Abstract
Bisphenol A (BPA) is a high production volume chemical and an endocrine disruptor. Developmental exposures to BPA have been linked to adult metabolic pathologies, but the pathways through which these disruptions occur remain unknown. This is a comprehensive interspecies association vs causal study to evaluate risks posed by prenatal BPA exposure and to facilitate discovery of biomarkers of relevance to BPA toxicity. Samples from human pregnancies during the first trimester and at term, as well as fetal and/or adult samples from prenatally BPA-treated sheep, rats, and mice, were collected to assess the impact of BPA on free fatty acid and oxidative stress dynamics. Mothers exposed to higher BPA during early to midpregnancy and their matching term cord samples displayed increased 3-nitrotyrosine (NY), a marker of nitrosative stress. Maternal samples had increased palmitic acid, which was positively correlated with NY. Sheep fetuses and adult sheep and rats prenatally exposed to a human-relevant exposure dose of BPA showed increased systemic nitrosative stress. The strongest effect of BPA on circulating free fatty acids was observed in adult mice in the absence of increased oxidative stress. This is the first multispecies study that combines human association and animal causal studies assessing the risk posed by prenatal BPA exposure to metabolic health. This study provides evidence of the induction of nitrosative stress by prenatal BPA in both the mother and fetus at time of birth and is thus supportive of the use of maternal NY as a biomarker for offspring health.
It is highly debated whether endocrine-disrupting chemicals at current exposure levels are detrimental to human health (1). In recent years, attention has focused on human exposure to bisphenol A (BPA), a widely used high production volume industrial endocrine-disrupting chemical. In fact, human exposure to BPA is virtually universal (2), including exposures during prenatal life, because it has been detected in maternal serum, amniotic fluid, and cord blood taken at birth and placental tissues (3–5). Exposure to BPA during these early developmental periods has been linked to intrauterine growth restriction/low birth weight and, hence, considered as a risk factor for adult reproductive and metabolic diseases (1, 6–8). Epidemiological studies have also reported positive correlations between increased BPA exposure and adult pathologies, including metabolic disorders (9, 10). The ubiquity of BPA and the range of target organs BPA impacts emphasize the need for a comprehensive evaluation of the health risks stemming from BPA exposure and expansion of investigations to endpoints of relevance to various systems and their integrative functions.
A human cross-sectional study involving 1455 subjects (data from National Health and Nutrition Examination Survey 2003–2004 survey) reported an association between urinary levels of BPA and diabetes (10) and metabolic syndrome (11). Studies in animal models have also shown that prenatal exposure to BPA leads to postnatal metabolic deficits, including disruption of glucose homeostasis (12), altered mitochondrial function (13), and changes in growth rate during early development (14). One of the precursors of these metabolic pathologies is insulin resistance, which in many instances is associated with inflammation. Known mediators of tissue specific insulin resistance and inflammation include imbalances in free fatty acids (FFAs) and oxidative stress (15). Indeed, oxidative stress may play a causal role in all forms of insulin resistance (16). These pathways are potential targets for insult from endocrine disruptors such as BPA. Evidence for endocrine-disrupting chemicals interfering with lipid metabolism, FFA balance and transport, and oxidative stress is available in various species (17). BPA augments the inflammatory response in rodents (18) and is associated with oxidative stress in postmenopausal women (19). Such findings of metabolic disruptions, especially after developmental exposure to BPA along with the fact that prenatal BPA exposure has the potential to induce epigenetic modifications (20), including changes in the miRNA machinery such as evidenced in other studies (21), provide support for BPA disruption of the glucose/insulin homeostasis and its link to in utero programming.
In assessing human risk posed by BPA, because sensitivity to BPA is likely to vary between species, causal studies in several species are needed. Furthermore, because route of administration, uptake, and metabolism of BPA are also likely to vary between species and from fetus to adult, it is important to relate internal levels in body fluids/tissues, as a reference point to assess human risk. Taking these into consideration, this study tested the hypothesis that gestational exposure to BPA, at levels humans are exposed to, disrupts the maternal and offspring metabolic homeostasis by perturbing FFA balance and oxidative stress system, contributors to the development of insulin resistance, forerunner of type 2 diabetes, metabolic syndrome, and cardiovascular disease. The study was developed as a comprehensive interspecies study to evaluate risks posed by prenatal BPA exposure and to facilitate discovery of biomarkers of relevance to BPA toxicity.
Materials and Methods
Human subjects
After institutional review board approval and written informed consent, women were recruited from the community surrounding the University of Michigan Von Voigtlander Women's Hospital. Women were contacted during their first trimester prenatal clinic visit and were included if they fit the next inclusion criteria: 18 years or older, natural conception (no infertility treatment), singleton pregnancy, and 8–14 weeks pregnant. Exclusion criteria included pregnancy complications such as gestational diabetes, preterm birth, preeclampsia, or newborn abnormalities. At this initial study visit, participants had blood drawn by venipuncture. After delivery of the placenta, cord blood samples were collected from the cord, which was clamped proximal to the placenta. To avoid BPA contamination from plastics, placenta was hung vertically, a needle was inserted into the cord and blood allowed to flow through the needle directly into glass tubes. All samples were collected and processed avoiding any contact with plastic to avoid BPA contamination. Based on first trimester maternal unconjugated BPA (uBPA) levels, maternal samples and pair-matched term umbilical cord were chosen from 12 low vs 12 high uBPA-exposed pregnant women. Complete demographic data of subjects included in the study and the uBPA levels in the high and low BPA groups are shown in Table 1. Subjects delivering both male and female offspring were included in the study; their distribution between the low and high BPA group did not vary (Table 1). Maternal samples were assayed for FFA analysis, and maternal and cord samples were both assayed for oxidized products of tyrosine (details below).
Table 1.
Human Subjects Characteristics
| Low BPA | High BPA | |
|---|---|---|
| Number of subjects | 12 | 12 |
| uBPA (ng/mL) | 0.08 ± 0.01 | 27.79 ± 8.38* |
| uBPA range (ng/mL) | 0.03–0.14 | 4.1–96.4 |
| Maternal age | 30.4 ± 1.1 | 32.9 ± 1.8 |
| Maternal BMI prepregnancy | 24.0 ± 1.0 | 24.3 ± 1.1 |
| Maternal BMI term | 29.8 ± 1.3 | 29.5 ± 1.3 |
| Race/ethnicity | ||
| Asian | 1 (8.3%) | 3 (25.0%) |
| African-American | 2 (16.7%) | 1 (8.3%) |
| Hispanic | 0 | 2 (16.7%) |
| White | 8 (66.7%) | 6 (50.0%) |
| Not available | 1 (8.3%) | 0 |
| Marital status (single/married) | 3/9 | 2/10 |
| Parity | 0.7 ± 0.7 | 0.6 ± 0.2 |
| Newborn gender (males/females) | 8/4 | 7/5 |
| Gestational length (d) | 275.7 ± 1.3 | 275.2 ± 2.4 |
Data expressed as mean ± SEM. Asterisk indicates significant difference (P <.01); note that statistical difference here is driven by study design.
Animal studies
Table 2 summarizes tissues, age and number of animals, BPA dose, vehicle used, exposure route, window of exposure, and outcome measures used in all animal species studied. Resource limitation and the gender focus of some of the grants that supported the sample generation restricted this investigation to females only. Due to limited sample volume in rodents, some outcome measures could not be undertaken at some time points. Specific details of animal experimentation by species are listed below.
Table 2.
Summary of Species Studied, Matrix Used, Age, Sample Size, BPA Dose, Vehicle and Exposure Route, Window of Exposure, and Outcome Measures Studied
| Species | Matrix | Age | Control (n) | BPA Low Dose (n) | BPA High Dose (n) | Vehicle Used and Route | Exposure Window | OX Stress | FFA |
|---|---|---|---|---|---|---|---|---|---|
| Sheep | Plasma | Mothers | Vehicle (7) | 0.5 mg/kg (5) | — | Corn oil (SC) | 30–65 d of pregnancy | Yes | No |
| Mothers | Vehicle (5) | 0.5 mg/kg (6) | — | 30–90 d of pregnancy | Yes | No | |||
| Fetal (GD65) | Vehicle (7) | 0.5 mg/kg (5) | — | GD30–GD65 | Yes | Yes | |||
| Fetal (GD90) | Vehicle (5) | 0.5 mg/kg (6) | — | GD30–GD90 | Yes | Yes | |||
| Adult (21 mo) | Vehicle (5) | 0.5 mg/kg (6) | 5 mg/kg (5) | Yes | Yes | ||||
| FatV | Adult (21 mo) | Vehicle (5) | 0.5 mg/kg (6) | 5 mg/kg (5) | Yes | Yes | |||
| Rat | Serum | Neonate (PND1) | Vehicle (4) | N/A | 50 mg/kg (4) | 95% EtOH-Cookie (oral) | GD6–GD21 | No | Yes |
| Adult (PND90) | Vehicle (6) | 50 mg/kg (6) | 50 mg/kg (6) | Yes | No | ||||
| FatGR | Adult (PND90) | Vehicle (6) | N/A | 50 mg/kg (6) | Yes | Yes | |||
| Mouse | Plasma | Adult (PND90) | Vehicle (4–6) | N/A | 50 mg/kg (4–6) | Diet (oral) | 2 wk premating to PND 21 | Yes | Yes |
| FatG | Adult (PND90) | Vehicle (6) | N/A | 50 mg/kg (6) | No | Yes |
Dose in sheep and rats is calculated per kilogram of body weight per day. Dose in mice is calculated per kilogram of diet per day. Number of females included in each time point is shown in parenthesis. Superscripts in matrix column refer to: V, visceral fat depot; G, gonadal fat depot; GR, pooled renal and gonadal fat depots. N/A indicates samples not available.
Sheep
Jugular blood and visceral adipose samples were collected from control and prenatal BPA-treated (0, 0.5, and 5 mg/kg · d from gestational day [GD]30 to GD90; term, ∼147 d) adult females (∼21 mo old) during the follicular phase of a synchronized cycle. Blood samples were collected before euthanasia, and adipose tissue was collected after a barbiturate overdose (Fatal Plus, Vortech Pharmaceuticals). Adipose tissue samples were flash frozen on dry ice and stored frozen until assay. Arterial umbilical cord samples from control and prenatal BPA-treated female fetuses (0- and 0.5-mg/kg · d BPA, other doses not studied), and jugular samples from their mothers were procured at days 65 and 90 of gestation (mid and end of BPA treatment, respectively) from a second set of animals. For treatment purposes, BPA (purity ≥99%, catalog 239658; Aldrich Chemical Co) was dissolved in corn oil (vehicle received by the control group). For reference, the 5-mg/kg · d dose BPA produces approximately 40-ng/mL free BPA in maternal blood (22), levels approximating twice the highest level found in pregnant United States women (23). Internal dose of uBPA achieved in umbilical arterial samples with the 0.5-mg/kg · d dose has been reported previously and averages 2.62 ± 0.52 ng/mL at GD90 (21), approaching the median level of BPA measured in maternal circulation (23) and urine (24) of United States women.
Rats
Serum from postnatal day (PND)1 and serum and adipose tissues from approximately 3-month-old (83–110 d) control and prenatal BPA-treated Wistar rats were obtained from an in-house colony at North Carolina State University. Animals were reared in an environment that minimizes exposure to BPA and other exogenous xenoestrogens (glass water bottles, polysulfone caging, woodchip bedding, and Harlan Teklad 2020 soy-free diet) (25). BPA (50 mg/kg body weight [BW], 50 μg/kg BW) or vehicle (95% ethanol) was dispensed (15-μL total volume) on a vanilla wafer cookie (Newman's Own) as described previously (26) and fed to the dams daily from GD6 to GD21. BPA was provided by the National Institute for Environmental Health Sciences (NIEHS) (BPA, 2,2-bis(4-hydroxyphenyl)propane; chemistry abstract service number 80-05-7; lot 11909; United States Environmental Protection Agency/NIEHS standard). Maternal levels of uBPA after a 100-μg/kg BW oral dose (twice the low dose used in this study) has been estimated to be around 0.07 ng/mL (27), which is within the exposure range of pregnant women (23). In addition, serum samples were obtained from female litter mates on PND1 animals and pooled as needed to generate sufficient volume for analysis. Serum and fat pads (right and left ovarian fat pads and right kidney fat pad) were obtained from the adults on the day of estrus (1 low-dose BPA female was acyclic at the time of killing). Fat pads were flash frozen on powdered dry ice. All materials were stored at −80°C until shipping.
Mice
Plasma and gonadal adipose tissue from approximately 3-month-old (82–110 d) control and prenatal BPA-treated female mice were obtained from a colony of Avy Agouti strain mice maintained for over 220 generations with forced heterozygosity for the Avy allele through the male line, resulting in a genetically invariant background with 93% similarity to C57BL/6J (28). Female wild-type (a/a) mice were killed and tissues collected on the first day of estrus (29). The Avy mutation initially arose spontaneously in the CH3/HeJ strain. Animals carrying the mutation were backcrossed for 1–3 generations with C57BL/6J animals, followed by over 200 generations of sibling mating, resulting in a genetically invariant background, which is 93% C57BL/6J overall (30). Thus, the animals evaluated in this study are quite similar to C57BL/6J mice, a standard mouse strain used in toxicant and metabolic studies. BPA was administered via the diet. Specifically, virgin a/a dams, 6 weeks of age, were randomly assigned to phytoestrogen-free AIN-93G diets (diet 95092, with 7% corn oil substituted for 7% soybean oil) supplemented with 50-mg BPA per kilogram of diet. All diet ingredients were supplied by Harlan Teklad, except BPA, which was supplied by the National Toxicology Program. At 8 weeks of age, exposed dams were mated to Avy/a sires. Dams and offspring were maintained on assigned diets until offspring weaning at 3 weeks of age, upon which the BPA-treated animals were maintained on the control diet. The 50-mg BPA per kilogram of diet is an order of magnitude lower than the dietary administered maximum nontoxic threshold in rodents (200 mg/kg BW/d) (31), and dams are expected to consume 10-mg BPA/kg BW per day, assuming consumption rates of 5 g/d. The internal BPA levels achieved in PND22 weanling offspring have been reported to range between 9.5 and 870 ng/g of total BPA in livers, which is comparable with the range observed in human fetal livers (less than limit of detection, 96.8 ng/g) (32). Other studies using a dose of 100-mg BPA/kg diet demonstrated that mean uBPA levels over a 24-hour period after BPA administration was 6.1 ng/mL (33), which falls under the range of human maternal exposure. In order to obtain enough material for the assays, female siblings from the same litter were pooled as needed.
The internal BPA doses discussed for the 3 species above are from previously published studies and carried out in different laboratories (21, 27, 32) using protocols established before the BPA Round Robin validation study (34).
BPA measures
uBPA and BPA glucuronide (BPA-G) were measured using a validated protocol published from the NIEHS-funded Round Robin study (34) in one of the laboratories that participated in the study. Plasma samples were extracted following methods described elsewhere, with some modifications (35). Briefly, after thawing at room temperature, serum (0.5 mL) was transferred to a 15-mL glass tube, and internal standards (d6-BPA and 13C12-BPA-G), ammonium acetate buffer, formic acid, and milli-Q water were added to a total volume of 3 mL. An Oasis mixed-mode cation exchange cartridge (60 mg/3 cc; Waters) was used for extraction and clean-up procedure. The cartridge was preconditioned with methanol and water. After loading the sample, the cartridge was washed with 15% methanol in water and eluted with methanol. The eluate was concentrated to 0.5 mL. The uBPA and BPA-G levels in samples were quantified using a HPLC coupled with API 5500 electrospray triple-quadrupole mass spectrometer (ESI-MS/MS). A total of 10 μL of the extract was injected onto an analytical column (Betasil C18, 100 × 2.1-mm column; Thermo Electron Corp), which was connected to a Javelin guard column (Betasil C18, 20 × 2.1 mm). The mobile phase was comprised of methanol and 10mM ammonium acetate in water. The ESI-MS/MS was operated in the electrospray negative ion mode. Data were acquired using multiple reaction monitoring for the transitions of 227 > 212 for BPA, 233 > 215 for d6-BPA, 403 > 113 for BPA-G, and 415 > 113 for 13C12-BPA-G.
Quality assurance and quality control parameters include validation of the method by spiking internal standards into the sample matrices and passing through the entire analytical procedure to calculate recoveries of target analytes through the analytical method. A procedural blank was analyzed with the samples to check for interferences or laboratory contamination. The limit of detection was 0.02 ng/mL for uBPA and BPA-G. The recovery of d6-BPA and 13C12-BPA-G spiked into samples was 95 ± 29% (mean ± SD) and 103 ± 37%, respectively. Reported concentrations were corrected for the recoveries of surrogate standard (isotope dilution method). The native standards spiked into procedural blank and selected sample matrices and passed through the entire analytical procedure yielded recoveries of 105 ± 5% and 102 ± 7% for BPA and 107 ± 19% and 95 ± 20% for BPA-G, respectively. An external calibration curve was prepared by injecting 10 μL of 0.01- to 100-ng/mL standards and calibration coefficient was more than 0.99.
FFA measures
FFAs were measured in plasma of human maternal samples as well as in fetal and adult sheep, PND1 rats, and adult mice. FFAs were also measured in adipose tissue of adult sheep, rat, and mice. FFA measurement in plasma and adipose tissue involved the next steps: 1) lipid extraction, 2) preparation of methyl ester and purification, 3) analysis of nonesterified FFA, and 4) gas chromatography (GC) identification of fatty acid methyl esters. In brief, total lipids from the fat samples were extracted using an organic solvent (36). The methyl esters were extracted with hexane and then purified by thin-layer chromatography and identified with respect to the retention time of the standard. The fatty acid compositions were then analyzed by GC. Analysis of nonesterified FFA of plasma samples was performed using a direct method of transesterification as described previously (37). Hexane was used to extract the methyl esters and then purified by thin-layer chromatography and analyzed by GC (6890N Model; Agilent) equipped with flame ionization detector, an auto sampler. Hydrogen was used as a carrier gas as well as for flame ionization detector and nitrogen as a makeup gas. A calibration curve was prepared using proportional amounts of C17:0 methyl ester standard. A mixture of standard methyl esters was also run to identify the components in unknown samples by comparing their retention times. The FFAs were quantified with respect to the amounts of C17:0 internal standard added, and the calibration curve was prepared. Intraassay coefficients of variation were 0.9% and 1.1% and interassay coefficients of variation were 2.6% and 3.3%. Individual FFAs and total amount of saturated, mono- and polyunsaturated FFAs were calculated. Desaturation indices were calculated as the ratio of the sum of the unsaturated to sum of saturated FFAs with the same number of carbons (ie, C16:1/C16:0).
Oxidative stress measures
Protein-bound oxidized tyrosine moieties (3-nitrotyrosine [NY], 3-chlorotyrosine [ClY], and o, o'-dityrosine [DiY]) in human, sheep, and mice plasma, rat serum, and adipose tissue were quantified by isotope dilution liquid chromatography electrospray ionization tandem mass spectrometry as described previously (38). Briefly, the plasma and tissue proteins were precipitated with ice-cold trichloroacetic acid (10% vol/vol) and then delipidated with water/methanol/water-washed diethyl ether (1:3:7; vol/vol/vol). Adipose tissue was homogenized before precipitation and delipidation. Known amounts of isotopically labeled internal standards 13C6-Y and 13C6-NY, 13C6-ClY, and 13C12-o, o'-DiY were added. The precipitated plasma proteins were hydrolyzed at 110°C for 24 hours in 4M methanesulfonic acid solution saturated with 1% benzoic acid. After solid-phase extraction, the oxidized amino acids were quantified by HPLC-ESI-MS/MS with multiple reaction monitoring by integrating peak areas of the labeled standards and the analytes. The levels of the oxidized amino acids were then normalized to the precursor amino acid tyrosine content. The levels of oxidized tyrosine products are expressed as the ratio of the oxidized product over the total tyrosine. Intraassay coefficients of variation for ClY, DiY, and NY were 2.42%, 3.31%, and 2.7%, respectively.
Statistical analysis
The impact of prenatal BPA treatment (all 3 animal species) on FFA and oxidative stress variables and the association of BPA levels in maternal circulation of human pregnancies with FFA and oxidative stress variables were analyzed using general linear model. For human studies, where both genders were involved, newborn gender was used as a covariate in the model. Pearson correlation was used to assess the association between 2 variables. Appropriate transformations were applied for all variables to account for heterogeneity of variances. Significance was defined as P < .05. All results are presented as mean ± SEM. All analyses were carried out using PASW Statistics for Windows release 18.0.1.
Results
Association between BPA and oxidative stress in human maternal and cord samples
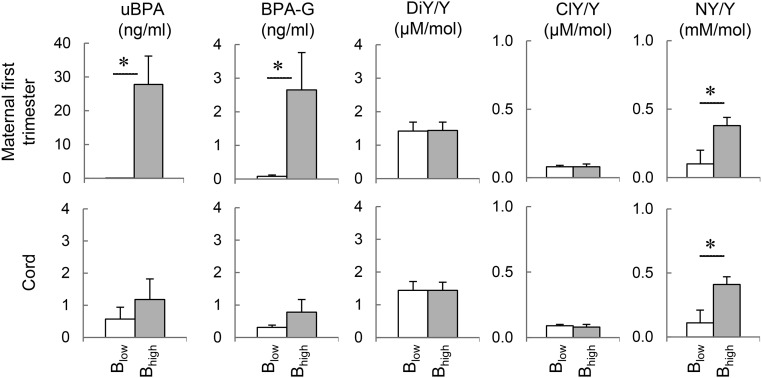
Mean (±SEM) of uBPA, BPA-G, and oxidized tyrosine products (NY [NY/tyrosine], ClY [ClY/tyrosine], and o,o'-DiY [DiY/tyrosine]), in first trimester maternal and term umbilical cord samples of low and high uBPA groups (based on first trimester maternal measures) are shown in Figure 1. As expected (due to categorization of subjects into high and low uBPA groups), uBPA levels in maternal samples of low BPA group were significantly different from the high uBPA group. This difference was also significant when BPA-G levels were compared (P < .05). In addition, maternal uBPA levels were significantly higher compared with term cord blood samples (P < .02). BPA-G was only numerically, but not statistically, higher in maternal compared with cord samples (P = .14). Numerical nonsignificant increases in uBPA and BPA-G concentrations were observed in cord samples of the high BPA group relative to the low BPA group. A positive correlation was found between BPA-G in first trimester samples and term cord samples but not in uBPA (r = 0.443; P < .05).
Figure 1.
BPA and tyrosine (Y) oxidation in human pregnancies. Mean (±SEM) plasma concentrations of uBPA, BPA-G, and oxidized products of tyrosine: DiY, ClY, and NY in 8- to 14-week maternal and their matching term umbilical cord samples in human pregnancies with low vs high uBPA levels. Results are expressed as the ratio between the oxidized product to tyrosine. Blow, low uBPA levels; Bhigh, high uBPA levels.
Pregnant mothers with high uBPA levels had higher levels of NY/tyrosine (P < .005) but not ClY/tyrosine or DiY/tyrosine compared with those with low uBPA. A significant Pearson correlation was found between BPA-G and NY/tyrosine levels (r = 0.440; P < .05). The correlation between uBPA and NY/tyrosine was marginally significant (r = 0.398; P = .054). Cord blood from mothers exposed to higher uBPA levels during 8–14 weeks of gestation had higher NY/tyrosine levels compared the low BPA group (P < .01). Analysis of samples from both groups combined found a positive correlation between maternal and cord NY levels (r = 0.475; P = .019) but not in ClY or DiY.
Association between BPA and FFAs in human maternal and cord samples
Mean (±SEM) values for FFAs are shown in Table 3. Levels of myristic acid (14:0), myristoleic acid (14:1), α-linolenic acid (18:3 (n-3)), arachidic acid (20:0), eicosapentaenoic acid (20:5), behenic acid (22:0), erucic acid (22:1), eicosapentaenoic acid (22:5), and docosahexaenoic acid (22:6) were undetectable in all the human specimens. γ-Linolenic acid (18:3 (n-6)), eicosenoic acid (20:1), and adrenic acid (22:4) were detected in less than or equal to 3 subjects and excluded from the analyses (not shown). There was no difference in palmitoleic acid (16:1), stearic (18:0), vaccenic acid (18:1(n-7)), linoleic acid (18;2), eicosadienoic acid (20:2), eicosatrienoic acid (20:3), and eicosatetraenoic acid (20:4) between women with low vs high uBPA levels in the first trimester, except for palmitic acid (16:0). Palmitic acid was higher in the high BPA group compared with the low BPA group (P = .012). Oleic acid (18:1(n-9)) and total FFAs also tended to be higher in women with higher BPA levels. In addition, a positive correlation was found between uBPA and palmitic acid (r = 0.779; P < .001) and between uBPA and total FFA (r = 0.645; P < .001) but not BPA-G. A trend towards positive correlation was observed between palmitic acid and NY/tyrosine (r = 0.396; P = .062).
Table 3.
FFA Levels (Mean ± SEM) in Maternal Samples of Humans
| FFA Name | FFA (nmol/mL) | Low uBPA | High uBPA | P Value |
|---|---|---|---|---|
| Maternal first trimester | ||||
| Palmitic | 16:0 | 52.7 ± 5.4a | 89.7 ± 12.3b | 0.012 |
| Palmitoleic | 16:1 | 1.5 ± 0.3 | 3.9 ± 1.8 | n.s. |
| Stearic | 18:0 | 53.8 ± 4.7 | 58.8 ± 3.9 | n.s. |
| Vaccenic | 18:1 (n-7) | 4.2 ± 0.6 | 6.6 ± 1.5 | n.s. |
| Oleic | 18:1 (n-9) | 56.6 ± 7.9 | 92.8 ± 17.4 | 0.078 |
| Linoleic | 18:2 | 55.9 ± 7.3 | 73.0 ± 11.2 | n.s. |
| Eicosadienoic | 20:2 | 1.9 ± 0.4 | 1.9 ± 0.4 | n.s. |
| Dihomo-γ-linolenic | 20:3 | 3.3 ± 0.6 | 2.2 ± 0.5 | n.s. |
| Arachidonic | 20:4 | 9.3 ± 1.3 | 8.2 ± 0.9 | n.s. |
| Total FFA | 240.0 ± 21.2 | 338.6 ± 46.1 | 0.065 |
The next FFAs were not detectable: myristic acid (14:0), myristoleic acid (14:1), α-linolenic acid (18:3 (n-3)), arachidic acid (20:0), eicosapentaenoic acid (20:5), behenic acid (22:0), erucic acid (22:1), eicosapentaenoic acid (22:5), and docosahexaenoic acid (22:6). The next FFAs were detected in less than or equal to 3 subjects and hence excluded from the analyses: γ-linolenic acid (18:3 (n-6)), eicosenoic acid (20:1), and adrenic acid (22:4). The bolded value highlights the significance difference in palmitic between groups.
Association between BPA and oxidative stress in animal studies
Impact of gestational BPA treatment on oxidized tyrosine products in the 3 animal species studied is shown in Figures 2 and 3. ClY was not detectable in all 3 animal species studied.
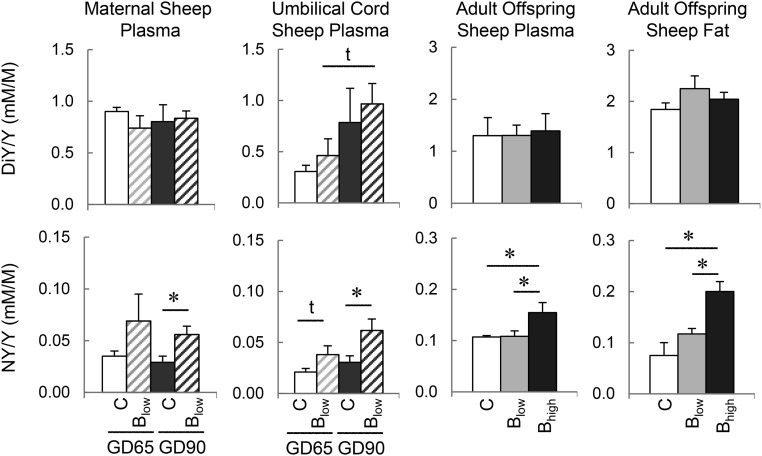
Figure 2.
Tyrosine (Y) oxidation in gestational BPA-treated sheep. Mean (±SEM) concentrations of oxidized products of tyrosine; DiY and NY in maternal plasma of control and BPA (B)-treated sheep, umbilical cord samples of female fetuses at GD65 and GD90, and in plasma and visceral adipose tissues from control and prenatal BPA-treated 21-month-old females sheep. Results are expressed as the ratio between the oxidized product and tyrosine. C, control; Blow, 0.5 mg/kg BW/d; Bhigh, 5 mg/kg BW/d.
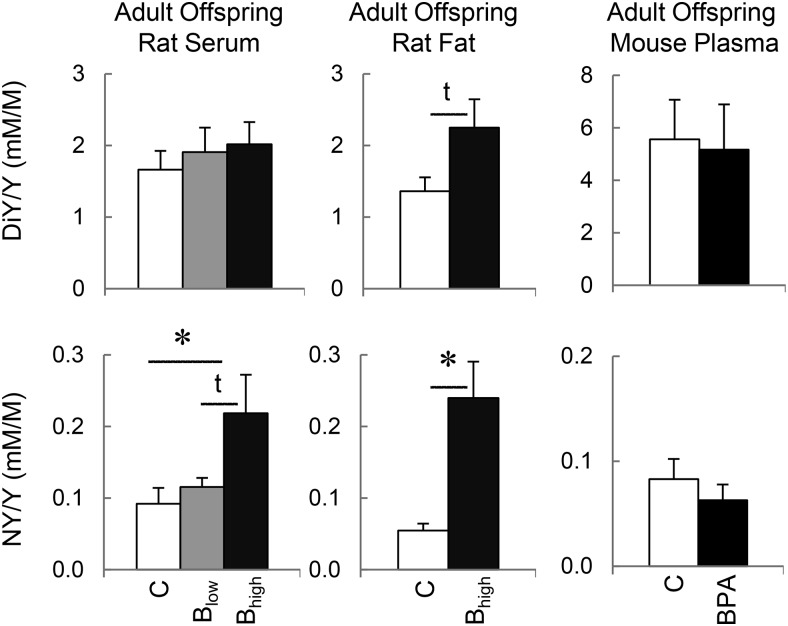
Figure 3.
Tyrosine (Y) oxidation in gestational BPA-treated rats and mice. Mean (±SEM) concentrations of oxidized products of tyrosine; DiY and NY in plasma/serum and adipose tissue (renal and/or gonadal fat pads) of control and prenatal BPA-treated adult (PND90) female rats (left and middle histograms). DiY and NY concentrations in plasma of adult (PND90) control (C) and prenatal BPA-treated female mice (right histograms). C, control; Blow, 50 μg/kg BW/d; and Bhigh, 50 mg/kg BW/d. For mouse, BPA was administered at 50 mg/kg diet/d.
Sheep
NY was higher in GD90 female fetuses of mothers treated with BPA (0.5 mg/kg BW/d). A trend for elevated NY was also evident in GD65 female fetuses of mothers treated with BPA. There was a tendency for a positive correlation between maternal and the fetal NY levels (r = 0.525; P = .079). DiY levels in maternal and fetal circulation of BPA-treated mothers did not differ from corresponding controls. However, a significant negative correlation was found between fetal and maternal DY levels (r = −0.649; P = .022). In prenatal BPA-treated adult females, NY was found to be elevated in both plasma and adipose tissue of higher BPA dose group (5 mg/kg BW/d) but not in the lower BPA dose group (0.5 mg/kg BW/d) relative to controls.
Rodents
NY levels were elevated in plasma and adipose tissue in BPA-treated rats exposed to the higher dose (50 mg/kg BW/d). DiY also tended to be higher in adipose tissue with the same dose of exposure. No differences in oxidized tyrosine products were found in the mouse.
Association between BPA and FFAs in animal studies
Results from circulating FFAs are shown in Table 4 (sheep fetal and mice) and Supplemental Table 1 (rat and adult sheep). Six FFA species were detected in at least 3 subjects per group in sheep fetuses and 8 FFAs in adult sheep. Of those, only arachidonic acid (20:4) was significantly higher in BPA-treated female fetuses at GD65 (C, 2.8 ± 0.9 vs BPA, 5.8 ± 0.5 nmol/mL; P < .05). No significant changes were observed in prenatal BPA-treated adult female sheep at either dose studied. A larger number of FFAs were detected in rats and mice (18 and 17, respectively) compared with human and sheep. Although prenatal BPA treatment had no effect on the circulating FFA profile in rats, it significantly reduced the concentrations of myristic acid in prenatal BPA-treated adult mice (C, 7.9 ± 1.6 vs BPA, 2.8 ± 0.9 nmol/mL; P < .05) and tended to increase γ-linolenic acid (18:3 (n-6)) (C, 1.3 ± 0.4 vs BPA, 5.1 ± 1.5 nmol/mL; P = .058).
Table 4.
Circulating FFA Levels in Fetal Sheep and Adult Mice
| Plasma FFA (nmol/mL) | Sheep |
Mice |
||||
|---|---|---|---|---|---|---|
| Fetal GD65 |
Fetal GD90 |
Adult (PND90) |
||||
| Control | BPA (0.5 mg/kg) | Control | BPA (0.5 mg/kg) | Control | BPA (50 mg/kg) | |
| 14:0 | ND | ND | ND | ND | 7.9 ± 1.6a | 2.8 ± 0.9b |
| 14:1 | ND | ND | ND | ND | ND* | ND* |
| 16:0 | 29.8 ± 2.9 | 22.5 ± 3.4 | 14.9 ± 2.0 | 17.0 ± 0.8 | 225.2 ± 23.9 | 194.2 ± 10.4 |
| 16:1 | ND | ND | ND | ND | 27.6 ± 5.6 | 22.0 ± 3.9 |
| 18:0 | 41.1 ± 7.8 | 37.0 ± 3.4 | 28.3 ± 1.6 | 24.2 ± 1.6 | 141.0 ± 15.6 | 158.1 ± 18.4 |
| 18:1 (n-7) | ND* | ND* | ND* | ND* | 18.6 ± 2.2 | 18.2 ± 4.5 |
| 18:1 (n-9) | 19.6 ± 2.1 | 24.0 ± 2.5 | 29.0 ± 3.4 | 30.9 ± 1.9 | 179.8 ± 21.9 | 226.8 ± 34.4 |
| 18:2 | ND* | ND* | ND* | ND* | 176.1 ± 22.5 | 218.7 ± 29.2 |
| 18:3 (n-3) | ND | ND | ND | ND | 3.0 ± 0.4 | 3.7 ± 0.7 |
| 18:3 (n-6) | ND* | ND* | ND* | ND* | 1.3 ± 0.4c | 5.1 ± 1.5d |
| 20:0 | ND | ND | ND | ND | ND | ND |
| 20:1 | ND* | ND* | ND* | ND* | 3.4 ± 0.5 | 4.5 ± 0.7 |
| 20:2 | 9.0 ± 1.6 | 10.2 ± 1.0 | 9.9 ± 1.6 | 6.5 ± 1.1 | 7.3 ± 0.4 | 11.3 ± 2.0 |
| 20:3 | ND* | ND* | ND* | ND* | 4.6 ± 0.8 | 5.9 ± 0.8 |
| 20:4 | 2.8 ± 0.9a | 5.8 ± 0.5b | 4.6 ± 1.6 | 6.7 ± 1.1 | 47.6 ± 7.7 | 59.8 ± 12.0 |
| 20:5 | ND* | ND* | ND* | ND* | ND | ND |
| 22:0 | ND | ND | ND | ND | ND | ND |
| 22:1 | ND | ND | ND | ND | ND | ND |
| 22:4 | ND* | ND* | ND* | ND* | 2.9 ± 0.3 | 4.1 ± 0.7 |
| 22:5 | ND* | ND* | ND* | ND* | ND* | ND* |
| 22:6 | ND* | ND* | ND* | ND* | 2.6 ± 0.9 | 3.5 ± 1.4 |
| 24:0 | 5.0 ± 1.2 | 6.6 ± 0.9 | 4.9 ± 0.7 | 4.8 ± 0.8 | 2.6 ± 0.2 | 4.4 ± 0.9 |
| 24:1 | ND* | ND* | ND* | ND* | 6.6 ± 0.9 | 9.3 ± 1.3 |
| Total FFA | 110.6 ± 13.5 | 108.0 ± 6.8 | 94.1 ± 6.7 | 98.2 ± 6.0 | 858.3 ± 94.5 | 952.9 ± 102.4 |
ND, not detected; ND*, detected in less than 3 sheep/mice per group. a ≠ b within age are significantly different (P < .05); c ≠ d within species and age tended to be different (P = .058). BPA doses expressed in kg of BW/d.
Results from FFAs in adipose tissue are summarized in Table 5. BPA had no effect on the adipose tissue FFA profile in adult sheep or mice. However, prenatal BPA treatment significantly increased erucid acid (C, 0.12 ± 0.05 vs BPA, 0.30 ± 0.06 nmol/mg; P < .05) and tended to increase eicosapentaenoic acid, behenic acid, and docosapentaenoic acid in adult rats. Prenatal exposure to BPA did not have an effect on total saturated, mono- and polyunsaturated FFAs, or desaturation index in plasma or fat in any of the 3 animal species studied.
Table 5.
Adipose Tissue FFA Levels (Mean ± SEM) in Adult Sheep, Rats, and Mice
| Fat FFA (nmol/mg) | Adult Sheep (21 mo old) |
Adult Rats (PND90) |
Adult Mice (PND90) |
||||
|---|---|---|---|---|---|---|---|
| Control | BPA (0.5 mg/kg) | BPA (5 mg/kg) | Control | BPA (50 mg/kg) | Control | BPA (50 mg/kg) | |
| 14:0 | 3.0 ± 0.5 | 2.9 ± 0.2 | 4.0 ± 1.2 | 4.5 ± 0.5 | 5.3 ± 0.5 | 8.3 ± 1.9 | 5.9 ± 0.7 |
| 14:1 | 0.4 ± 0.1 | 0.3 ± 0.0 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.3 ± 0.0 |
| 16:0 | 32.7 ± 7.4 | 27.2 ± 1.6 | 39.8 ± 11.6 | 86.4 ± 6.5 | 94.9 ± 7.4 | 148.8 ± 24.7 | 119.4 ± 14.2 |
| 16:1 | 3.1 ± 1.1 | 1.9 ± 0.2 | 2.6 ± 0.8 | 22.0 ± 1.7 | 21.4 ± 2.6 | 42.0 ± 13.7 | 28.0 ± 4.1 |
| 18:0 | 48.7 ± 7.6 | 41.1 ± 2.5 | 62.6 ± 13.0 | 11.1 ± 0.9 | 13.0 ± 0.9 | 27.0 ± 4.8 | 20.7 ± 5.0 |
| 18:1 (n-7) | 1.1 ± 0.4 | 1.0 ± 0.1 | 1.4 ± 0.5 | 2.2 ± 1.1 | 0.7 ± 0.1 | 0.3 ± 0.2 | 0.5 ± 0.2 |
| 18:1 (n-9) | 69.8 ± 15.7 | 51.7 ± 4.3 | 70.6 ± 17.9 | 85.6 ± 17.8 | 97.6 ± 14.1 | 219.4 ± 46.1 | 230.3 ± 22.8 |
| 18:2 | 11.3 ± 7.2 | 3.6 ± 0.3 | 5.5 ± 2.2 | 95.9 ± 13.0 | 101.9 ± 7.9 | 204.6 ± 31.0 | 160.7 ± 14.9 |
| 18:3 (n-3) | 1.2 ± 0.4 | 0.8 ± 0.1 | 1.1 ± 0.3 | 5.7 ± 0.9 | 5.0 ± 0.5 | 1.7 ± 0.2 | 2.0 ± 0.5 |
| 18:3 (n-6) | 0.17 ± 0.05 | 0.11 ± 0.05 | 0.05 ± 0.02 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| 20:0 | ND | ND | ND | 0.24 ± 0.03 | 0.24 ± 0.02 | 0.12 ± 0.02 | 0.13 ± 0.01 |
| 20:1 | 0.52 ± 0.27 | 0.22 ± 0.03 | 0.32 ± 0.09 | 0.64 ± 0.10 | 0.77 ± 0.19 | 5.2 ± 0.3 | 4.4 ± 0.4 |
| 20:2 | 0.18 ± 0.11 | 0.08 ± 0.01 | 0.12 ± 0.04 | 0.73 ± 0.08 | 0.75 ± 0.15 | 1.3 ± 0.2 | 1.2 ± 0.1 |
| 20:3 | 0.09 ± 0.06 | 0.03 ± 0.00 | 0.05 ± 0.02 | 0.41 ± 0.06 | 0.44 ± 0.05 | 1.1 ± 0.2 | 0.9 ± 0.1 |
| 20:4 | 0.31 ± 0.16 | 0.13 ± 0.02 | 0.20 ± 0.07 | 2.3 ± 0.4 | 2.1 ± 0.3 | 2.9 ± 0.3 | 2.5 ± 0.3 |
| 20:5 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.21 ± 0.07c | 0.4 ± 0.04d | 0.12 ± 0.01 | 0.09 ± 0.01 |
| 22:0 | ND | ND | ND | 0.01 ± 0.01c | 0.04 ± 0.01d | ND | ND |
| 22:1 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.12 ± 0.05a | 0.30 ± 0.06b | 0.2 ± 0.04 | 0.17 ± 0.04 |
| 22:4 | 0.08 ± 0.04 | 0.04 ± 0.00 | 0.07 ± 0.01 | 0.45 ± 0.08 | 0.45 ± 0.11 | 0.70 ± 0.05 | 0.59 ± 0.06 |
| 22:5 | 0.13 ± 0.05 | 0.08 ± 0.01 | 0.12 ± 0.04 | 0.34 ± 0.05c | 0.50 ± 0.06d | 0.16 ± 0.02 | 0.13 ± 0.03 |
| 22:6 | 0.05 ± 0.04 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.31 ± 0.08 | 0.27 ± 0.04 | 0.27 ± 0.06 | 0.16 ± 0.03 |
| 24:0 | 0.08 ± 0.05 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.20 ± 0.11 | 0.12 ± 0.02 | 0.16 ± 0.02 | 0.17 ± 0.03 |
| 24:1 | 0.06 ± 0.03 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.16 ± 0.05 | 0.30 ± 0.09 | 0.05 ± 0.01 | 0.04 ± 0.00 |
| Total FFA | 172.9 ± 39.7 | 131.4 ± 7.5 | 162.4 ± 19.2 | 320.4 ± 35.8 | 347.3 ± 24.8 | 665.3 ± 105.9 | 578.6 ± 59.6 |
ND, not detected; a ≠ b within species and age are significantly different (P < .05); c ≠ d within species and age tended to be different (.05 > P < .06). BPA doses expressed in kilogram of BW per day for sheep and rats and kilogram of diet per day for mice. Adipose tissue origin: gonadal and renal (rats) and gonadal (mice).
Discussion
This is the first BPA exposure study to comprehensively combine and compare an association study in human and a targeted BPA treatment study involving 3 different mammalian species (sheep, rats, and mice). Findings from this study demonstrate a positive association between increased BPA exposure and increased systemic nitrosative stress, as well as increased palmitic acid during the first trimester of pregnancy in humans. Cord blood of subjects exposed to higher BPA levels during 8–14 weeks of gestation also had higher nitrosative stress. Complementary studies with multiple animal species treated prenatally with BPA at relevant human exposure doses provide the causal link of BPA induction of nitrosative stress.
Impact of prenatal BPA on oxidative stress
Oxidized tyrosine moieties were used to study pathways involved in oxidative stress after BPA exposure. These specific stable products of tyrosine oxidation have been demonstrated in numerous animal and human studies to yield mechanistic information on oxidant stress pathways. ClY is a specific marker of myeloperoxidase pathway, NY is a marker for nitrosative stress, and DiY is a marker for hydroxyl radical and peroxidase oxidation (38, 39). This is the first report of a positive association between circulating BPA levels and oxidative stress in maternal-cord blood pairs in humans. The similar circulating NY levels in both maternal and cord milieu found in this study differ from previous findings, where umbilical cord levels of NY were found to be higher than in the term maternal samples (40). These differences may relate the timing when maternal samples were collected, 8–14 weeks in the present study and term in Weber et al study (40). Our study documenting a positive correlation between maternal NY levels with cord NY levels at term lends credence for the prediction by Weber et al (40) that maternal oxidative stress, specifically NY, could be used as a predictor of fetal oxidative stress. Perinatal exposure to other endocrine-disrupting compounds, such as thimerosal, tributyltin, and benzene, also have been shown to induce oxidative stress (41–43). Controversial evidence about the induction of nitrosative stress through prenatal dietary deficits, such as low protein (44, 45), is also available.
Increased protein tyrosine nitration occurs during states that lead to high oxidant rates, such as inflammation. Elevated NY during pregnancy were found in severe diseases associated with preterm birth, such as bronchopulmonary dysplasia (46), perinatal asphyxia (47), or chronic hypoxia (48). Because no preterm births were evident in the chosen subject population, the relationship of prenatal BPA exposure and NY may be more subtle and needs to be examined in larger cohorts. Although the association between BPA and NY in the human study does not prove causality, it points to a potential link. Animal studies demonstrating the induction of oxidative stress by BPA treatment provide data supportive of a causal link. For instance, evidence exists linking BPA exposure with oxidative stress in fish spermatozoa (49), zebrafish embryos (50), and in the circulation of Wistar rats (51). Induction of nitrosative stress in fetal circulation of sheep by prenatal BPA treatment (this study) also provides a causal link for the associations seen in the human study. Importantly, the impact of BPA in inducing oxidative stress in sheep was evident at both gestational stages studied (gestational day [GD] 65 and GD90) but stronger in the later gestational age. This may be a reflection of the cumulative effect of BPA throughout the window of exposure (GD30–GD90), because BPA has been found to accumulate in adipose tissue (52). Unfortunately, the fetal size in rats and mice limited the assessment of oxidative stress during fetal time points to validate findings from sheep to other species. Although evidence exists suggestive of BPA being causative of oxidative stress, very little is known about the underlying mechanisms by which BPA induces oxidative stress. A recent study in 3T3 fibroblasts suggests that G protein-coupled receptor 30 may be one of the receptors involved in mediating oxidative stress (53).
Because tyrosine in proteins is 3–4 mol%, thus allowing the presence of several tyrosine residues (54), the implications of increased tyrosine oxidation are broad. Importantly, several clinical and animal studies have shown that both oxidized tyrosines are associated with associated with a proinflammatory state, such as atherosclerosis (55), diabetes (56), lupus (57), and rheumatoid arthritis (58). The selective elevation in NY, but not ClY, evidenced in the current study suggests that BPA may enhance reactive nitrogen species through mechanisms that do not use myeloperoxidase such as peroxynitrite formation through direct reaction between superoxide and nitric oxide or through endothelial nitric oxide synthase uncoupling. Indeed, generation of NY by such mechanisms has been postulated in vasculopathy associated with diabetes, cardiovascular disease, and Fabry's disease (59). Although some proteins and tyrosine residues are known to be preferentially nitrated (60, 61), further research is warranted to address specific protein targets of BPA; the present study only provides an overall measure of protein nitration.
As opposed to measurable levels of CIY found in human samples, circulating ClY levels were undetectable in animal studies. Because previous studies in rats (62) and mice (63) were able to measure CIY, lack of detection in the current study may relate to strain/species differences or sensitivity of detection method. Similar studies have not been carried out in sheep. The finding of an association between BPA and NY in human pregnancies and BPA induced increase in NY, but not DiY, in animal studies in this study is suggestive of a common mechanism that is conserved across species (human, sheep, and rats) by which BPA may lead to systemic nitrosative stress. Evolutionary conserved mechanisms across species are known for other endocrine distupting chemicals (diethylstibestrol and dichlorodiphenyltrichloroethane) on reproductive outcomes (cancer and precocious puberty) (64), as well as other environmental endocrine disrupting chemicals with estrogenic, androgenic, and antiandrogenic activity (65).
The increase in systemic oxidative stress evidenced in adult sheep and rat females is clearly programmed via prenatal BPA treatment, although the underlying mechanisms are unclear. The increase in circulating NY seen during GD65 and GD90 of gestation in sheep indicates that a broad range of organs/systems may be impacted during critical windows of development. Targets of oxidative stress include phospholipid membranes, proteins, and nucleic acids. As such, increased systemic oxidative stress can lead to irreversible changes in these molecules, as well as in mitochondria (66). For instance, a short exposure of adult mice to BPA has been shown to induce mitochondrial dysfunction and an associated increase in oxidative stress (67).
In the context of impact of prenatal BPA on adult outcomes, the increased nitrosative stress was dose dependent in both sheep and rats with only the highest dose having an effect. Although the low-dose BPA did not evoke a similar adult response, an increase in NY was evident in both maternal and fetal sheep plasma at GD90 (end of BPA treatment), suggesting that this may be a direct response to low BPA exposure. The low BPA dose used in this study may have been below the threshold to induce postnatal systemic nitrosative stress in the adult. Importantly, the systemic nitrosative stress evident postnatally in the high prenatal BPA-treated group demonstrates that prenatal BPA exposure can disrupt oxidative stress pathways in postnatal life. The finding that prenatal BPA increased NY in adipose tissue of adult sheep and rats points to tissue-specific programming effects as well, although the consequences of this finding are still unclear. Although not investigated in the current study, prenatal systemic oxidative stress is implicated in the development of an array of disease states. Fetal programming of oxidative stress has been mostly studied in the context of cardiovascular outcomes resulting from prenatal hypoxia (68), protein restriction (45), and dexamethasone treatment (69). Interestingly, the impact of prenatal BPA in increasing NY was not evident in adult mice. Given the dose dependency seen in both rats and sheep, and that all species target similar relevant human dose exposures, it is possible that a different threshold for BPA exists for induction of oxidative stress in mice. Such interspecies comparison studies are not available to extrapolate our findings.
Impact of prenatal BPA on FFAs
Increasing evidence suggests that maternal fatty acid status during pregnancy can modulate the development of the immune system in the offspring (70, 71). The finding of high palmitic acid and a trend for an increase in total FFA in women exposed to higher BPA levels suggest that offspring outcomes may be affected in these pregnancies. The lack of impact of maternal exposure on gestational age and birth weight in the human study is likely the function of the small sample size (n = 12 per group); clearly the study is underpowered to discern such associations. Because higher FFAs have been implicated in increased risk of preterm delivery (72) and higher palmitic acid during the first trimester linked to preeclamptic pregnancies (73), follow-up studies using a larger cohort are warranted to address pregnancy and newborn outcomes. It needs to be recognized that the association between BPA and FFA levels during the first trimester may be affected by diet, because both can be strongly affected by daily intake and source of food (2, 74).
In animal studies, the impact of BPA on FFA profile in prenatal BPA-treated females was less consistent across species compared with those observed with oxidative stress. The strongest effect of prenatal BPA treatment on FFA profile was observed in plasma of adult mice. BPA induced an approximately 3-fold reduction in myristic acid, whose increase is associated with metabolic syndrome (75), and an approximately 4-fold increase in the omega 6 γ-linolenic acid, precursor of dihomo-γ-linolenic acid, a FFA proposed to have a therapeutic effect in proliferative diseases (76). Thus, the FFA profile observed in prenatally BPA-treated mice does not reflect a metabolic impairment, which is in agreement with a lack of metabolic syndrome, but increased activity and energy expenditure found in these BPA-treated female mice (77).
A major strength of this study addressing impact of BPA on oxidative stress and FFA index is that BPA measures in humans were performed using a method validated in a NIEHS-funded Round Robin study, in which the accuracy and precision of the analytical chemistry methods to measure uBPA and BPA-G were evaluated in several laboratories across the United States (34). uBPA levels in cord blood observed in our study (range, 0.31–0.78 ng/mL) are in the range measured in other studies (5, 78–80). The higher uBPA levels observed in maternal circulation compared with cord blood are also consistent with other studies carried out in the United States (5, 79), Europe (81), and Korea (78). As opposed to lower BPA levels in cord blood, higher uBPA levels have been found in human amniotic fluid, suggesting that fetuses are likely exposed to higher BPA levels (3), and cord blood measures may underestimate fetal BPA exposure. This is further supported by studies in animal models, which indicate that BPA levels in tissues can build up to 10-fold higher than that found in circulation (82). Levels of BPA-G, the major BPA metabolite found in blood, reported in this study are also in the range of those seen in midterm cord blood of elective pregnancy terminations (80). The relationship between uBPA and BPA-G in the present study was approximately 1:1, which contrasts with the study in which BPA-G was 2–3 times less than the active form (80). Although our samples number is small (n = 24), our data do not support the conclusion by Gerona et al (82) that the fetal uridine 5′-diphospho-glucuronosyltransferase system is immature. Altogether, further research is needed to determine the consequences of BPA bioaccumulation in tissues on fetal development and newborn outcomes.
Conclusions
The current study provides first unequivocal evidence that developmental exposure to BPA can induce nitrosative stress and that this effect is conserved across humans and 2 of the animal species studied. It also demonstrates that prenatal BPA exposure leads to long-term nitrosative stress in adults, which points to the specificity of NY as a biomarker for BPA exposure. Overall, these findings are supportive of the potential use of maternal NY as a biomarker for future offspring health, although further studies are required to pin point the specific pathways by which this is programmed developmentally.
Acknowledgments
We thank Jann Howell and Lucy Allbaugh for their support during human subjects' recruitment; Samantha Milewski for help with clinical data; Douglas Doop for his expert animal care, facility management, and help with generation of the experimental lambs; and Carol Herkimer, Evan M. Beckett, Jacob Moeller, and Dr Bachir Abi-Salloum for assistance with prenatal treatments and fetal collection procedures; Olivia S. Anderson for generation of experimental mice and Tamara R. Jones and Kari E. Sant for mouse sample procurement; and Arun Das for his help with the free fatty acid measures.
Present address for A.V.-L.: Department of Animal Sciences, Michigan State University, East Lansing, MI 48824.
This work was supported by an American Recovery and Reinvestment Act supplement to National Institute for Environmental Health Sciences (NIEHS) Grant R01 ES016541 (to V.P.), NIEHS grant P01 ES022844, and the United States Environmental Protection Agency (US EPA) Grant RD 83543601. Analytical support for this research was also provided by the NIEHS grant P30ES017885. Plasma and tissue samples used in this investigation were generated as part of the following grants: human, NIEHS R01 ES017005 (to V.P.); sheep, NIEHS R01 ES016541 (to V.P.); rat, NIEHS R01 ES016001 (to H.B.P.); and mice, NIEHS R01 ES017524 (D.C.D.). Metabolomic measurements were processed through the Molecular Phenotyping Core, MI Nutrition and Obesity Center (The National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] grant P30DK089503) and Michigan Regional Metabolomics Resource Core (NIDDK grant U24 DK097153).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- BPA-G
- BPA glucuronide
- BW
- body weight
- C
- carbon atom
- ClY
- 3-chlorotyrosine
- DiY
- dityrosine
- ESI-MS/MS
- electrospray triple-quadrupole mass spectrometer
- FFA
- free fatty acid
- GC
- gas chromatography
- GD
- gestational day
- NIEHS
- National Institute for Environmental Health Sciences
- NY
- 3-nitrotyrosine
- PND
- postnatal day
- uBPA
- unconjugated BPA.
References
- 1. WHO Report World Health Organization (WHO)/United Nations Environment Programme (UNEP). State of the science of endocrine disrupting chemicals-2012. Bergman A, Heindel JJ, Jobling S, Kidd KA, Zoeller RT, eds. 2014 Available at http://www.who.int/ceh/publications/endocrine/en/ Accessed December 18, 2014.
- 2. Vandenberg LN, Hunt PA, Myers JP, Vom Saal FS. Human exposures to bisphenol A: mismatches between data and assumptions. Rev Environ Health. 2013;28(1):37–58. [DOI] [PubMed] [Google Scholar]
- 3. Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17(11):2839–2841. [DOI] [PubMed] [Google Scholar]
- 4. Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang T, Sun H, Kannan K. Blood and urinary bisphenol A concentrations in children, adults, and pregnant women from china: partitioning between blood and urine and maternal and fetal cord blood. Environ Sci Technol. 2013;47(9):4686–4694. [DOI] [PubMed] [Google Scholar]
- 6. Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127(3–5):204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fenichel P, Chevalier N, Brucker-Davis F. Bisphenol A: an endocrine and metabolic disruptor. Ann Endocrinol (Paris). 2013;74(3):211–220. [DOI] [PubMed] [Google Scholar]
- 8. Peretz J, Vrooman L, Ricke WA, et al. Bisphenol A and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect. 2014;122(8):775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lang IA, Galloway TS, Scarlett A, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300(11):1303–1310. [DOI] [PubMed] [Google Scholar]
- 10. Silver MK, O'Neill MS, Sowers MR, Park SK. Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003–2008. PLoS One. 2011;6(10):e26868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Teppala S, Madhavan S, Shankar A. Bisphenol A and metabolic syndrome: results from NHANES. Int J Endocrinol. 2012;2012:598180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alonso-Magdalena P, Vieira E, Soriano S, et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118(9):1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang Y, Liu J, Li Y, et al. Prenatal exposure to bisphenol A at the reference dose impairs mitochondria in the heart of neonatal rats. J Appl Toxicol. 2014;34(9):1012–1022. [DOI] [PubMed] [Google Scholar]
- 14. Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice. Endocrinology. 2010;151(6):2603–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–139. [DOI] [PubMed] [Google Scholar]
- 16. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. [DOI] [PubMed] [Google Scholar]
- 17. Silins I, Högberg J. Combined toxic exposures and human health: biomarkers of exposure and effect. Int J Environ Res Public Health. 2011;8(3):629–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116(12):1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang YJ, Hong YC, Oh SY, et al. Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ Res. 2009;109(6):797–801. [DOI] [PubMed] [Google Scholar]
- 20. Kim JH, Sartor MA, Rozek LS, et al. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC Genomics. 2014;15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Veiga-Lopez A, Luense LJ, Christenson LK, Padmanabhan V. Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology. 2013;154(5):1873–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147(12):5956–5966. [DOI] [PubMed] [Google Scholar]
- 23. Padmanabhan V, Siefert K, Ransom S, et al. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol. 2008;28(4):258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calafat AM, Weuve J, Ye X, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117(4):639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patisaul HB, Sullivan AW, Radford ME, et al. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 2012;7(9):e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patisaul HB, Roberts SC, Mabrey N, et al. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27(2):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doerge DR, Twaddle NC, Vanlandingham M, Brown RP, Fisher JW. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicol Appl Pharmacol. 2011;255(3):261–270. [DOI] [PubMed] [Google Scholar]
- 28. Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23(15):5293–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Becker JB, Arnold AP, Berkley KJ, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146(4):1650–1673. [DOI] [PubMed] [Google Scholar]
- 30. Weinhouse C, Anderson OS, Bergin IL, et al. Dose-dependent incidence of hepatic tumors in adult mice following perinatal exposure to bisphenol A. Environ Health Perspect. 2014;122(5):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takahashi O, Oishi S. Testicular toxicity of dietarily or parenterally administered bisphenol A in rats and mice. Food Chem Toxicol. 2003;41(7):1035–1044. [DOI] [PubMed] [Google Scholar]
- 32. Sieli PT, Jašarevic E, Warzak DA, et al. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ Health Perspect. 2011;119(9):1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson OS, Nahar MS, Faulk C, et al. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ Mol Mutagen. 2012;53(5):334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vandenberg LN, Gerona RR, Kannan K, et al. A round robin approach to the analysis of bisphenol a (BPA) in human blood samples. Environ Health. 2014;13(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liao C, Kannan K. Determination of free and conjugated forms of bisphenol A in human urine and serum by liquid chromatography-tandem mass spectrometry. Environ Sci Technol. 2012;46(9):5003–5009. [DOI] [PubMed] [Google Scholar]
- 36. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. [DOI] [PubMed] [Google Scholar]
- 37. Tserng KY, Kliegman RM, Miettinen EL, Kalhan SC. A rapid, simple, and sensitive procedure for the determination of free fatty acids in plasma using glass capillary column gas-liquid chromatography. J Lipid Res. 1981;22(5):852–858. [PubMed] [Google Scholar]
- 38. Vivekanandan-Giri A, Byun J, Pennathur S. Quantitative analysis of amino acid oxidation markers by tandem mass spectrometry. Methods Enzymol. 2011;491:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vivekanadan-Giri A, Wang JH, Byun J, Pennathur S. Mass spectrometric quantification of amino acid oxidation products identifies oxidative mechanisms of diabetic end-organ damage. Rev Endocr Metab Disord. 2008;9(4):275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weber D, Stuetz W, Bernhard W, et al. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur J Clin Nutr. 2014;68(2):215–222. [DOI] [PubMed] [Google Scholar]
- 41. Badham HJ, Renaud SJ, Wan J, Winn LM. Benzene-initiated oxidative stress: effects on embryonic signaling pathways. Chem-Biol Interact. 2010;184(1–2):218–221. [DOI] [PubMed] [Google Scholar]
- 42. Sulkowski ZL, Chen T, Midha S, Zavacki AM, Sajdel-Sulkowska EM. Maternal thimerosal exposure results in aberrant cerebellar oxidative stress, thyroid hormone metabolism, and motor behavior in rat pups; sex- and strain-dependent effects. Cerebellum. 2012;11(2):575–586. [DOI] [PubMed] [Google Scholar]
- 43. Mitra S, Srivastava A, Khanna S, Khandelwal S. Consequences of tributyltin chloride induced stress in Leydig cells: an ex-vivo approach. Environ Toxicol Pharmacol. 2014;37(2):850–860. [DOI] [PubMed] [Google Scholar]
- 44. Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 2005;68(5):2180–2188. [DOI] [PubMed] [Google Scholar]
- 45. Cambonie G, Comte B, Yzydorczyk C, et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1236–R1245. [DOI] [PubMed] [Google Scholar]
- 46. Banks BA, Ischiropoulos H, McClelland M, Ballard PL, Ballard RA. Plasma 3-nitrotyrosine is elevated in premature infants who develop bronchopulmonary dysplasia. Pediatrics. 1998;101(5):870–874. [DOI] [PubMed] [Google Scholar]
- 47. Groenendaal F, Lammers H, Smit D, Nikkels PG. Nitrotyrosine in brain tissue of neonates after perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006;91(6):F429–F433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Escobar J, Teramo K, Stefanovic V, et al. Amniotic fluid oxidative and nitrosative stress biomarkers correlate with fetal chronic hypoxia in diabetic pregnancies. Neonatology. 2013;103(3):193–198. [DOI] [PubMed] [Google Scholar]
- 49. Hulak M, Gazo I, Shaliutina A, Linhartova P. In vitro effects of bisphenol A on the quality parameters, oxidative stress, DNA integrity and adenosine triphosphate content in sterlet (Acipenser ruthenus) spermatozoa. Comp Biochem Physiol C Toxicol Pharmacol. 2013;158(2):64–71. [DOI] [PubMed] [Google Scholar]
- 50. Xu H, Yang M, Qiu W, Pan C, Wu M. The impact of endocrine-disrupting chemicals on oxidative stress and innate immune response in zebrafish embryos. Environ Toxicol Chem. 2013;32(8):1793–1799. [DOI] [PubMed] [Google Scholar]
- 51. Popa DS, Bolfa P, Kiss B, et al. Influence of Genista tinctoria L. or methylparaben on subchronic toxicity of bisphenol A in rats. Biomed Environ Sci. 2014;27(2):85–96. [DOI] [PubMed] [Google Scholar]
- 52. Nunez AA, Kannan K, Giesy JP, Fang J, Clemens LG. Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere. 2001;42(8):917–922. [DOI] [PubMed] [Google Scholar]
- 53. Nishimura Y, Nakai Y, Tanaka A, Nagao T, Fukushima N. Long-term exposure of 3T3 fibroblast cells to endocrine disruptors alters sensitivity to oxidative injury. Cell Biol Int. 2014;38(7):868–874. [DOI] [PubMed] [Google Scholar]
- 54. Bartesaghi S, Ferrer-Sueta G, Peluffo G, et al. Protein tyrosine nitration in hydrophilic and hydrophobic environments. Amino Acids. 2007;32(4):501–515. [DOI] [PubMed] [Google Scholar]
- 55. Pennathur S, Heinecke JW. Mechanisms of oxidative stress in diabetes: implications for the pathogenesis of vascular disease and antioxidant therapy. Front Biosci. 2004;9:565–574. [DOI] [PubMed] [Google Scholar]
- 56. Pennathur S, Ido Y, Heller JI, et al. Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem. 2005;280(24):22706–22714. [DOI] [PubMed] [Google Scholar]
- 57. Smith CK, Vivekanandan-Giri A, Tang C, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66(9):2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vivekanandan-Giri A, Slocum JL, Byun J, et al. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Ann Rheum Dis. 2013;72(10):1725–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shu L, Vivekanandan-Giri A, Pennathur S, et al. Establishing 3-nitrotyrosine as a biomarker for the vasculopathy of Fabry disease. Kidney Int. 2014;86(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Souza JM, Daikhin E, Yudkoff M, Raman CS, Ischiropoulos H. Factors determining the selectivity of protein tyrosine nitration. Arch Biochem Biophys. 1999;371(2):169–178. [DOI] [PubMed] [Google Scholar]
- 61. Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am J Physiol-Heart C. 2005;288(1):H371–H381. [DOI] [PubMed] [Google Scholar]
- 62. Kunitomo M, Yamaguchi Y, Kagota S, Otsubo K. Beneficial effect of coenzyme Q10 on increased oxidative and nitrative stress and inflammation and individual metabolic components developing in a rat model of metabolic syndrome. J Pharmacol Sci. 2008;107(2):128–137. [DOI] [PubMed] [Google Scholar]
- 63. Kumar Y, Liang C, Limmon GV, et al. Molecular analysis of serum and bronchoalveolar lavage in a mouse model of influenza reveals markers of disease severity that can be clinically useful in humans. PLoS One. 2014;9(2):e86912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hotchkiss AK, Rider CV, Blystone CR, et al. Fifteen years after “Wingspread”–environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci. 2008;105(2):235–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757(5–6):509–517. [DOI] [PubMed] [Google Scholar]
- 67. Moon MK, Kim MJ, Jung IK, et al. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Korean Med Sci. 2012;27(6):644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rueda-Clausen CF, Dolinsky VW, Morton JS, Proctor SD, Dyck JR, Davidge ST. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes. 2011;60(2):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roghair RD, Miller FJ, Jr, Scholz TD, Lamb FS, Segar JL. Endothelial superoxide production is altered in sheep programmed by early gestation dexamethasone exposure. Neonatology. 2008;93(1):19–27. [DOI] [PubMed] [Google Scholar]
- 70. Prescott SL, Dunstan JA. Prenatal fatty acid status and immune development: the pathways and the evidence. Lipids. 2007;42(9):801–810. [DOI] [PubMed] [Google Scholar]
- 71. Lumia M, Luukkainen P, Tapanainen H, et al. Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediatr Allergy Immunol. 2011;22(8):827–835. [DOI] [PubMed] [Google Scholar]
- 72. Chen X, Scholl TO. Association of elevated free fatty acids during late pregnancy with preterm delivery. Obstet Gynecol. 2008;112(2 pt 1):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lorentzen B, Drevon CA, Endresen MJ, Henriksen T. Fatty acid pattern of esterified and free fatty acids in sera of women with normal and pre-eclamptic pregnancy. Br J Obstet Gynaecol. 1995;102(7):530–537. [DOI] [PubMed] [Google Scholar]
- 74. Wolever TM, Bentum-Williams A, Jenkins DJ. Physiological modulation of plasma free fatty acid concentrations by diet. Metabolic implications in nondiabetic subjects. Diabetes Care. 1995;18(7):962–970. [DOI] [PubMed] [Google Scholar]
- 75. Mayneris-Perxachs J, Guerendiain M, Castellote AI, et al. Plasma fatty acid composition, estimated desaturase activities, and their relation with the metabolic syndrome in a population at high risk of cardiovascular disease. Clin Nutr. 2014;33(1):90–97. [DOI] [PubMed] [Google Scholar]
- 76. Wang X, Lin H, Gu Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis. 2012;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Anderson OS, Peterson KE, Sanchez BN, Zhang ZZ, Mancuso P, Dolinoy DC. Perinatal bisphenol A exposure promotes hyperactivity with corresponding hormonal responses. FASEB J. 2013;27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wan Y, Choi K, Kim S, et al. Hydroxylated polybrominated diphenyl ethers and bisphenol A in pregnant women and their matching fetuses: placental transfer and potential risks. Environ Sci Technol. 2010;44(13):5233–5239. [DOI] [PubMed] [Google Scholar]
- 79. Unal ER, Lynn T, Neidich J, et al. Racial disparity in maternal and fetal-cord bisphenol A concentrations. J Perinatol. 2012;32(11):844–850. [DOI] [PubMed] [Google Scholar]
- 80. Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Persp. 2002;110(11):A703–A707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Uchida K, Suzuki A, Kobayashi Y, et al. Bisphenol-A administration during pregnancy results in fetal exposure in mice and monkeys. J Health Sci. 2002;48(6):579–582. [Google Scholar]
- 82. Gerona RR, Woodruff TJ, Dickenson CA, et al. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environ Sci Technol. 2013;47(21):12477–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]