Abstract
Translocator protein (TSPO) is a mitochondrial outer membrane protein of unknown function with high physiological expression in steroidogenic cells. Using TSPO gene–deleted mice, we recently demonstrated that TSPO function is not essential for steroidogenesis. The first link between TSPO and steroidogenesis was established in studies showing modest increases in progesterone production by adrenocortical and Leydig tumor cell lines after treatment with PK11195. To reconcile discrepancies between physiological and pharmacological interpretations of TSPO function, we generated TSPO-knockout MA-10 mouse Leydig tumor cells (MA-10:TspoΔ/Δ) and examined their steroidogenic potential after exposure to either dibutyryl-cAMP or PK11195. Progesterone production in MA-10:TspoΔ/Δ after dibutyryl-cAMP was not different from control MA-10:Tspo+/+ cells, confirming that TSPO function is not essential for steroidogenesis. Interestingly, when treated with increasing concentrations of PK11195, both control MA-10:Tspo+/+ cells and MA-10:TspoΔ/Δ cells responded in a similar dose-dependent manner showing increases in progesterone production. These results show that the pharmacological effect of PK11195 on steroidogenesis is not mediated through TSPO.
Translocator protein (TSPO), previously known as peripheral benzodiazepine receptor, is a highly conserved protein across kingdoms (1). It was first identified for its high binding affinity to benzodiazepines in distinct peripheral sites, as opposed to the central benzodiazepine receptor (γ-aminobutyric acid type A receptor) sites in the brain (2–5). The isoquinoline carboxamine, PK11195 [N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide], was then identified as a selective high-affinity TSPO/peripheral benzodiazepine receptor binding chemical (6), and has been widely used in most binding/functional studies. Binding sites of TSPO ligands have been found in multiple tissues including heart, brain, adrenal, kidney, salivary gland, platelets, brown adipose tissue, skin, and liver (7), but were noted to be highest in steroidogenic cells (8–10).
Subcellular fractionation and drug displacement studies showed that TPSO was enriched in the outer mitochondrial membrane (8, 11). A putative cholesterol recognition amino acid consensus (CRAC) sequence was identified at the C terminal region (12), distinct from its PK11195 binding site (13). The structure of murine TSPO is described as a five-transmembrane alpha helix (14) that was initially modeled to form a hydrophobic interior core containing the CRAC domain for cholesterol translocation (15). However, a more recent nuclear magnetic resonance structure of TSPO showed that the CRAC domain is located on the outside of the TSPO pointing toward the membrane environment (13). Therefore, the ability of cholesterol to dimerize has been proposed to induce oligomerization of TSPO leading to the potential transporter function (13).
For the past 25 years, TSPO has been depicted in the steroidogenic pathway as a critical transporter of cholesterol from the outer mitochondrial membrane to the mitochondrial matrix (16). Conversion of cholesterol to pregnenolone by the enzyme CYP11A1 is restricted to the matrix side of the inner mitochondrial membrane; therefore, mitochondrial cholesterol import forms the first and the rate-limiting step for the acute production of all steroid hormones. The very first link between TSPO and steroid hormone biosynthesis emerged from a pharmacological study of different high-affinity TSPO-binding chemicals on steroidogenesis. In Y1 mouse adrenal tumor cells, three of nine chemicals, including PK11195, could induce steroid production at maximum 2-fold of the baseline (17). A follow-up report using the MA-10 mouse Leydig tumor cell line showed the same phenomenon with PK11195 being capable of inducing a 4-fold increase in baseline steroid production (18). These responses were independent of induction of the steroidogenic acute regulatory protein (STAR), a key player in mitochondrial cholesterol transport required for steroidogenesis (19, 20). A subsequent publication reported that mono-allelic targeted disruption of the Tspo gene by homologous recombination in the R2C rat Leydig tumor cell line abolished TSPO protein expression and consequently inhibited progesterone level to only approximately 5% of control values (21); it was concluded that TSPO has an indispensable role in steroidogenesis.
Using Leydig cell–specific TSPO conditional knockout mice, we recently demonstrated that TSPO does not have a role in T production (22). In direct contrast with a previous study (23), we subsequently found that global TSPO knockout mice were viable and fertile with no effects on steroidogenesis (24). We also showed in different steroidogenic cell lines: Y1, MA-10, MLTC, and R2C, that TSPO knockdown did not affect their ability to produce steroid hormones (24). In fact, the steroidogenic human adrenocortical cell line H295R, is deficient of TSPO, yet is capable of making steroid hormones (24). These results provided compelling genetic evidence that TSPO physiology is not associated with steroidogenesis. However, this did not agree with TSPO pharmacology because TSPO binding chemicals are reported to induce steroid hormone production (17, 18, 25).
To reconcile this discrepancy, we generated TSPO-knockout MA-10 Leydig tumor (MA-10:TspoΔ/Δ) cells and tested the ability of PK11195 to induce steroid hormone production. Our results demonstrate that TSPO deletion in MA-10 cells had no effect on its ability to produce steroid hormones and that the pharmacological effect of PK11195 on steroidogenesis is not mediated through TSPO.
Materials and Methods
Generation of TSPO-deleted MA-10 cells
MA-10 cells were cultured in DMEM with glutamine and pyruvate containing 10% fetal bovine serum as previously described (10). The clustered regularly interspaced short palindromic repeats (CRISPR) system was used to generate MA-10:TspoΔ/Δ cells by targeting exon 2 of the Tspo gene (NCBI: Gene ID: 12 257; Reference Sequence: NM_009775.4). The specific guide RNA sequence 5′-GCCTACTTTGTACGTGGCGA-3′ was cloned in the pX330-U6-Chimeric_BB-cBh-hSpCas9 plasmid (26), transfected into MA-10 cells using TransIT-X2 (Mirus Bio) and cultured for 48 hours. Transfected cells were then seeded in 96-well plates at the density of approximately 1 cell/well for clonal selection. Clones originating from single cells were screened for absence of TSPO protein expression by performing Western blots using a monoclonal antibody (Abcam) that recognizes amino acid 156–169 coded by the TSPO exon 4. Three MA-10:TspoΔ/Δ cell clones were identified and mutations in the Tspo gene were confirmed by sequencing after cloning both the respective Tspo cDNAs and Tspo exon 2 genomic DNA into TOPO®-TA vectors (Life Technologies), and ZR Plasmid Miniprep (Zymo Research) purification. The list of primers used for cloning and sequencing is provided in Supplemental Table 1.
Immunocytochemistry
Cells were incubated with 300nM Mitotracker Deep Red FM (Life Technologies) in culture medium for 1 hour at 37°C, washed with PBS, and fixed with 2% paraformaldehyde. Cells were blocked with 5% horse serum in PBS to prevent nonspecific binding, stained with a primary rabbit monoclonal TSPO antibody (Abcam) and secondary Alexa Fluor 488 antibody as previously described (10). Images were captured using a LSM 510 confocal microscope (Zeiss) and visualized using Image J.
Hormone assays
For dibutyryl-cAMP (Bt2cAMP) stimulation experiments, 5 × 104 cells were plated per well in a 96-well plate and allowed to attach overnight. Cells were then washed with PBS and incubated with 0.5mM Bt2cAMP (Sigma) in DMEM medium without serum for 6 hours. For PK11195 stimulation experiments, 15 × 104 cells were plated per well in a 12-well plate and allowed to attach overnight. Cells were washed with PBS, changed to DMEM medium without serum, and different doses of PK11195 (Sigma) in equal volume of vehicle (ethanol, 0.1% v/v final) was added (Treatments: Vehicle only, 100nM, 1μM, and 10μM final concentrations of PK11195), and incubated for 2 hours. For both stimulations, supernatants were collected and progesterone was measured as previously described (27), and values were normalized to total protein in each well.
Immunoblots
Proteins were separated by SDS-PAGE and immunoblotted using rabbit monoclonal primary antibodies against TSPO (Abcam) and a rabbit polyclonal STAR antibody (19). Each primary antibody was multiplexed with the control for protein loading, mouse monoclonal β-actin (Li-Cor). Simultaneous detection was performed using IRDye 700 and 800 labeled secondary antibodies using a laser fluorescence scanner (Li-Cor) and quantified using ImageJ as described previously (10, 22).
Statistics
Numeric differences between groups were compared using a Student t test; comparisons for more than two groups were performed using ANOVA and post hoc Tukey's test; P < .05 was considered significant. All analyses were performed using Prism 5 (GraphPad).
Results
CRISPR/Cas9-mediated deletion of TSPO in MA-10 cells
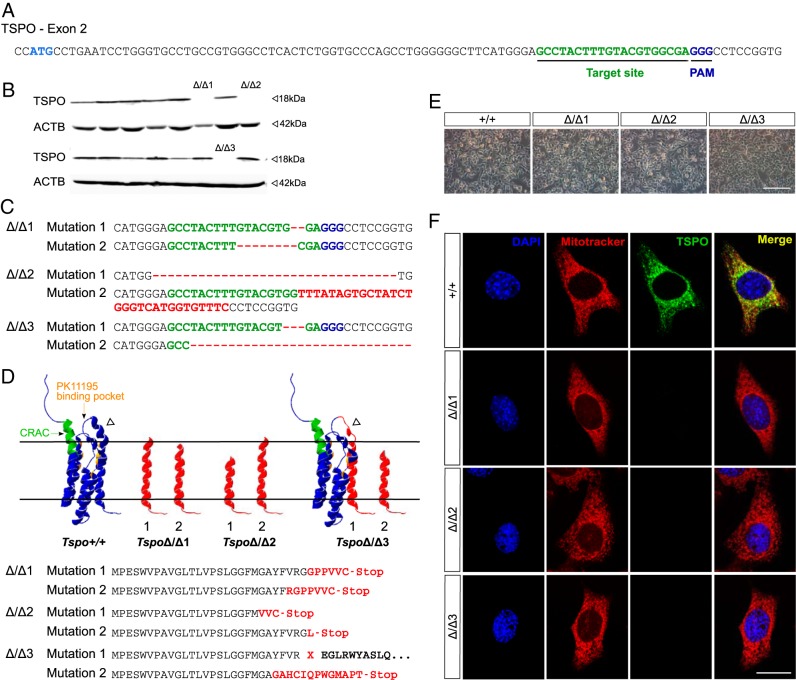
Tspo has four exons with the translation start codon in exon 2. Using the CRISPR/Cas9 system, a guide RNA was used with sequence complementary to 20 nucleotides downstream of the start codon in Tspo exon 2 for targeting Cas9 nuclease activity (Figure 1A). The resulting DNA double-stranded breaks, which are subject to the error-prone nonhomologous end joining, caused random indel mutations that induced frame shifts and/or generated a premature stop codon in the mRNA, leading to a truncated and nonfunctional TSPO protein.
Figure 1.
CRISPR/Cas9-mediated deletion of TSPO in steroidogenic MA-10 Leydig cells. A, Sequence of Tspo exon 2 showing the start codon and targeted sequence followed by the protospacer adjacent motif. B, After transfection with Tspo-CRISPR/Cas9 construct, MA-10 clones were screened for TSPO protein expression using a monoclonal TSPO antibody that recognizes amino acids 156–169 encoded by Tspo exon 4. Western blots that identified the three TSPO-deleted clones are shown. Control bands are β-Actin (ACTB). C, Sequencing results of Tspo cDNA in 3 MA-10:TspoΔ/Δ clones confirmed indel mutations in the target sites of expressed alleles. Regions corresponding to guide RNA sequences are shown in green, deletions and insertions are shown in red. Deleted regions ranged from 2 bp in MA-10:TspoΔ/Δ1 to 88 bp in MA-10:TspoΔ/Δ3. D, Mutations in Tspo induced a frame shift and/or generated a stop codon, which resulted in a truncation with fragments restricted to TSPO N-terminal 28 and 25 amino acids for alleles in MA-10:TspoΔ/Δ1, 21 and 28 amino acids for alleles in MA-10:TspoΔ/Δ2, and 23 amino acids for the one allele in MA-10:TspoΔ/Δ3. The second allele in MA-10:TspoΔ/Δ3 was a Gly28 mutation that induced a significant change to the short α helix loop (arrowhead) that connects transmembrane region 1 and 2 that ultimately led to complete loss of TSPO protein expression. E, Representative light microscopy images of MA-10:Tspo+/+ and MA-10:TspoΔ/Δ cells showed that MA-10:TspoΔ/Δ cells were healthy with no apparent morphological changes (Scale bar: 200 μm). F, Immunocytochemistry confirmed complete absence of TSPO in the different MA-10:TspoΔ/Δ clones (Scale bar: 20 μm).
In this study, after transfection with the Tspo-CRISPR/Cas9 construct, we screened 36 MA-10 cell clones derived from single transfected cells for TSPO protein expression using a TSPO monoclonal antibody that recognizes amino acids 156–169 at the C-terminal tail of TSPO (coded by exon 4). We identified three TSPO-deleted clones: MA-10:TspoΔ/Δ1, MA-10:TspoΔ/Δ2, and MA-10:TspoΔ/Δ3 (Figure 1B). Sequencing confirmed that deletion mutations occurred in the targeted region in exon 2 in the expressed alleles of the three clones resulting in loss of TSPO protein expression (Figure 1, C and D). In MA-10:TspoΔ/Δ1, truncations resulting from frame shifts resulted in fragments restricted to TSPO N-terminal 28 and 25 amino acids respectively for expressed alleles; similarly, MA-10:TspoΔ/Δ2 resulted in fragments restricted to the N-terminal 21 and 28 amino acids respectively for expressed alleles. In MA-10:TspoΔ/Δ3, one allele was truncated with a fragment restricted to TSPO N-terminal 23 amino acids, and the other allele showed a deletion of Gly28. No mutant clones expressed the cholesterol-binding CRAC domain and the PK11195 binding pocket of the TSPO protein. The Gly28 deletion in one allele of MA-10:TspoΔ/Δ3 was a three-base pair (bp) deletion that could potentially allow the remainder of the TSPO protein to remain in frame; however, we did not detect any TSPO protein expression from this clone (Figure 1B). Based on modeling this deletion in the TSPO structure (13), we identified a significant change to the short α helix loop that connects transmembrane region 1 and 2 (Figure 1D), which could potentially trigger the misfolded protein response. However, treatment with the proteasome inhibitor MG132 did not recover TSPO protein detection (data not shown), suggesting that cellular quality control mechanisms for TSPO are yet to be understood. Sequencing the gDNA Tspo loci from the three MA-10:TspoΔ/Δ clones detected five to six copies of TSPO in each clone that were all mutated (Supplemental Table 2). Although the potential possibility of multiple alleles exists (Supplemental Figure 1), it is also possible that persistent or repeated cutting by Cas9 at the Tspo exon 2 loci that may have occurred during clonal selection, could have resulted in some minor subclonal populations. These different mutations included those that were detected in Tspo cDNA from these clones. All MA-10:TspoΔ/Δ cells seemed healthy with no morphological differences compared with the control MA-10:Tspo+/+ cells (Figure 1E). Immunocytochemical staining confirmed that TSPO protein was not detectable in MA-10:TspoΔ/Δ cells (Figure 1F).
Loss of TSPO does not affect progesterone production in MA-10 cells
We examined the effect of complete TSPO protein deficiency on steroid hormone production in MA-10:TspoΔ/Δ cells compared with control MA-10:Tspo+/+ cells. At baseline, progesterone levels were not different between the three MA-10:TspoΔ/Δ clones and control MA-10:Tspo+/+ cells (Figure 2A). After stimulation with Bt2cAMP, progesterone levels increased in both MA-10:TspoΔ/Δ and control MA-10:Tspo+/+ cells to identical levels showing no defects in steroid hormone production (Figure 2A). In fact, progesterone levels in one clone MA-10:TspoΔ/Δ3, were even significantly higher than control MA-10:Tspo+/+ cells. Expression of the STAR protein, a critical player in the steroid hormone synthesis pathway, was similar at both baseline and after Bt2cAMP stimulation in MA-10:TspoΔ/Δ clones and control MA-10:Tspo+/+ cells (Figure 2B). These results suggested that TSPO is not involved in the steroid hormone biosynthetic pathway in MA-10 cells.
Figure 2.
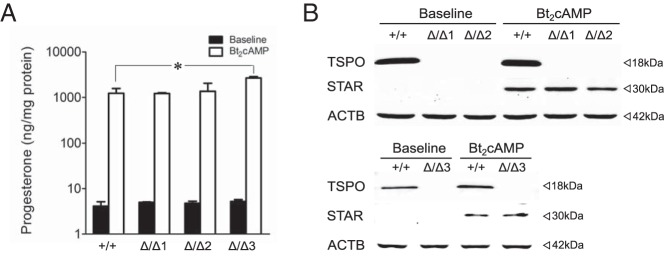
TSPO deficiency does not affect progesterone production in MA-10 cells. A, TSPO deletion in the three MA-10:TspoΔ/Δ clones did not affect baseline or Bt2cAMP-stimulated progesterone production. Bt2cAMP stimulation increased progesterone levels to a similar extent in both intact MA-10:Tspo+/+, and TSPO-deleted clones: MA-10:TspoΔ/Δ1, MA-10:TspoΔ/Δ2, and MA-10:TspoΔ/Δ3. Progesterone levels after Bt2cAMP treatment in clone MA-10:TspoΔ/Δ3 were significantly higher than MA-10:Tspo+/+ cells. Mean values were generated from two to three experiments conducted in triplicate. B, Western blots showing absence of TSPO and the induction of STAR after Bt2cAMP stimulation of MA-10:TspoΔ/Δ clones and MA-10:Tspo+/+ cells. Induction of STAR expression after Bt2cAMP stimulation were identical in the different MA-10:TspoΔ/Δ clones compared with MA-10:Tspo+/+ cells. Control bands are β-Actin (ACTB).
Pharmacological effect of PK11195 on steroidogenesis is not mediated by TSPO
Using MA-10:TspoΔ/Δ cells as a tool, we attempted to validate the specific relationship between PK11195 and TSPO in the induction of steroidogenesis, a pharmacological effect demonstrated in previous studies (17, 18). We examined whether PK11195 would induce a steroidogenic response in MA-10:TspoΔ/Δ clones. Our results showed that PK11195 could stimulate progesterone production in a dose-dependent manner in both MA-10:TspoΔ/Δ clones and control MA-10:Tspo+/+ cells (Figure 3). In control MA-10:Tspo+/+ cells, the maximal progesterone levels observed at 10μM PK11195 was approximately 3–4-fold higher than baseline; this was identical in MA-10:TspoΔ/Δ clones 1 and 3. MA-10:TspoΔ/Δ clone 2 also showed a similar response, but the progesterone production was induced only approximately 2-fold of the baseline. These results suggested that the pharmacological effect of the TSPO ligand PK11195 on steroid hormone synthesis in MA-10 cells is not mediated via TSPO.
Figure 3.
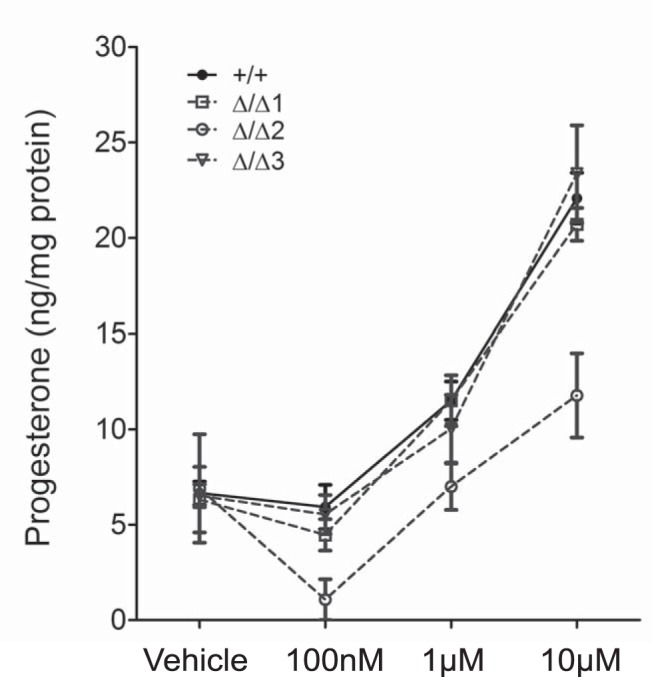
PK11195 induced steroidogenesis in the absence of TSPO. PK11195 dose-dependent increase in progesterone production was observed in both MA-10:TspoΔ/Δ clones (1, 2, and 3) and control MA-10:Tspo+/+ cells. Mean values were generated from three to four independent experiments conducted in triplicate.
Discussion
Recent evidence that there is no physiological need for TSPO in mitochondrial cholesterol transport for steroid hormone biosynthesis has raised critical questions regarding the pharmacology of TSPO binding chemicals (24, 28, 29). The core basis for TSPO involvement in steroidogenesis was suggested by early studies reporting that TSPO binding chemicals can induce steroid hormone production (17, 18). Based on our results, it is apparent that the effect of PK11195 on MA-10 cells resulting in the induction of steroid hormone production is not mediated through TSPO.
Hormone production by steroidogenic cells upon treatment with TSPO binding chemicals has been offered as proof of TSPO involvement in steroidogenesis (17, 18). The effect of PK11195 was previously shown to induce progesterone production in a steep dose response curve that plateaued at concentrations higher than 1μM. However, it is interesting to note that the response itself was very modest and transient; in a time course, there was no progressive increase in progesterone accumulation in cell culture supernatants after the first hour of PK11195 treatment, and levels remained unchanged over the next 4 hours (18). This is in contrast with stimulation with trophic hormones, which show a progressive accumulation of progesterone in the supernatant over the entire 5-hour period (18). We initially interpreted this distinction as an indicator that the effect of TSPO binding chemicals is a physical response to TSPO binding rather than a physiological effect. In a recent commentary, Papadopoulos also offered such an explanation to address why physiological TSPO function might be different from pharmacology (30). In addition, structural evidence that PK11195-binding stabilizes TSPO also contributed to the logic that such an event might be possible (13).
In our treatments, maximal induction of progesterone production was 2–4-fold higher than baseline at 10μM PK11195 in both MA-10:TspoΔ/Δ and MA-10:Tspo+/+ cells, which is comparable to the extent of induction previously demonstrated for MA-10 cells (18). One of the three clones MA-10:TspoΔ/Δ2 was less responsive and reached only approximately 2-fold above baseline, a difference that could be explained by inherent variability that exists in clonal populations within MA-10 cells (31). Interestingly, we did not find induction of progesterone production in either MA-10:TspoΔ/Δ or MA-10:Tspo+/+ cells at nanomolar PK11195 concentrations; values for progesterone also did not seem to plateau at 1μM for these cells as previously reported (18), suggesting an experimental difference in effective ranges for this steroidogenic response by PK11195. We observed that PK11195 ability to induce progesterone production was inversely affected by cell density, and a prominent response could be observed only when cells were less than 50% confluent (not shown). This also suggested that the PK11195 exposure did not generate a typical drug-ligand-type response in these cells. Nevertheless, the fact that we observed a PK11195 response in both MA-10:TspoΔ/Δ and MA-10:Tspo+/+ cells confirms that TSPO is not involved in this phenomenon.
One possibility for this disconnect is that the pharmacological effect of PK11195 on steroidogenesis could be due to a chemical-membrane interaction. It has been recently demonstrated that TSPO ligands like PK11195 can insert themselves into lipid bilayers affecting membrane properties (32), a phenomenon that could modulate cholesterol availability to the steroidogenic machinery. Another study reported that high concentrations of PK11195 could rapidly displace fluorescent NBD-cholesterol [(22-(N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-yl)Amino)-23,24-Bisnor-5-Cholen-3β-Ol)] from different membrane compartments into lipid droplets, affecting intracellular cholesterol in astrocytes and fibroblasts (33). Although it was not clear whether this displacement was mediated by TSPO in these cell types, one plausible explanation for our results in steroidogenic cells is that a similar displacement could potentially release a limited amount of cholesterol from mitochondrial membranes and hence trigger a modest and transient increase in steroid hormone production.
In addition, whereas there are reports in the literature of TSPO-independent action of several putative binding chemicals, specific cellular targets other than TSPO have not been extensively characterized. PK11195 has been demonstrated to target the F1F0-ATP synthase and inhibit mitophagy without the involvement of TSPO (34). In another study, an apoptosis-sensitizing effect for PK11195 was observed even after TSPO was completely knocked down (35). The antitumor effect of TSPO-binding chemicals Ro5–4864 and FGIN-1–27 was identical irrespective of the presence or absence of TSPO in different tumors (36). In hematologic cancers, PK11195 showed broad inhibition of adenosine triphosphate (ATP)-binding cassette transporters by binding to plasma membrane sites without involvement of TSPO (37). In cells of mesenchymal origin, PK11195 and Ro5–4864 could inhibit cell proliferation through decreases in activation of ERK and c-Jun independent of TSPO (38). Therefore, we can speculate that the effect of PK11195 on steroidogenesis may be mediated through binding to another unidentified cellular target in steroid hormone–producing cells.
Results in this study also contradicted the previous study on R2C cells that disruption of one Tspo allele dramatically reduced steroid production to only approximately 5% of control values (21). This disruption also caused significant adverse morphological changes and 2–3-fold slower cell proliferation in these R2C cells. We did not observe any differences in morphology, viability, or proliferation in the three MA-10:TspoΔ/Δ clones generated in this study. Therefore, previous results from the solitary mono-allelic Tspo-disrupted R2C clone may require reinvestigation.
In summary, our results show that TSPO deletion in MA-10 cells has no effect on steroid hormone biosynthesis and that the pharmacological activity of PK11195 on the induction of steroidogenesis is not mediated through TSPO.
Acknowledgments
We thank Dr Mario Ascoli for providing us with MA-10 cells, and Dr Feng Zhang at the Broad Institute of Massachusetts Institute of Technology and Harvard for the plasmid coding the CRISPR/Cas9 targeting system.
This work was supported by funds from Cornell College of Agriculture and Life Sciences (startup) to V.S., National Institutes of Health Grant HD-17481, and Robert A. Welch Foundation Grant B1–0028 to D.M.S. and a fellowship from the Vietnam Education Foundation to L.N.T.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- bp
- base pair
- Bt2cAMP
- dibutyryl-cAMP
- CRAC
- cholesterol recognition amino acid consensus
- MA-10:TspoΔ/Δ
- TSPO-knockout MA-10 mouse Leydig tumor cells
- STAR
- steroidogenic acute regulatory protein
- TSPO
- translocator protein.
References
- 1. Yeliseev AA, Kaplan S. A sensory transducer homologous to the mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J Biol Chem. 1995;270:21167–21175. [DOI] [PubMed] [Google Scholar]
- 2. Braestrup C, Squires RF. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci U S A. 1977;74:3805–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braestrup C, Albrechtsen R, Squires RF. High densities of benzodiazepine receptors in human cortical areas. Nature. 1977;269:702–704. [DOI] [PubMed] [Google Scholar]
- 4. Regan JW, Yamamura HI, Yamada S, Roeske WR. High affinity [3H]flunitrazepam binding: Characterization, localization, and alteration in hypertension. Life Sci. 1981;28:991–998. [DOI] [PubMed] [Google Scholar]
- 5. Davies LP, Huston V. Peripheral benzodiazepine binding sites in heart and their interaction with dipyridamole. Eur J Pharmacol. 1981;73:209–211. [DOI] [PubMed] [Google Scholar]
- 6. Le Fur G, Perrier ML, Vaucher N, et al. Peripheral benzodiazepine binding sites: Effect of PK 11195, 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide. I. In vitro studies. Life Sci. 1983;32:1839–1847. [DOI] [PubMed] [Google Scholar]
- 7. Gavish M, Bachman I, Shoukrun R, et al. Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev. 1999;51:629–650. [PubMed] [Google Scholar]
- 8. Anholt RR, De Souza EB, Oster-Granite ML, Snyder SH. Peripheral-type benzodiazepine receptors: Autoradiographic localization in whole-body sections of neonatal rats. J Pharmacol Exp Ther. 1985;233:517–526. [PubMed] [Google Scholar]
- 9. Wang HJ, Fan J, Papadopoulos V. Translocator protein (Tspo) gene promoter-driven green fluorescent protein synthesis in transgenic mice: An in vivo model to study Tspo transcription. Cell Tissue Res. 2012;350:261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morohaku K, Phuong NS, Selvaraj V. Developmental expression of translocator protein/peripheral benzodiazepine receptor in reproductive tissues. PloS One. 2013;8:e74509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anholt RR, Pedersen PL, De Souza EB, Snyder SH. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem. 1986;261:576–583. [PubMed] [Google Scholar]
- 12. Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139:4991–4997. [DOI] [PubMed] [Google Scholar]
- 13. Jaremko L, Jaremko M, Giller K, Becker S, Zweckstetter M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science. 2014;343:1363–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murail S, Robert JC, Coïc YM, et al. Secondary and tertiary structures of the transmembrane domains of the translocator protein TSPO determined by NMR. Stabilization of the TSPO tertiary fold upon ligand binding. Biochim Biophys Acta. 2008;1778:1375–1381. [DOI] [PubMed] [Google Scholar]
- 15. Korkhov VM, Sachse C, Short JM, Tate CG. Three-dimensional structure of TspO by electron cryomicroscopy of helical crystals. Structure. 2010;18:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Midzak A, Rone M, Aghazadeh Y, Culty M, Papadopoulos V. Mitochondrial protein import and the genesis of steroidogenic mitochondria. Mol Cell Endocrinol. 2011;336:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mukhin AG, Papadopoulos V, Costa E, Krueger KE. Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proc Natl Acad Sci U S A. 1989;86:9813–9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papadopoulos V, Mukhin AG, Costa E, Krueger KE. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J Biol Chem. 1990;265:3772–3779. [PubMed] [Google Scholar]
- 19. Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- 20. Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci U S A. 1997;94:11540–11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem. 1997;272:32129–32135. [DOI] [PubMed] [Google Scholar]
- 22. Morohaku K, Pelton SH, Daugherty DJ, Butler WR, Deng W, Selvaraj V. Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology. 2014;155:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papadopoulos V, Amri H, Boujrad N, et al. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62:21–28. [DOI] [PubMed] [Google Scholar]
- 24. Tu LN, Morohaku K, Manna PR, et al. Peripheral Benzodiazepine Receptor/Translocator Protein Global Knock-out Mice Are Viable with No Effects on Steroid Hormone Biosynthesis. J Biol Chem. 2014;289:27444–27454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chung JY, Chen H, Midzak A, Burnett AL, Papadopoulos V, Zirkin BR. Drug ligand-induced activation of translocator protein (TSPO) stimulates steroid production by aged brown Norway rat Leydig cells. Endocrinology. 2013;154:2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manna PR, Cohen-Tannoudji J, Counis R, et al. Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: Its role in the regulation of the steroidogenic acute regulatory protein. J Biol Chem. 2013;288:8505–8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stocco DM. The Role of PBR/TSPO in Steroid Biosynthesis Challenged. Endocrinology. 2014;155:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banati RB, Middleton RJ, Chan R, et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat Commun. 2014;5:5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Papadopoulos V. On the Role of the Translocator Protein (18-kDa) TSPO in Steroid Hormone Biosynthesis. Endocrinology. 2014;155:15–20. [DOI] [PubMed] [Google Scholar]
- 31. Gocze PM, Freeman DA. Factors underlying the variability of lipid droplet fluorescence in MA-10 Leydig tumor cells. Cytometry. 1994;17:151–158. [DOI] [PubMed] [Google Scholar]
- 32. Hatty CR, Le Brun AP, Lake V, et al. Investigating the interactions of the 18kDa translocator protein and its ligand PK11195 in planar lipid bilayers. Biochim Biophys Acta. 2014;1838:1019–1030. [DOI] [PubMed] [Google Scholar]
- 33. Falchi AM, Battetta B, Sanna F, et al. Intracellular cholesterol changes induced by translocator protein (18 kDa) TSPO/PBR ligands. Neuropharmacology. 2007;53:318–329. [DOI] [PubMed] [Google Scholar]
- 34. Seneviratne MS, Faccenda D, De Biase V, Campanella M. PK11195 inhibits mitophagy targeting the F1Fo-ATPsynthase in Bcl-2 knock-down cells. Curr Mol Med. 2012;12:476–482. [DOI] [PubMed] [Google Scholar]
- 35. Gonzalez-Polo RA, Carvalho G, Braun T, et al. PK11195 potently sensitizes to apoptosis induction independently from the peripheral benzodiazepin receptor. Oncogene. 2005;24:7503–7513. [DOI] [PubMed] [Google Scholar]
- 36. Hans G, Wislet-Gendebien S, Lallemend F, et al. Peripheral benzodiazepine receptor (PBR) ligand cytotoxicity unrelated to PBR expression. Biochem Pharmacol. 2005;69:819–830. [DOI] [PubMed] [Google Scholar]
- 37. Walter RB, Pirga JL, Cronk MR, Mayer S, Appelbaum FR, Banker DE. PK11195, a peripheral benzodiazepine receptor (pBR) ligand, broadly blocks drug efflux to chemosensitize leukemia and myeloma cells by a pBR-independent, direct transporter-modulating mechanism. Blood. 2005;106:3584–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kletsas D, Li W, Han Z, Papadopoulos V. Peripheral-type benzodiazepine receptor (PBR) and PBR drug ligands in fibroblast and fibrosarcoma cell proliferation: Role of ERK, c-Jun and ligand-activated PBR-independent pathways. Biochem Pharmacol. 2004;67:1927–1932. [DOI] [PubMed] [Google Scholar]