were similar to female WT but significantly higher than male WT mice, whereas in the upper quartile, Sst-KO females had higher rank values than all the other groups (Figure 2A).
Deconvolution analysis of the GH data from the Sst-KO mice revealed no genotype differences in most of the calculated parameters. However, the basal GH secretion rate for both sexes of Sst-KO mice was significantly higher than that of male WT but not different from female WT mice or each other (Table 1). The ApEn for male Sst-KO mice was increased to female WT and Sst-KO levels, indicating a decrease in pulse reproducibility (Table 1).
Sexually dimorphic hepatic gene expression profiles are altered by the absence of SST
Our microarray analysis identified 195 annotated genes/cDNAs and 11 unannotated sequences that were differentially expressed between the livers of WT male and female mice using the parameters of a FDR less than 5% and a greater than 2-fold change (Supplemental Table 2). This is similar to the number of sexual dimorphic hepatic genes found by other groups using alternative microarray chips and statistical analyses (41–44). Table 2 lists the genes with the highest fold change (>8) between WT male and female livers, and these trends are mostly in agreement with the previously published reports. Two highly female-predominant sequences were identified that have not been previously reported, likely because they are not represented by a probe on Agilent microarrays. They are transcription factor 24 (Tcf24), about which little has been published, and the unannotated sequence AI132709.
Table 2.
Highly Sexually Dimorphic Hepatic Genes in Wild-Type C57BL/6J Mice Revealed by Microarray
| Gene symbol | Gene name | P value (FDR) | Male:female Log2 FC | Previous reference |
|---|---|---|---|---|
| Female predominant | ||||
| Xist | Inactive X-specific transcripts | 6.82E-07 | −7.85 | 43, 44 |
| Cyp2b9 | Cytochrome P450, family 2, subfamily b, polypeptide 9 | 2.29E-09 | −7.18 | 41, 43, 44 |
| Sult3a1 | Sulfotransferase family 3A, member 1 | 8.56E-08 | −7.13 | 41–44 |
| Slc22a26 | Solute carrier family 22 (organic cation transporter), member 26 | 2.51E-12 | −7.10 | 41–44 |
| Fmo3 | Flavin containing monooxygenase 3 | 3.26E-08 | −6.94 | 41–44 |
| Cyp2b13 | Cytochrome P450, family 2, subfamily b, polypeptide 13 | 3.69E-06 | −6.80 | 41, 43, 44 |
| Sult2a2 | Sulfotransferase family 2A, dehydroepiandrosterone (DHEA)-preferring, member 2 | 2.46E-04 | −5.93 | 41–44 |
| Hao2 | Hydroxyacid oxidase 2 | 5.86E-09 | −5.92 | 41–44 |
| AI132709 | Expressed sequence AI132709 | 7.15E-10 | −5.73 | None |
| Acot3 | Acyl-CoA thioesterase 3 | 1.94E-04 | −4.83 | 41, 43, 43 |
| Cyp3a41 | Cytochrome P450, family 3, subfamily a, polypeptide 41 | 4.56E-04 | −4.78 | 41, 43, 44 |
| Rad51b | RAD51 homolog B | 9.28E-05 | −4.70 | 41, 44 |
| Slc22a27 | Solute carrier family 22, member 27 | 5.84E-08 | −4.56 | 41 |
| Slc22a29 | Solute carrier family 22. member 29 | 2.09E-06 | −4.19 | 44 |
| Tcf24 | Transcription factor 24 | 7.46E-10 | −4.15 | None |
| Abcd2 | ATP-binding cassette, subfamily D (ALD), member 2 | 3.82E-07 | −4.05 | 41–44 |
| Cux2 | Cut-like homeobox 2 | 7.35E-06 | −3.69 | 41–44 |
| Nipal1 | NIPA-like domain containing 1 | 9.94E-04 | −3.10 | 41–44 |
| Cyp17a1 | Cytochrome P450, family 17, subfamily a, polypeptide 1 | 0.004 | −3.07 | 41–44 |
| Fam19a2 | Family with sequence similarity 19, member A2 | 7.17E-07 | −3.05 | 43, 44 |
| Cyp3a16 | Cytochrome P450, family 3, subfamily a, polypeptide 16 | 0.016 | −3.00 | 41–44 |
| Nt5e | 5′ nucleotidase, ecto | 1.14E-05 | −3.00 | 41–44 |
| Male predominant | ||||
| Cyp4a12 | Cytochrome P450, family 4, subfamily a, polypeptide 12 | 2.68E-06 | 7.37 | 41–44 |
| Ddx3y | DEAD (Asp-Glu-Ala-Asp) box polypeptide 3, Y-linked | 5.71E-12 | 6.75 | 41–44 |
| Hsd3b5 | Hydroxy-δ-5-steroid dehydrogenase, 3 β- and steroid δ-isomerase 5 | 3.24E-04 | 6.58 | 41–44 |
| Eif2s3y | Eukaryotic translation initiation factor 2, subunit 3, structural gene Y linked | 9.44E-11 | 6.18 | 41, 43, 44 |
| Ugt2b38 | UDP glucuronosyltransferase 2 family, polypeptide B38 | 2.68E-06 | 5.26 | 41–44 |
| Scara5 | Scavenger receptor class A, member 5 (putative) | 4.71E-04 | 4.73 | 41–44 |
| Nat8 | N-acetyltransferase8 (GCN5 related, putative) | 3.84E-08 | 4.67 | 41–44 |
| Elovl3 | Elongation of very long chain fatty acids (FEN1/Elo2, SUR4/Elo3, yeast)-like 3 | 0.003 | 4.38 | 41–44 |
| Cyp2d9 | Cytochrome P450, family 2, subfamily d, polypeptide 9 | 0.003 | 3.52 | 41, 43, 44 |
| Kdm5d | Lysine (K)-specific demethylase 5D | 5.84E-08 | 3.33 | 41–44 |
| C6 | Complement component 6 | 3.48E-06 | 3.20 | 41–44 |
| Cyp7b1 | Cytochrome P450, family 7, subfamily b, polypeptide 1 | 1.07E-08 | 3.10 | 41–44 |
All genes with FDR < 0.05 and Log2(fold change) > I3I are shown and listed in order of fold change from highest to lowest. Genes with multiple probes on the microarrays are listed only once. The indicated references are for previous studies that reported the same pattern of expression.
In general, the loss of SST was associated with feminized hepatic gene expression profiles in male mice while having little effect on female mice. The microarray results of the 206 sexually dimorphic genes were visualized by a heatmap (Figure 3). After clustering by gene, a separation was readily apparent between the WT female predominant (group I) and the WT male predominant (group II) groups. The Sst-KO male mRNA levels more closely matched both groups of female rather than WT male mice. Upon closer examination, 78% of the 73 male-predominant and 71% of the 133 female-predominant genes showed a feminization in Sst-KO males, whereas 85% and 87% of these gene groups, respectively, were unchanged in Sst-KO females (Supplemental Table 2). Of the 28 sexually dimorphic genes whose levels were changed in Sst-KO females, only 10 were shifted towards a more masculinized profile, whereas 18 were “hyperfeminized.” In contrast, all of the 151 genes with altered levels in Sst-KO male mice were feminized and none were “hypermasculinized.”
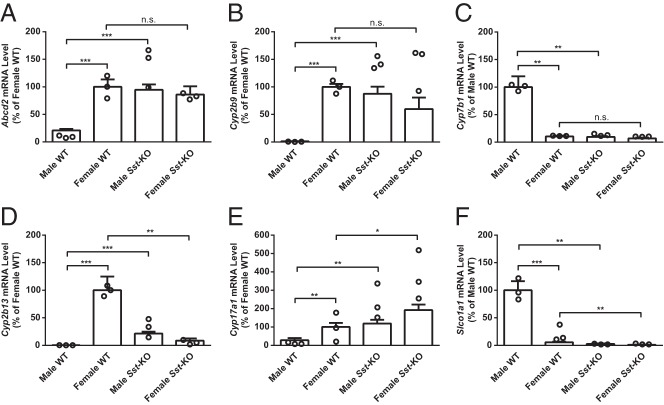
Figure 3.
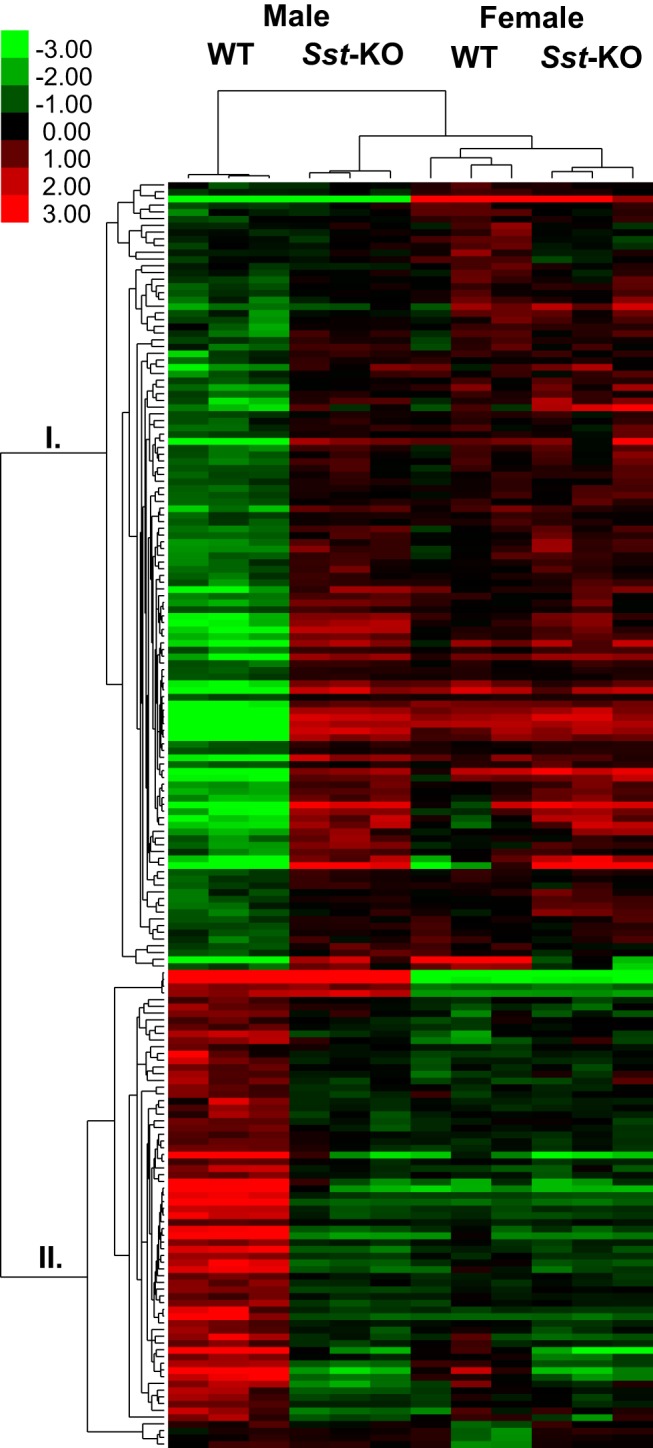
Heatmap of the microarray data visualizing mRNA levels of the 206 sexually dimorphic genes in WT mice compared with Sst-KO mice of both sexes. Relatively high levels are indicated in red and low levels in green. Values were clustered according to gene and sample using the hierarchical clustering method in Cluster3. Female-predominant genes clustered to branch I and male-predominant genes clustered to branch II, and the 3 biological replicates from each group clustered together.
To validate the microarray results, we performed quantitative RT-PCR for a subset of the differentially expressed genes using RNA from livers of an independent cohort of animals. We chose genes from each of 5 categories (male or female predominant in WT and direction of change in each sex of Sst-KO mice) that exhibited robust differences in the microarrays. Relative mRNA levels of Abcd2, Cyp2b9, Cyp7b1, Cyp2b13, Cyp17a1, and Slco1a1 showed comparable sex and genotype differences and identical directions of change measured by qRT-PCR and by microarray, although the absolute fold changes among groups was sometimes larger by the microarray method (Figure 4).
Figure 4.
qRT-PCR validation of the microarray results. mRNA levels relative to a control group (either WT male or female, depending on the sex predominance of the gene) as measured by qRT-PCR are shown by the open bars. The open circles indicate the 3 biological replicates from the corresponding microarray results. A–C, Three “typical” genes were unaffected in Sst-KO females but feminized in Sst-KO males. D–F, Three “atypical” genes showed changes in both sexes of Sst-KO mice. RT-qPCR values shown are the mean + SEM (n = 5–11) and were analyzed by unpaired Student's t test; *, P < .05; **, P < .01; ***, P < .001. Statistical analyses of the microarray results are presented in Supplemental Table 2.
SST deficiency affects the hypothalamic-pituitary-adrenal axis
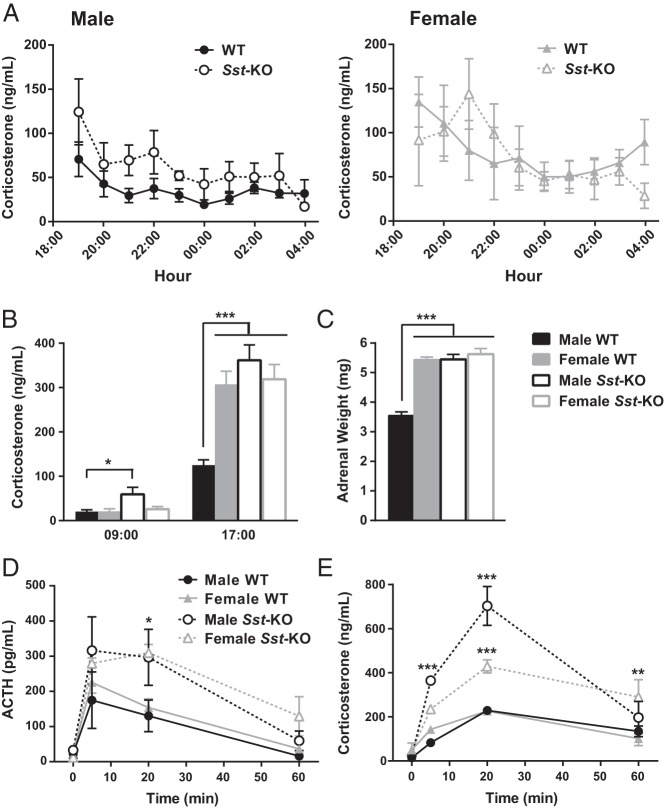
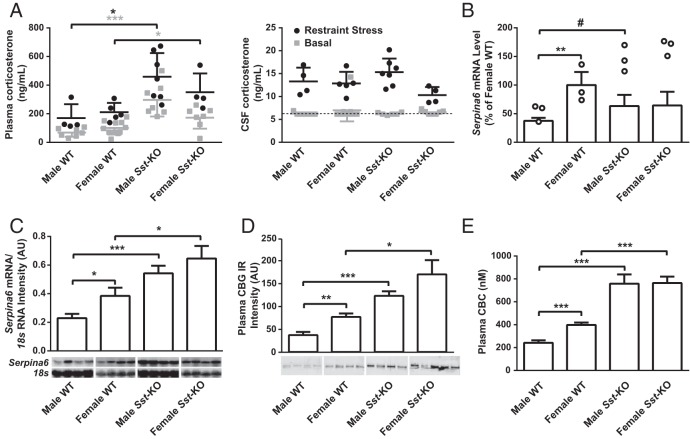
Stress is known to affect the GH axis, and previous publications have reported significantly elevated daytime corticosterone levels in another strain of Sst-KO compared with WT mice (21, 45), prompting us to also examine the HPA axis of the Sst-KO mice. First, we measured corticosterone once per hour to evaluate stress in the cannulated mice during blood collection. Corticosterone levels of WT mice were within the expected basal range and exhibited the typical circadian pattern of higher levels at the onset of darkness that declined until the end of the dark cycle (Figure 5A) (46, 47). RMANOVA of all 4 groups revealed a significant main effect of time. Although corticosterone levels of the male Sst-KO mice were almost double that of WT mice at several time points, these differences across the 11-hour time frame were not statistically significant (P = .07, Sidak post hoc test, effect of genotype). However, in a separate cohort of mice, we found elevated plasma corticosterone levels in unstressed male Sst-KO mice at both the daytime nadir and peak (Figure 5B). In addition, male Sst-KO mice had enlarged adrenal glands (Figure 5C) due to hypertrophy of the zona fasiculata (data not shown). Next, we measured plasma ACTH and corticosterone at several time points during and after acute restraint stress. ACTH was doubled in Sst-KO males compared with WT males after the 20-minute stress (Figure 5D). Sst-KO mice of both sexes had exaggerated elevations in corticosterone during and after stress compared with their WT controls (Figure 5E). Despite elevated basal corticosterone levels, enlarged adrenals, and exaggerated hormonal stress responses, Sst-KO mice do not exhibit behavioral signs of anxiety or depression (V. Otero-Corchon and M.J. Low, unpublished results) (20, 21), and their thymus glands do not involute (29.6 ± 4.4 vs 27.1 ± 4.0 mg [mean ± SD], female WT and Sst-KO, respectively, P = .15; and 21.1 ± 3.7 vs 27.1 ± 4.0 mg, male WT and Sst-KO, respectively, P = .20).
Figure 5.
Characterization of the HPA axis in Sst-KO mice. A, Hourly overnight plasma corticosterone levels of mice from the Culex study. RMANOVA on all 4 groups showed no difference between groups but a significant effect of time (F10,200 = 6.12, P < .0001). RMANOVA restricted to males (left panel) revealed a significant effect of time (F10,110 = 5.01, P < .001) and a near significant effect of genotype (F1,11 = 5.06, P = .07), whereas RMANOVA restricted to females showed only a significant effect of time (F10,90 = 2.69, P < .01). RMANOVA between WT male and WT female mice revealed a significant effect of time (F10,110 = 4.17, P < .001) and a near significant effect of sex (F1,11 = 4.57, P = .06). B, Corticosterone measurements from unstressed mice at the diurnal nadir (9 am) and peak (5 pm). Data were analyzed by unpaired Student's t test, *, P < .05; ***, P < .001. C, Adrenal weights analyzed by unpaired Student's t test, ***, P < .001. D and E, Hormone responses to 20 minutes of acute restraint stress starting at time t = 0. D, RMANOVA of the ACTH response to stress revealed a significant effect of time (F3,60 = 25.7, P < .001) and group (F3,20 = 5.5, P < .01) but no interaction. Tukey's multiple comparisons test found a significant difference between male WT and male Sst-KO mice at 20 minutes (*, P < .05). E, RMANOVA of the corticosterone response to stress found a significant effect of time (F3,60 = 62.0, P < .001), group (F3,20 = 24.1, P < .0001) and an interaction (F9,60 = 7.8, P < .001). Tukey's multiple comparisons test identified pairwise differences between Sst-KO and WT males at 5 and 20 minutes after stress and between Sst-KO and WT females at 20 and 60 minutes after stress (**, P < .01; ***, P < .001). All data shown are the mean ± SEM.
Most circulating corticosterone is biologically unavailable to tissues, because it is bound to CBG, encoded by the Serpina6 gene. CBG does not cross the blood brain barrier (48), and corticosterone in the brain has been shown to reflect free corticosterone levels in the plasma (49). We therefore estimated circulating free corticosterone levels by measuring corticosterone in the CSF of unstressed and stressed mice. In contrast to the genotype differences found in total plasma corticosterone levels, free corticosterone levels in CSF did not differ between WT and Sst-KO mice (Figure 6A), consistent with the lack of evidence for functional glucocorticoid excess in the mutant animals. We did not initially identify Serpina6 as a sexually dimorphic gene due to the stringent restrictions on statistical analysis of the microarray data, but using quantitative RT-PCR, we found it to be female predominant (Figure 6B), in agreement with previous literature (25, 42–44). Furthermore, hepatic mRNA levels of Serpina6 were increased in Sst-KO compared with WT males, suggesting a feminized expression pattern in Sst-KO males (Figure 6B). Northern blottings further confirmed female-predominant Serpina6 mRNA levels in WT mice, feminization in Sst-KO males, and “ultrafeminization” in Sst-KO females (Figure 6C). The same differential expression patterns held true for plasma CBG protein levels (Figure 6D) and CBC (Figure 6E), indicating that the group differences in Serpina6 mRNA and CBG protein levels parallel the observed differences in total corticosterone levels.
Figure 6.
Plasma and CSF corticosterone, liver Serpina6 and plasma CBG levels. A, Plasma corticosterone (left) and CSF corticosterone (right) both basally (gray squares) and after restraint stress (black circles). The dotted line represents the assay sensitivity of 6.3 ng/mL. B, Hepatic Serpina6 mRNA levels as measured by qRT-PCR (open bars) and microarrays (open circles; #, P < .001, see Supplemental Table 2). C, Northern blot analysis of Serpina6 mRNA normalized to 18s. D, Western blottings of plasma CBG immunoreactivity (IR). E, CBC was measured at 9 am and 5 pm, but no diurnal rhythm was found and results displayed are collapsed across time points. All data shown, except microarray data in B, are mean ± SEM (n = 4–11) and were analyzed by unpaired Student's t test; *, P < .05; **, P < .01; ***, P < .001.
Discussion
Although serial blood sampling from indwelling cannulae is considered the gold standard method to measure GH pulsatility, it has only been performed in mice by one other group to our knowledge (8), due primarily to the technical challenges of the surgery. An alternative method was developed recently involving the collection of 4-μL blood samples from the tail tip every 10 minutes for 6 hours from awake, but gently restrained, mice (50). Intrinsic to this method is frequent handling of the mice during bleeding, which is likely to activate the stress response. Although the handling stress was claimed to be negligible, reported corticosterone levels were relatively high at 370 ng/mL (51). Thus, our automated method has the advantage of obtaining serial blood samples from individual mice for extended periods of time during the day or night for the analysis of hormone patterns in the absence of activating the stress axis.
Human males secrete more GH at night than during the day (52), and nocturnal GH pulses are tightly correlated with slow-wave sleep (53), leading to the hypothesis that rodents might secrete more GH in the daytime when they are sleeping. Early studies in rats found no difference in male GH pulsatility patterns in the night vs the day (4, 5) but increased pulsatility in females at night (54), suggesting that diurnal GH patterns may be more related to light than to sleep-wake patterns. In support of this, a more recent study found that light stimulation in the middle of the night rapidly down-regulated spontaneous GH secretion (55). Together, these studies also suggest the possibility of circadian variation in GH pulsatility in mice, but no previous study has measured nocturnal GH pulsatility. However, the GH secretory parameters determined by deconvolution analysis in the present nighttime study are very similar to those reported in the published daytime studies using WT male mice of comparable age (3, 50, 56, 57), suggesting that GH pulsatility is not diurnally regulated, at least in male mice.
The only other studies that have examined sex differences in GH pulsatility in mice collected serial blood samples during the daytime from outbred CD-1 mice (8, 58). Using a similar but older deconvolution algorithm to ours, MacLeod et al (8) found more frequent GH peaks and significantly shorter nadir trough periods between peaks, resulting in a shortened total cycle length, in the female mice. There were no sex differences in the range of GH values or the width and area of GH release in each pulse. Our data analysis is in agreement that the defining sex difference in GH secretion in mice is the prolonged interpulse trough period in males compared with females and suggests that any possible circadian GH variation does not affect the sex difference in this parameter. However, our analysis also revealed a significantly lower ApEn for GH secretion in male mice, indicating that GH secretory bursts are less random in male compared with female mice. This difference is reflected in the pattern of GH secretory events. We found that the GH secretory bursts in female C57BL/6 mice usually occurred as isolated events lasting 15–30 minutes, whereas the bursts in male C57BL/6 mice frequently occurred in overlapping or tightly related volleys of 2 or more bursts. In contrast, the GH secretory peaks described previously in CD-1 mice were isolated and equally narrow in both sexes (8, 58). These differences might reflect the different mouse strains.
A possible confounding factor of our results is that the female mice could have been in different stages of the estrous cycle at the time of sampling. Early studies indicated no difference in GH secretory patterns across estrous stage in rats (54, 59). However, reports in humans have been contradictory. Some groups report no change in GH pulsatility across the menstrual cycle (60, 61), whereas others report elevated mean GH in the late follicular phase (62) and increased pulse frequency in the ovulatory phase (63), both of which positively correlate with plasma estrogen. The effect of estrous stage on GH pulsatility in mice has never been studied, but it is possible that some parameters that we measured could have been influenced by estrous stage, leading to increased variability within females and thus a lack of power to detect a difference from males. Nevertheless, collapsing all females regardless of estrous stage, we still found several significant sex differences that are in agreement with previous literature.
The next goal of our study was to identify the role of SST in mediating sex-specific GH secretory patterns. Overall mean and median GH values were increased above WT in both sexes of Sst-KO mice, and further analysis revealed sex-specific effects of Sst deletion in the distribution of GH values over different ranges. The major effect of SST deficiency, however, was to greatly reduce the fraction of individual GH levels less than 2 ng/mL in male Sst-KO mice, consequently eliminating the masculinized pattern of prolonged GH troughs. Theoretically, SST could inhibit GH secretion through any of 5 known SST receptors (Sst1–Sst5). GHRH neurons in the arcuate nucleus express Sst2A in a sexually dimorphic distribution (64), and GH administration in Sst2-KO mice failed to decrease the activity of GHRH neurons (65), suggesting that SST might contribute to sexually dimorphic GH-mediated negative feedback via Sst2 at the level of the hypothalamus. The anterior pituitary expresses all 5 SST receptors, but Sst2 and Sst5 are thought to be most important in regulating GH release from somatotrophs and are more highly expressed in male vs female mice (66). However, knockout mice for each of the SST receptors have been generated with no reports of elevated circulating GH, suggesting either functional redundancy among the receptors or developmental compensations in the knockout models. Future experiments are needed to characterize GH pulsatility in mice with individual or compound deletions of the SST receptors and to determine the relative importance of SST effects in the hypothalamus and pituitary gland.
Although GH pulsatility in Sst-KO mice has not been directly studied previously, Luque et al (67) examined several other aspects of the GH axis using an alternative strain of Sst-KO mice, also shown to have elevated random GH levels in both sexes (21, 67). They found elevated plasma IGF-I, increased pituitary Gh, Ghrhr, and Ghsr expression, and decreased hypothalamic Ghrh expression only in female Sst-KO mice, leading them to conclude that endogenous SST plays a predominant role in females in the regulation of the GH axis (67). Those findings are consistent with our observations that female Sst-KO mice exhibited the highest levels of mean GH, a skewed distribution of ranked GH levels, and the largest GH secretion rate compared with the other 3 groups. However, our results suggest that a lack of SST more profoundly affects the GH interpulse trough interval in males than it does in females. It is possible that in normal females, estrogen signaling interacts with SST signaling in the hypothalamus and the pituitary to organize the GHRH-GH signaling axis. In males, testosterone directly activates SST neurons, which act as a brake on GH release from somatotrophs. Thus, the sex steroid milieu dictates a differential role for SST and, while in females, a lack of SST affects expression of several key genes in the GH axis, SST is more physiologically relevant in males because of the profound effects of Sst deletion on male GH secretory patterns and GH-regulated hepatic mRNA levels.
An intriguing observation from our analysis of ultradian GH secretion and sexually dimorphic hepatic gene expression was the elevation of CBG production in both sexes of Sst-KO mice and in WT female compared with WT male mice. Although higher Serpina6 mRNA levels have been noted previously in the livers of female mice compared with males (25, 42–44), and serum CBG levels have been shown to be dependent on GH pulse patterns (68), to our knowledge, a potential mechanistic relationship between sexually dimorphic GH secretion and sexual dimorphism of the HPA axis has never been proposed. Previous reports of elevated corticosterone levels in Sst-KO mice attributed the increase to a putative loss of SST inhibition of ACTH release from pituitary corticotrophs (45). That study demonstrated in vitro effects of SST to inhibit basal ACTH release and to attenuate CRH-induced ACTH release from primary cultured pituitary cells of both rats and nonhuman primates. Our in vivo data showing exaggerated ACTH and corticosterone responses to acute restraint stress in Sst-KO mice and increased adrenal gland weight in male Sst-KO mice are consistent with this possibility. Paradoxically, the mutant mice do not exhibit phenotypic signs of glucocorticoid excess, and free corticosterone levels in CSF are the same in both sexes and genotypes of mice after acute restraint stress, despite their differences in total corticosterone levels in plasma.
Therefore, we propose that SST has 2 distinct and parallel actions in regulation of the HPA axis. SST indirectly contributes to the sexual dimorphism of the HPA axis through its shaping of sexually dimorphic GH secretion and hepatic gene expression. Sex differences in the levels of circulating CBG produced by the liver contribute to the higher total corticosterone levels (bound and free) measured in WT female mice. Due to feminized GH pulsatility, Sst-KO male mice have feminized levels of CBG and hence elevated total corticosterone levels but do not exhibit a Cushingoid phenotype, because their biologically available free corticosterone remains normal. In addition, SST can inhibit ACTH release from the pituitary (45). A normally higher pituitary SST tone in male mice, proposed as a mechanism for the sexual dimorphism of GH secretion, may therefore lead to chronically slightly increased basal ACTH levels in male Sst-KO mice, which we have been unable to confirm due to assay limitations (data not shown). This could explain the hypertrophied adrenal zona fasciculata in male Sst-KO mice. However, SST inhibition of ACTH release may be more physiologically relevant in the context of simultaneous CRH stimulation as occurs during stress, as we have shown.
In summary, we used an automated blood sampling system to characterize the sex differences in GH pulsatility in WT C57BL/6J mice and to determine the necessity of SST for this sexual dimorphism. Carotid artery cannulation and automated sampling were well tolerated by the mice, as reflected by normal corticosterone levels, and the results accurately portrayed physiological changes of plasma hormones. We found that male mice have lower mean and median GH, more nonrandom GH secretory bursts, and more consecutive nadir GH values of less than 2 ng/mL than female mice during the nocturnal period. In the absence of SST, male mice exhibited a feminized pattern of GH secretion, associated with a global feminization of sexually dimorphic hepatic mRNA levels. SST deficiency in female mice also increased mean and median GH levels but produced no measurable change in GH pulsatility and only affected hepatic mRNA levels in a handful of sexually dimorphic genes, including Serpina6. Together, these results suggest that the default GH secretory pattern is feminine and that SST is necessary to masculinize the hypothalamic-pituitary-liver axis. As a consequence, decreased Serpina6 expression and plasma CBG contribute to the lower circulating total corticosterone levels in WT male compared with female mice.
Acknowledgments
We thank Richard Miller for providing us with plasma from dw/dw mice, Craig Jaffe for insightful discussion, Amanda Sharpe for CSF collections from the cisterna magna, and Courtney Attard and Jeffrey Jian for technical assistance and the University of Michigan Center for Statistical Consultation and Research for advice on statistical analyses.
This work was supported by the National Institutes of Health (NIH) Early Stage Training in the Neurosciences Training Grant T32-NS076401 (to J.M.A.). G.L.H. was supported by a Canada Research Chair in Reproductive Health and an operating grant from Canadian Institutes of Health Research (MOP-111102). M.J.L. was supported by a NIH Grant R01-DK066604. This work used core services provided by the University of Michigan Animal Phenotyping Core supported by the Michigan Nutrition and Obesity Research Center and the Michigan Diabetes Research Center (NIH Grants P30-DK089503 and P30-DK020572) and the Molecular Biology Core supported by the Michigan Gastrointestinal Peptide Research Center (NIH Grant P30-DK034933).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ApEn
- approximate entropy
- CBC
- corticosteroid-binding capacity
- CBG
- corticosteroid-binding globulin
- CSF
- cerebrospinal fluid
- CV
- coefficient of variability
- FDR
- false discovery rate
- HPA
- hypothalamic-pituitary-adrenal
- KO
- knockout
- RMANOVA
- repeated measures ANOVA
- SST
- somatostatin
- WT
- wild type.
References
- 1. Gahete MD, Durán-Prado M, Luque RM, et al. Understanding the multifactorial control of growth hormone release by somatotropes: lessons from comparative endocrinology. Ann NY Acad Sci. 2009;1163:137–153. [DOI] [PubMed] [Google Scholar]
- 2. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. [DOI] [PubMed] [Google Scholar]
- 3. Hassouna R, Zizzari P, Tomasetto C, et al. An early reduction in GH peak amplitude in preproghrelin-deficient male mice has a minor impact on linear growth. Endocrinology. 2014;155(9):3561–3571. [DOI] [PubMed] [Google Scholar]
- 4. Tannenbaum GS, Martin JB. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology. 1976;98(3):562–570. [DOI] [PubMed] [Google Scholar]
- 5. Edén S. Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology. 1979;105(2):555–560. [DOI] [PubMed] [Google Scholar]
- 6. Hartman ML, Veldhuis JD, Vance ML, Faria AC, Furlanetto RW, Thorner MO. Somatotropin pulse frequency and basal concentrations are increased in acromegaly and are reduced by successful therapy. J Clin Endocrinol Metab. 1990;70(5):1375–1384. [DOI] [PubMed] [Google Scholar]
- 7. Pincus SM, Gevers EF, Robinson I, et al. Females secrete growth hormone with more process irregularity than males in both humans and rats. Am J Physiol. 1996;270(1):E107–E115. [DOI] [PubMed] [Google Scholar]
- 8. MacLeod JN, Pampori NA, Shapiro BH. Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol. 1991;131(3):395–399. [DOI] [PubMed] [Google Scholar]
- 9. Mode A, Norstedt G, Simic B, Eneroth P, Gustafsson JA. Continuous infusion of growth hormone feminizes hepatic steroid metabolism in the rat. Endocrinology. 1981;108(6):2103–2108. [DOI] [PubMed] [Google Scholar]
- 10. Norstedt G, Palmiter R. Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell. 1984;36(4):805–812. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Klein K, Sugathan A, et al. Transcriptional profiling of human liver identifies sex-biased genes associated with polygenic dyslipidemia and coronary artery disease. Plos One. 2011;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76(2):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waxman DJ, Pampori NA, Ram PA, Agrawal AK, Shapiro BH. Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome p450. Proc Natl Acad Sci USA. 1991;88(15):6868–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wehrenberg WB, Brazeau P, Luben R, Böhlen P, Guillemin R. Inhibition of the pulsatile secretion of growth hormone by monoclonal antibodies to the hypothalamic growth hormone releasing factor (GRF). Endocrinology. 1982;111(6):2147–2148. [DOI] [PubMed] [Google Scholar]
- 15. Lumpkin MD, McDonald JK. Blockade of growth hormone-releasing factor (GRF) activity in the pituitary and hypothalamus of the conscious rat with a peptidic GRF antagonist. Endocrinology. 1989;124(3):1522–1531. [DOI] [PubMed] [Google Scholar]
- 16. Tannenbaum GS, Epelbaum J, Colle E, Brazeau P, Martin JB. Antiserum to somatostatin reverses starvation-induced inhibition of growth hormone but not insulin secretion. Endocrinology. 1978;102(6):1909–1914. [DOI] [PubMed] [Google Scholar]
- 17. Terry LC, Martin JB. The effects of lateral hypothalamic-medial forebrain stimulation and somatostatin antiserum on pulsatile growth hormone secretion in freely behaving rats: evidence for a dual regulatory mechanism. Endocrinology. 1981;109(2):622–627. [DOI] [PubMed] [Google Scholar]
- 18. Argente J, Chowen JA, Zeitler P, Clifton DK, Steiner RA. Sexual dimorphism of growth hormone-releasing hormone and somatostatin gene expression in the hypothalamus of the rat during development. Endocrinology. 1991;128(5):2369–2375. [DOI] [PubMed] [Google Scholar]
- 19. Chowen-Breed JA, Steiner RA, Clifton DK. Sexual dimorphism and testosterone-dependent regulation of somatostatin gene expression in the periventricular nucleus of the rat brain. Endocrinology. 1989;125(1):357–362. [DOI] [PubMed] [Google Scholar]
- 20. Low MJ, Otero-Corchon V, Parlow AF, et al. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest. 2001;107(12):1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeyda T, Diehl N, Paylor R, Brennan MB, Hochgeschwender U. Impairment in motor learning of somatostatin null mutant mice. Brain Res. 2001;906(1–2):107–114. [DOI] [PubMed] [Google Scholar]
- 22. Liu L, Herukka SK, Minkeviciene R, van Groen T, Tanila H. Longitudinal observation on CSF Aβ42 levels in young to middle-aged amyloid precursor protein/presenilin-1 doubly transgenic mice. Neurobiol Dis. 2004;17(3):516–523. [DOI] [PubMed] [Google Scholar]
- 23. Gruenewald DA, Hess DL, Wilkinson CW, Matsumoto AM. Excessive testicular progesterone secretion in aged male Fischer 344 rats: a potential cause of age-related gonadotropin suppression and confounding variable in aging studies. J Gerontol. 1992;47(5):B164–B170. [DOI] [PubMed] [Google Scholar]
- 24. Hammond GL, Lähteenmäki PL. A versatile method for the determination of serum cortisol binding globulin and sex hormone binding globulin binding capacities. Clin Chim Acta. 1983;132(1):101–110. [DOI] [PubMed] [Google Scholar]
- 25. Scrocchi LA, Orava M, Smith CL, Han VK, Hammond GL. Spatial and temporal distribution of corticosteroid-binding globulin and its messenger ribonucleic acid in embryonic and fetal mice. Endocrinology. 1993;132(2):903–909. [DOI] [PubMed] [Google Scholar]
- 26. Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2008;29(7):823–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keenan DM, Iranmanesh A, Veldhuis JD. Analytical construct of reversible desensitization of pituitary-testicular signaling: illustrative application in aging. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R349–R360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akaike H. A new look at statistical model identification. IEEE Trans. 1974;AC19(6):716–723. [Google Scholar]
- 29. Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. [DOI] [PubMed] [Google Scholar]
- 30. Ritchie ME, Diyagama D, Neilson J, et al. Empirical array quality weights in the analysis of microarray data. BMC Bioinformatics. 2006;7:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article 3. [DOI] [PubMed] [Google Scholar]
- 32. Benjamini Y, Hochberg Y. Controlling the false discovery rate - A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 33. Gautier L, Cope L, Bolstad BM, Irizarry RA. affy–analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307–315. [DOI] [PubMed] [Google Scholar]
- 34. Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey V, Huber W, Irizarry R, Dudoit S, eds. Bioinformatics and Computational Biology Solution Using R and Bioconductor. Springer, New York; 2005:397–420. [Google Scholar]
- 35. Rozen S, Skaletsky HJ. Primer3. Code available at http://www-genome.wi.mit.edu/genome_software/other/primer3.html 1998.
- 36. Smith CL, Hammond GL. Rat corticosteroid binding globulin: primary structure and messenger ribonucleic acid levels in the liver under different physiological conditions. Mol Endocrinol. 1989;3(2):420–426. [DOI] [PubMed] [Google Scholar]
- 37. Smith CL, Hammond GL. Ontogeny of corticosteroid-binding globulin biosynthesis in the rat. Endocrinology. 1991;128(2):983–988. [DOI] [PubMed] [Google Scholar]
- 38. Xu J, Bekaert AJ, Dupont J, et al. Exploring endocrine GH pattern in mice using rank plot analysis and random blood samples. J Endocrinol. 2011;208(2):119–129. [DOI] [PubMed] [Google Scholar]
- 39. Fuh G, Cunningham BC, Fukunaga R, Nagata S, Goeddel DV, Wells JA. Rational design of potent antagonists to the human growth hormone receptor. Science. 1992;256(5064):1677–1680. [DOI] [PubMed] [Google Scholar]
- 40. Matthews DR, Hindmarsh PC, Pringle PJ, Brook CGD. A distribution method for analyzing the baseline of pulsatile endocrine signals as exemplified by 24-hour growth hormone profiles Clin Endocrinol (Oxf). 1991;35(3):245–252. [DOI] [PubMed] [Google Scholar]
- 41. Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20(6):1333–1351. [DOI] [PubMed] [Google Scholar]
- 42. Yang X, Schadt EE, Wang S, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16(8):995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gatti DM, Zhao N, Chesler EJ, et al. Sex-specific gene expression in the BXD mouse liver. Physiol Genomics. 2010;42(3):456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Conforto TL, Zhang Y, Sherman J, Waxman DJ. Impact of CUX2 on the female mouse liver transcriptome: activation of female-biased genes and repression of male-biased genes. Mol Cell Biol. 2012;32(22):4611–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luque RM, Gahete MD, Hochgeschwender U, Kineman RD. Evidence that endogenous SST inhibits ACTH and ghrelin expression by independent pathways. Am J Physiol. 2006;291(2):E395–E403. [DOI] [PubMed] [Google Scholar]
- 46. Saito M, Bray GA. Diurnal rhythm for corticosterone in obese (ob/ob) diabetes (db/db) and gold-thioglucose-induced obesity in mice. Endocrinology. 1983;113(6):2181–2185. [DOI] [PubMed] [Google Scholar]
- 47. Zaki MS, Katayama T, Murata T, Konishi H, Shiota K, Takahashi M. The regulation of food intake and correlated energy balance in mice. J Vet Med Sci. 1991;53(2):249–254. [DOI] [PubMed] [Google Scholar]
- 48. Schwarz S, Pohl P. Steroid hormones and steroid hormone binding globulins in cerebrospinal fluid studied in individuals with intact and with disturbed blood-cerebrospinal fluid barrier. Neuroendocrinology. 1992;55(2):174–182. [DOI] [PubMed] [Google Scholar]
- 49. Qian X, Droste SK, Gutièrrez-Mecinas M, et al. A rapid release of corticosteroid-binding globulin from the liver restrains the glucocorticoid hormone response to acute stress. Endocrinology. 2011;152(10):3738–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Steyn FJ, Huang L, Ngo ST, et al. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011;152(8):3165–3171. [DOI] [PubMed] [Google Scholar]
- 51. Steyn FJ, Leong JW, Huang L, et al. GH does not modulate the early fasting-induced release of free fatty acids in mice. Endocrinology. 2012;153(1):273–282. [DOI] [PubMed] [Google Scholar]
- 52. Jaffe CA, Ocampo-Lim B, Guo W, et al. Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J Clin Invest. 1998;102(1):153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vancauter E, Kerkhofs M, Caufriez A, Vanonderbergen A, Thorner MO, Copinschi G. A quantitative estimation of growth hormone secretion in normal man - Reproducibility and relation to sleep and time of day J Clin Endocrinol Metab. 1992;74(6):1441–1450. [DOI] [PubMed] [Google Scholar]
- 54. Clark RG, Carlsson LM, Robinson IC. Growth hormone secretory profiles in conscious female rats. J Endocrinol. 1987;114(3):399–407. [DOI] [PubMed] [Google Scholar]
- 55. Davies JS, Carter DA, Wells T. Photic stimulation inhibits growth hormone secretion in rats: a hypothalamic mechanism for transient entrainment. Endocrinology. 2004;145(6):2950–2958. [DOI] [PubMed] [Google Scholar]
- 56. Steyn FJ, Lee K, Fogarty MJ, et al. Growth hormone secretion is correlated with neuromuscular innervation rather than motor neuron number in early-symptomatic male amyotrophic lateral sclerosis mice. Endocrinology. 2013;154(12):4695–4706. [DOI] [PubMed] [Google Scholar]
- 57. Tan HY, Huang L, Simmons D, Veldhuis JD, Steyn FJ, Chen C. Hypothalamic distribution of somatostatin mRNA expressing neurones relative to pubertal and adult changes in pulsatile growth hormone secretion in mice. J Neuroendocrinol. 2013;25(10):910–919. [DOI] [PubMed] [Google Scholar]
- 58. Pampori NA, Shapiro BH. Effects of neonatally administered monosodium glutamate on the sexually dimorphic profiles of circulating growth hormone regulating murine hepatic monooxygenases. Biochem Pharmacol. 1994;47(7):1221–1229. [DOI] [PubMed] [Google Scholar]
- 59. Takahashi S, Gottschall PE, Quigley KL, Goya RG, Meites J. Growth hormone secretory patterns in young, middle-aged and old female rats. Neuroendocrinology. 1987;46(2):137–142. [DOI] [PubMed] [Google Scholar]
- 60. Klein NA, Battaglia DE, Miller PB, Soules MR. Circulating levels of growth hormone, insulin-like growth factor-I and growth hormone binding protein in normal women of advanced reproductive age. Clin Endocrinol (Oxf). 1996;44(3):285–292. [DOI] [PubMed] [Google Scholar]
- 61. Jaffe CA, Ocampo-Lim B, Guo W, et al. Growth hormone secretory dynamics over the menstrual cycle. Endocr J. 2000;47(5):549–556. [DOI] [PubMed] [Google Scholar]
- 62. Faria ACS, Bekenstein LW, Booth RA, et al. Pulsatile growth hormone release in normal women during the menstrual cycle. Clin Endocrinol (Oxf). 1992;36(6):591–596. [DOI] [PubMed] [Google Scholar]
- 63. Ovesen P, Vahl N, Fisker S, Veldhuis JD, Christiansen JS, Jørgensen JO. Increased pulsatile, but not basal, growth hormone secretion rates and plasma insulin-like growth factor I levels during the periovulatory interval in normal women. J Clin Endocrinol Metab. 1998;83(5):1662–1667. [DOI] [PubMed] [Google Scholar]
- 64. Bouyer K, Loudes C, Robinson IC, Epelbaum J, Faivre-Bauman A. Sexually dimorphic distribution of sst2A somatostatin receptors on growth hormone-releasing hormone neurons in mice. Endocrinology. 2006;147(6):2670–2674. [DOI] [PubMed] [Google Scholar]
- 65. Zheng H, Bailey A, Jiang MH, et al. Somatostatin receptor subtype 2 knockout mice are refractory to growth hormone-negative feedback on arcuate neurons. Mol Endocrinol. 1997;11(11):1709–1717. [DOI] [PubMed] [Google Scholar]
- 66. Cordoba-Chacon J, Gahete MD, Castano JP, Kineman RD, Luque RM. Somatostatin and its receptors contribute in a tissue-specific manner to the sex-dependent metabolic (fed/fasting) control of growth hormone axis in mice. Am J Physiol. 2011;300(1):E46–E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Luque RM, Kineman RD. Gender-dependent role of endogenous somatostatin in regulating growth hormone-axis function in mice. Endocrinology. 2007;148(12):5998–6006. [DOI] [PubMed] [Google Scholar]
- 68. Jansson JO, Oscarsson J, Mode A, Ritzén EM. Plasma growth hormone pattern and androgens influence the levels of corticosteroid-binding globulin in rat serum. J Endocrinol. 1989;122(3):725–732. [DOI] [PubMed] [Google Scholar]