Abstract
Hypothalamic kisspeptin (Kiss1) neurons express estrogen receptor α (ERα) and exert control over GnRH/LH secretion in female rodents. It has been proposed that estradiol (E2) activation of ERα in kisspeptin neurons in the arcuate nucleus (ARC) suppresses GnRH/LH secretion (negative feedback), whereas E2 activation of ERα in kisspeptin neurons in the anteroventral periventricular nucleus (AVPV) mediates the release of preovulatory GnRH/LH surges (positive feedback). To test these hypotheses, we generated mice bearing kisspeptin cell–specific deletion of ERα (KERαKO) and treated them with E2 regimens that evoke either negative or positive feedback actions on GnRH/LH secretion. Using negative feedback regimens, as expected, E2 effectively suppressed LH levels in ovariectomized (OVX) wild-type (WT) mice to the levels seen in ovary-intact mice. Surprisingly, however, despite the fact that E2 regulation of Kiss1 mRNA expression was abrogated in both the ARC and AVPV of KERαKO mice, E2 also effectively decreased LH levels in OVX KERαKO mice to the levels seen in ovary-intact mice. Conversely, using a positive feedback regimen, E2 stimulated LH surges in WT mice, but had no effect in KERαKO mice. These experiments clearly demonstrate that ERα in kisspeptin neurons is required for the positive, but not negative feedback actions of E2 on GnRH/LH secretion in adult female mice. It remains to be determined whether the failure of KERαKO mice to exhibit GnRH/LH surges reflects the role of ERα in the development of kisspeptin neurons, in the active signaling processes leading to the release of GnRH/LH surges, or both.
Ovulatory cyclicity in mammals is maintained by the stimulatory and feedback actions of hormonal signals within the hypothalamic-pituitary-gonadal axis. Hypothalamic neurosecretion of GnRH stimulates release of the pituitary gonadotropins, LH and FSH, which circulate to control ovarian folliculogenesis and steroidogenesis. In turn, ovarian steroids exert critically important feedback actions in the hypothalamus and anterior pituitary gland to control the cyclical release of GnRH and the gonadotropins. Negative feedback effects of estradiol (E2) and progesterone prevail throughout most of the ovulatory cycle to maintain restraint of GnRH and gonadotropin secretion. As the cycle progresses, maturing ovarian follicles produce a surge of E2, evoking a positive feedback action on the hypothalamus that culminates in the stimulation of a preovulatory GnRH surge, followed by an LH surge. Failure of either feedback mechanism is invariably associated with impaired or absent ovulatory cyclicity and sub- or infertility. Estrogen receptor α (ERα) mediates most these feedback actions in rodents (1–4), yet the specific cell populations and neural circuitries in which these receptors convey these effects remain uncertain.
The scarcity of ERα in GnRH neurons (5, 6) suggests that E2 governs GnRH neurosecretion by targeting ERα-expressing cells in afferent circuitries, such as those that express the potent GnRH secretagogue, kisspeptin. Kisspeptin is mainly expressed in the arcuate (ARC) and anteroventral periventricular (AVPV) nuclei in the rodent hypothalamus, neural regions shown to be critically important sites for E2-mediated negative (7, 8) and positive (9–12) feedback, respectively, on GnRH secretion. Suggestive of kisspeptin's involvement in both feedback mechanisms, E2 acts through ERα to inhibit kisspeptin expression in the ARC and stimulate it in the AVPV (13). In addition, both E2-mediated inhibition of kisspeptin expression in the ARC and GnRH/LH secretion work through nonclassical, estrogen response element (ERE)-independent ERα-signaling pathways, whereas both E2-mediated stimulation of kisspeptin expression in the AVPV and GnRH/LH surges require classical, ERE-dependent ERα-signaling pathways (14, 15). The foregoing findings, taken together with the observations that, in female rodents, most kisspeptin neurons in both the ARC and AVPV coexpress ERα (13), has prompted several groups to propose the hypotheses that i) E2 mediates its negative feedback effects by inhibiting kisspeptin release from the ARC to suppress GnRH secretion, and ii) E2 mediates its positive feedback effects by stimulating kisspeptin release from the AVPV to induce the generation of a GnRH surge (13, 16).
Implicit in the foregoing model is the idea that feedback actions of E2 are conveyed via ERα in kisspeptin neurons. However, there is currently no evidence demonstrating that E2-mediated ERα signaling in ARC and AVPV kisspeptin neurons is integral to the control of kisspeptin expression or negative or positive feedback regulation of GnRH, and therefore, LH secretion. Here, we use a mouse model in which ERα is selectively ablated in kisspeptin cells to address these questions. We show that ERα expression in kisspeptin neurons is required for both stimulation of Kiss1 mRNA expression and the positive feedback regulation of GnRH/LH surges. Although we also find that ERα in kisspeptin neurons is required for suppression of Kiss1 mRNA expression in the ARC, we demonstrate that negative feedback effects of E2 on GnRH/LH secretion can be exerted through mechanisms that operate independently of ERα in kisspeptin neurons.
Materials and Methods
Animals
Mice were housed in animal facilities located at either Northwestern University (Evanston, Illinois) or the University of Wisconsin-Madison (UW-Madison; Madison, Wisconsin). All experimental procedures adhered to guidelines provided in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Northwestern and at UW-Madison. Mice were maintained on a 12:12-h light:dark cycle (Northwestern: 6:00 am lights on; UW-Madison: 7:00 am lights on) and given access to water and standard rodent chow (Northwestern: 7912 Teklad; UW-Madison: 2019 Teklad; Harlan Laboratories) ad libitum.
To specifically ablate ERα from kisspeptin cells, mice with Cre recombinase knocked-in downstream of the Kiss1 promoter (Kiss-Cre mice) were bred to mice with exon 3 of the ERα gene (Esr1) flanked by loxP sites (ERαlox/lox mice) as previously described (17). As a result of cre recombinase-mediated excision of exon 3 of Esr1, ERα protein is absent from almost all neurons expressing kisspeptin (17). All experiments were performed in adult (2.0–4.5 months old) female Kiss-Cre+/ERαlox/lox (KERαKO) mice and their Kiss-Cre−/ERαlox/lox and Kiss-Cre−/ERαlox/wt (wild-type [WT]) littermates.
Treatments
Experiment 1: Evaluation of E2-mediated negative feedback effects on Kiss1 mRNA expression and LH levels after short-term ovariectomy
Adult female mice were anesthetized by isoflurane inhalation and bilaterally ovariectomized (OVX). Each animal received one Silastic capsule (0.058” inner diameter, 0.077” outer diameter; 1.5 cm in length with each end plugged with Silastic medical grade adhesive) filled to an effective length of 1.0 cm with sesame oil (vehicle) or E2 dissolved in sesame oil (1 mg/mL) placed sc under one flank. Mice were anesthetized 7 days (1 week) after OVX between 8:00 am–12:30 pm, blood was withdrawn by cardiac puncture and plasma stored at −20°C for hormone assays. Brains were rapidly removed, fresh frozen, and processed for in situ hybridization (ISH) procedures (n = 6–12 per group).
Experiment 2: Evaluation of E2-mediated negative feedback effects on LH levels after long-term OVX
Long-term (> 2 weeks) OVX increases LH to significantly higher levels than after short-term OVX (18). Adult female mice were anesthetized by isoflurane inhalation and sham-operated or bilaterally OVX. Each OVX animal received one Silastic capsule (same as Experiment 1) filled to an effective length of 1.0 cm with sesame oil (vehicle) or E2 dissolved in sesame oil (1 mg/mL) placed sc under one flank. Capsules were replaced 14 days after OVX to maintain circulating E2 levels. Estrous cyclicity in sham-operated mice was monitored by daily vaginal smears. Most ovary-intact KERαKO mice remain in persistent estrus or diestrus (17), therefore sham-operated WT and KERαKO mice were euthanized at either cycle stage. No differences between diestrus and estrus LH levels were found among WT or KERαKO mice; therefore, all sham-operated mice were analyzed as a single group for each genotype. Mice were anesthetized 21 days after OVX or 18–26 days after sham operation between 7:30 am–12:00 pm. Blood was withdrawn by cardiac puncture and plasma stored at −20°C for LH assay (n = 7–18 per group).
Experiment 3: Evaluation of E2-mediated positive feedback effects on LH levels
Adult female mice were anesthetized and OVX as in Experiment 1. Each animal received one VWR Select Silicone capsule (0.04” inner diameter, 0.085” outer diameter) containing crystalline E2 dissolved in Silastic medical grade adhesive (0.1 mg/mL) placed sc under one flank. The length of each capsule varied such that each animal received 1 μg E2/20 g body weight (1 μg E2/1.0 cm of capsule). Six days after OVX between 10:00–10:10 am, mice were injected sc with either sesame oil (vehicle) or E2 benzoate (EB; Sigma; 1 μg in 0.1 mL sesame oil) to mimic increasing E2 levels that precede the preovulatory GnRH/LH surge. After lights off the following evening between 7:10–7:30 pm, mice were anesthetized, blood was withdrawn by cardiac puncture, and plasma stored at −20°C for hormone assays (n = 6–8 per group). Surge levels of LH were defined as plasma LH levels greater than 2 SDs from the mean LH level of vehicle-treated mice of the same genotype (19).
Experiment 4: Evaluation of pituitary sensitivity to E2 and pituitary responsiveness to GnRH
Adult female mice were anesthetized and OVX as in Experiment 1. Each animal received one Silastic capsule (same as Experiment 1) filled to an effective length of 1.0 cm with E2 dissolved in sesame oil (1 mg/mL) placed sc under one flank. Six days after OVX between 10:00–11:00 am, mice were injected sc with either sesame oil (vehicle) or EB (1 μg in 0.1 mL sesame oil). The following morning between 8:00–10:30 am, mice were injected sc with saline (vehicle) or GnRH (Sigma; 200 ng/kg body weight in 0.1 mL saline) and anesthetized 10 minutes postinjection (20). Blood was withdrawn by cardiac puncture and plasma stored at −20°C for LH assay (n = 9–11 per group).
Hormone assays
Hormone assays were performed at Northwestern University (Experiment 1; Evanston, Illinois), or at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Experiments 2–4, Charlottesville, Virginia). Hormone levels that were undetectable were recorded to be the lower limit of detection of the associated assay.
Plasma from mice studied in Experiment 1 was assayed for LH using RIA reagents obtained from the National Institute of Diabetes and Digestive and Kidney Diseases, including the LH reference (RP-3) and S-11 antibody. The assay had a lower limit of detection of 0.2 ng/mL. The intra-assay and interassay coefficients of variation (CVs) were 4.1% and 7.9%, respectively. Plasma from mice studied in Experiments 2–4 was assayed for LH using the mouse LH sandwich assay. The assay had a lower limit of detection of 0.04 ng/mL and an intra-assay CV of 6.4%.
E2 measurements from mice studied in Experiments 1 and 3 were performed using an ELISA kit (Calbiotech). The assay had a lower limit of detection of 3.0 pg/mL and an intra-assay CV of 6.0%.
ISH for Kiss1 mRNA
Coronal brain sections (20 μm) were prepared using a cryostat. Sections were collected in two series from the rostral border of the diagonal band of Broca through the premammillary nucleus, thaw-mounted onto gelatin-subbed slides, and stored at −80°C.
The plasmid containing the cDNA used to generate the riboprobe to detect Kiss1 mRNA was generously provided by Dr Robert Steiner, University of Washington, and was described previously (16). Antisense Kiss1 probe was transcribed from 1.8 μg linearized plasmid in an in vitro reaction containing 250 μCi 33P-UTP (PerkinElmer), 2.5mM each ATP, CTP, and GTP, 16 U T7 RNA polymerase (Promega), 60 U RNAsin (Promega), 10mM dithiothreitol (DTT), and transcription buffer (Promega). Residual DNA was digested with 1 μl RQ1 DNase (Promega). The probe was purified and quantified using a scintillation counter.
All slides (AVPV and ARC) were processed together. Sections were postfixed in 4% paraformaldehyde for 5 minutes, washed in phosphate buffered saline, acetylated with 0.25% acetic anhydride in 0.1M triethanolamine for 10 minutes, rinsed in 1× standard saline citrate (SSC), dehydrated with graded alcohols, delipidated in chloroform for 5 minutes, dehydrated again, and air-dried. 33P-UTP labeled probe was mixed with 5 μl/100 μl salmon sperm DNA (Sigma) in buffer containing 10mM Tris, 1mM EDTA, and 10mM DTT, heat denatured, and then added to hybridization mixture (50% formamide, 10% dextran sulfate, 0.3M NaCl, 10mM Tris, 1mM EDTA, 1× Denhardt's solution, 10mM DTT) for a final probe concentration of 0.05 pmol/mL. Slides were covered with 100 μl of probe/hybridization mixture, fitted with silane-coated coverslips, and incubated overnight at 55°C. Slides were washed in 1× SSC for 30 minutes at room temperature, treated with RNase A (Sigma; 20 μg/mL in 10mM Tris, 0.5M NaCl, 1M EDTA) for 30 minutes at 37°C, washed in 1× SSC for 30 minutes at room temperature followed by four washes in 0.1× SSC for 15 minutes at 62°C and one wash in 0.1× SSC for 30 minutes at room temperature. Slides were then dehydrated through graded alcohols, air dried, and dipped in Kodak NTB emulsion (Carestream Health) diluted with 0.6M ammonium acetate. Slides were kept at −20°C for 20 days (AVPV) or 42 days (ARC) until developed (Kodak D-19). Slides were counterstained with 0.2% cresyl violet acetate, dehydrated in graded alcohols, and rinsed in Histo-Clear (National Diagnostics U.S.A.). Coverslips were applied using Permount (Fisher Scientific).
Quantification of Kiss1 mRNA
Two brain regions of interest (AVPV and ARC) were analyzed separately in a blinded fashion. Quantification was performed under dark-field illumination using NIS-Elements Basic Research software (Nikon Instruments). For each animal, eight sections between Bregma 0.38 and −0.04 were analyzed for the AVPV and eight sections between Bregma −1.46 and −2.54 (21) were analyzed for the ARC. All sections were analyzed unilaterally using a region of interest (ROI) drawn manually around the cells expressing Kiss1 mRNA, which were identified as dense clusters of silver grains. The absolute mean signal intensity of the silver grains within the ROI was recorded. The final mean signal intensity was determined for each section by subtracting the average of three background measurements, each the same size as the ROI, from the absolute mean signal intensity.
Statistical analyses
Data are presented as the mean ± SEM. Mann-Whitney U nonparametric tests were performed using R for Mac OS X (The R Foundation for Statistical Computing) to determine statistical significance in Experiment 1. Two-way ANOVA followed by Bonferroni post-hoc tests were performed using Prism 5 for Mac OS X (GraphPad Software) to determine statistical significance in Experiments 2–4. Differences were considered significant when P < .05.
Results
Experiment 1: Kiss1 mRNA expression and LH response to short-term OVX and E2 treatment
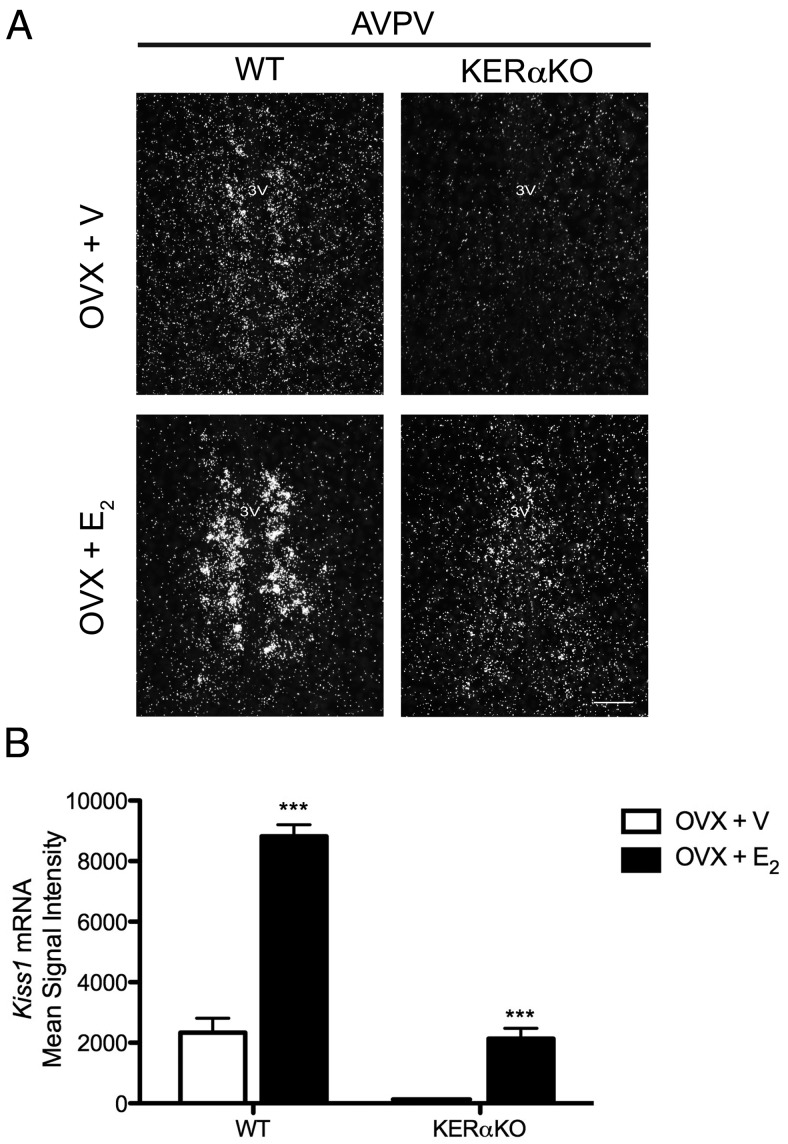
To determine whether E2 directly activates ERα in kisspeptin neurons to control kisspeptin expression in the hypothalamus, Kiss1 mRNA levels were measured in the ARC and AVPV of OVX WT and KERαKO mice treated with or without E2. In the ARC, Kiss1 mRNA levels did not differ significantly between WT and KERαKO vehicle-treated mice 7 days after OVX (Figure 1). Although E2 treatment significantly suppressed Kiss1 mRNA to undetectable levels in WT mice (P < .001), it had no effect on Kiss1 mRNA expression in the ARC of KERαKO mice and Kiss1 mRNA levels remained high in these mice (Figure 1). In the AVPV, Kiss1 mRNA levels were low in WT mice and undetectable in KERαKO mice 7 days after OVX (Figure 2). As seen previously (13, 22), E2 treatment significantly increased Kiss1 mRNA to much higher levels in WT mice compared with vehicle-treated WT mice (P < .001). However, E2 stimulated only a low level of detectable Kiss1 mRNA expression in KERαKO mice (P < .001), which did not differ from basal Kiss1 mRNA levels in vehicle-treated WT mice (Figure 2).
Figure 1.
E2 decreases Kiss1 mRNA expression in the ARC of OVX WT mice, but has no effect on Kiss1 mRNA expression in the ARC of female KERαKO mice. A, Representative photomicrographs of Kiss1 mRNA expression in the ARC of WT (left panels) and KERαKO (right panels) mice at Bregma −1.94 (21). Mice were bilaterally OVX and implanted with a capsule containing vehicle (V) or E2. Brains were collected 7 days after OVX. B, Mean signal intensity of Kiss1 mRNA expression in the ARC. Scale bar = 100 μm. ***, P < .001 compared with V-treated mice. All data represented as mean ± SEM.
Figure 2.
E2 increases Kiss1 mRNA expression in the AVPV of OVX WT and, to a lesser extent, KERαKO mice. A, Representative photomicrographs of Kiss1 mRNA expression in the AVPV of WT (left panels) and KERαKO (right panels) mice at Bregma 0.26 (21). Mice were bilaterally OVX and implanted with a capsule containing vehicle (V) or E2. Brains were collected 7 days after OVX. B, Mean signal intensity of Kiss1 mRNA expression in the AVPV. Scale bar = 100 μm. ***, P < .001 compared with V-treated mice. All data represented as mean ± SEM.
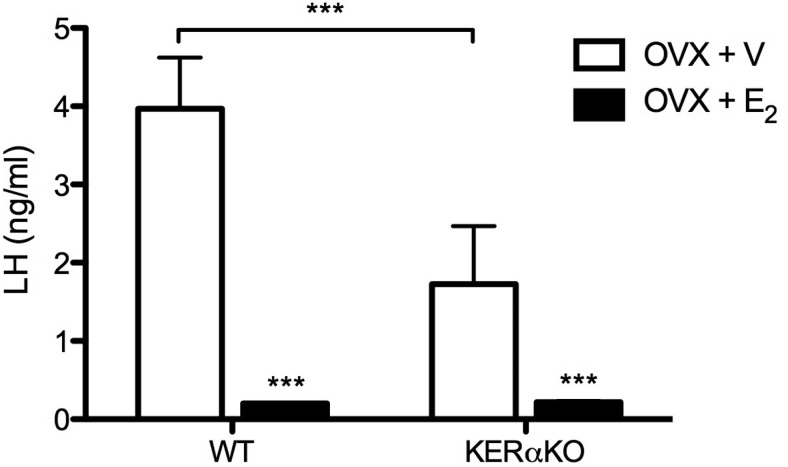
To examine whether E2-mediated negative feedback on GnRH/LH secretion is abrogated in adult KERαKO mice, plasma LH levels were compared 7 days after OVX in vehicle- and E2-treated adult WT and KERαKO mice. As expected, LH levels were elevated in WT mice after OVX and were significantly decreased with E2 treatment (P < .001; Figure 3). As seen previously (17), KERαKO mice exhibited lower LH levels than WT mice after OVX (P < .001). Nevertheless, E2 treatment significantly decreased LH levels to largely undetectable levels in KERαKO mice (P < .05; Figure 3).
Figure 3.
E2 exerts negative feedback effects on LH in short-term OVX WT and KERαKO mice. Mean plasma LH levels in WT and KERαKO female mice that were bilaterally OVX and implanted with a capsule containing vehicle (V) or E2. Blood was collected 7 days after OVX. ***, P < .001 compared with V-treated mice. All data represented as mean ± SEM.
Circulating E2 levels differed between treatment groups. Vehicle-containing capsules resulted in largely undetectable levels of circulating E2 (≤ 3.0 pg/mL) in both genotypes, whereas E2-containing capsules produced similar levels of E2 in WT and KERαKO mice (WT: 51.4 ± 6.2 pg/mL; KERαKO: 50.5 ± 5.4 pg/mL).
Experiment 2: LH response to long-term OVX and E2 treatment
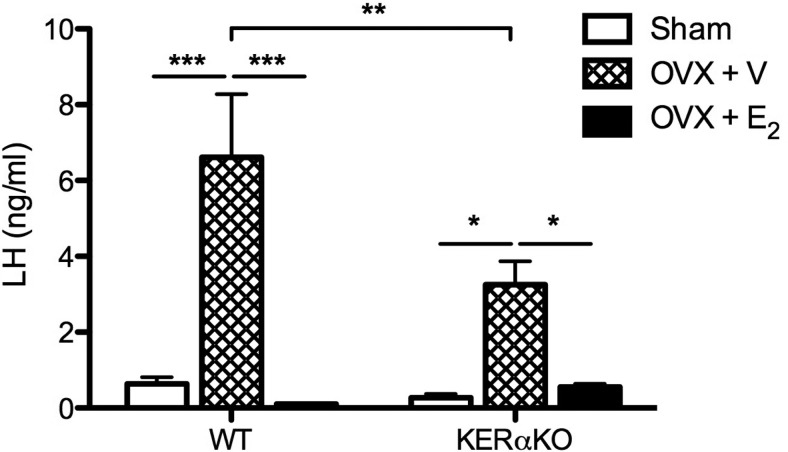
To examine whether the blunted increase in LH exhibited by KERαKO mice 7 days after OVX precluded the characterization of some fraction of E2-mediated negative feedback in KERαKO mice, E2-mediated negative feedback was tested in a group of mice that underwent long-term (3 weeks) OVX. LH levels in WT mice were significantly elevated 3 weeks after OVX (P < .001) and were effectively decreased to ovary-intact (sham) levels with E2 treatment (P < .001; Figure 4). Although KERαKO mice still exhibited a slightly blunted increase in LH levels 3 weeks after OVX compared with WT mice (P < .05), E2 treatment significantly suppressed LH to levels seen in ovary-intact (sham) KERαKO mice (P < .05; Figure 4).
Figure 4.
E2 exerts negative feedback effects on LH in long-term OVX WT and KERαKO mice. Mean plasma LH levels from WT and KERαKO female mice that were bilaterally OVX and implanted with a capsule containing vehicle (V) or E2. Blood was collected 3 weeks after OVX. *, P < .05; **, P < .01; ***, P < .001. All data represented as mean ± SEM.
Experiment 3: LH surge induction by E2
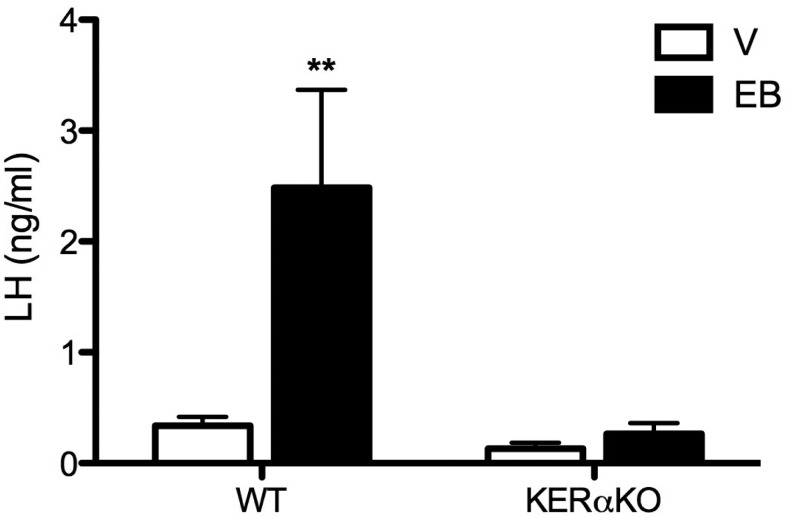
To determine whether ERα in kisspeptin cells is required for stimulation of E2-induced LH surges, adult WT and KERαKO mice were OVX, treated with E2, and given an injection of vehicle or EB 6 days after OVX. After lights off the following evening, 75% (6 of 8) WT mice treated with EB exhibited an LH surge (P < .01; Figure 5), whereas LH levels remained suppressed in all WT mice treated with vehicle. Conversely, EB failed to induce LH surges in KERαKO mice as LH levels in EB-treated KERαKO mice did not differ from vehicle-treated KERαKO mice (Figure 5).
Figure 5.
E2 induces LH surges in OVX WT, but not KERαKO, mice. Mean plasma LH levels from female WT and KERαKO mice that were bilaterally OVX, implanted with a capsule containing vehicle (V) or E2, and injected sc with vehicle or EB 6 days after OVX. Blood was collected after lights off (7:10–7:30 pm) 7 days after OVX. **, P < .01 compared with V-treated mice. All data represented as mean ± SEM.
Circulating E2 levels differed between vehicle and EB treatment groups. Vehicle-treated mice exhibited low circulating levels of E2 (WT: 12.0 ± 2.7 pg/mL; KERαKO: 13.9 ± 4.1 pg/mL), whereas EB-treated mice of each genotype exhibited higher levels of E2 (WT: 33.0 ± 5.3 pg/mL; KERαKO: 26.8 ± 7.5 pg/mL).
Experiment 4: LH response to exogenous GnRH treatment
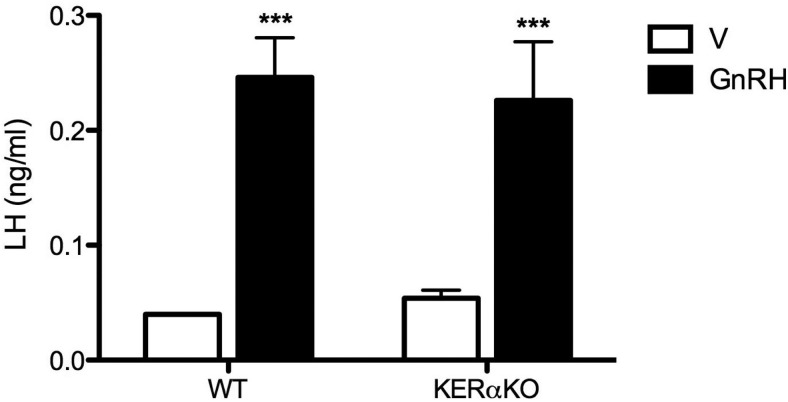
To examine whether the results observed in Experiments 1–3 were due to altered pituitary sensitivity to E2 and/or responsiveness to GnRH, adult WT and KERαKO mice were OVX, treated with E2, and challenged with exogenous GnRH or vehicle 7 days after OVX. In vehicle-treated mice of both genotypes, LH levels were low, reminiscent of the E2-mediated negative feedback effects seen in Experiments 1 and 2 (Figure 6). Treatment with GnRH significantly elevated LH to similar levels in both genotypes (P < .001; Figure 6), indicating that pituitary sensitivity to E2 and pituitary responsiveness to GnRH are normal in KERαKO mice.
Figure 6.
Pituitary sensitivity to E2 and pituitary responsiveness to GnRH are normal in female KERαKO mice. Mean plasma LH levels from female WT and KERαKO mice that were bilaterally OVX, implanted with an E2-containing capsule, and injected sc with vehicle (V) or exogenous GnRH 7 days after OVX. Blood was collected 10 minutes after vehicle or GnRH injection. ***, P < .001 compared with V-treated mice. All data represented as mean ± SEM.
Discussion
These studies tested the hypothesis that both positive and negative feedback effects of E2 on GnRH and LH release are mediated by ERα in kisspeptin neurons. Toward this end, we assessed the effect of kisspeptin cell-specific ERα ablation on the capacity of E2 to evoke LH, and presumably GnRH, surges and to suppress basal GnRH/LH secretions. Our findings clearly demonstrate, for the first time, an absolute requirement for ERα in kisspeptin neurons in the generation of GnRH/LH surges, but do not lend support for their requisite involvement in negative feedback in adult female mice.
Previous studies in gene knockout mice have established that the positive feedback actions of E2 depend upon activation of neuronal ERα (15, 23) and require kisspeptin and its cognate receptor, Kiss1r (19, 24–27). In our studies, we demonstrate that normal positive feedback actions of E2 specifically depend upon ERα expression in kisspeptin neurons because E2 failed to elicit late-afternoon GnRH/LH surges in KERαKO mice. It is likely that GnRH/LH surges in these animals were entirely eliminated, and not simply altered in timing, given that we previously found that ovary-intact KERαKO mice exhibit near-complete or complete anovulation (17).
Although our studies do not distinguish between the hormonal consequences of ERα deletion in either the AVPV or ARC kisspeptin neuronal groups, our finding that KERαKO mice fail to generate GnRH/LH surges while also exhibiting greatly reduced E2 stimulation of Kiss1 mRNA in the AVPV suggests that ERα in AVPV kisspeptin neurons mediates positive feedback in normal animals. Given that E2 treatment has no effect on AVPV Kiss1 mRNA expression in complete ERα knockout (ERαKO) mice (13, 25), residual stimulation of Kiss1 mRNA in the AVPV of KERαKO mice by E2 may be mediated by upstream ERα-expressing neurons that transynaptically stimulate Kiss1 mRNA expression. Regardless, the absence of a complete response to E2 in the AVPV of KERαKO mice suggests that ERα in AVPV kisspeptin neurons is required for the generation of GnRH/LH surges in rodents.
Our findings add to the considerable body of evidence supporting the idea that positive feedback actions of E2 are mediated by ERα-expressing kisspeptin neurons in the AVPV. Early lesion studies first established that the AVPV is required for ovulatory cyclicity and the generation of gonadotropin surges (9–11). The AVPV is sexually dimorphic, with a larger volume in the female associated with the capacity to respond to E2-mediated positive feedback actions (28). Similarly, the kisspeptin neuronal population within the AVPV is enriched at least 10-fold in females vs males (14, 29), and these neurons are activated coincident with the release of preovulatory GnRH/LH surges (25, 30, 31). E2 stimulates Kiss1 gene expression in the AVPV neurons through an ERα-dependent mechanism (13, 25), and here we demonstrate that this action is largely mediated by ERα in kisspeptin neurons. The importance of these neurons in the generation of preovulatory GnRH/LH surges has been underscored by the existence of direct projections from AVPV kisspeptin to GnRH neurons, which express Kiss1r (32–34). Collectively, these observations provide a compelling argument that stimulation of Kiss1 gene expression and positive feedback actions of E2 on GnRH release are conveyed via activation of ERα in kisspeptin neurons located in the AVPV.
The timing of preovulatory GnRH/LH surges in rodents is controlled by a daily neuronal signal, which is conveyed in part from the master circadian clock in the suprachiasmatic nucleus (SCN) to the AVPV (35, 36). Disruption of the clock mechanism or interruption of signals in neural pathways from the SCN to the AVPV induces acyclicity and sub- or infertility (9). Vasopressin is believed to function as a major neurotransmitter link from SCN clock neurons to the AVPV (37–39), and recent studies in rodents have documented that vasopressinergic SCN-AVPV projections target E2-sensitive kisspeptin neurons in this region (40, 41). This vasopressinergic input onto kisspeptin neurons is increased with E2 treatment (44), which also increases GnRH neuron responsiveness to kisspeptin in a circadian manner (40, 42). These findings suggest that E2 acts to maximize the SCN-AVPV kisspeptin-GnRH signal at the time of the preovulatory GnRH surge. Our current observations suggest that E2 also acts via ERα in AVPV kisspeptin neurons to heighten their responsiveness to the incoming circadian signal and allow for the subsequent release of a preovulatory signal to GnRH neurons.
E2 may act on AVPV kisspeptin neurons to enable the release of a preovulatory signal by evoking one or more of several ERα-mediated transcriptional mechanisms. Similar to E2 regulation of GnRH/LH surges (15), E2-mediated stimulation of Kiss1 gene expression in the AVPV depends upon classical ERα transcriptional regulation via binding to EREs (22). Recruitment of ERα to the predicted Kiss1 promoter region to stimulate Kiss1 transcription may be regulated epigenetically by E2 via decreased histone acetylation of the Kiss1 promoter (43). Presumably, up-regulation of Kiss1 transcription would increase the releasable synaptic pool of kisspeptin and allow for an enhanced kisspeptin signal to be transimtted to GnRH neurons. In addition to the modulation of kisspeptin expression, E2 may act through ERα in kisspeptin neurons to regulate the expression of ion channels and the magnitude of ionic currents that increase the excitability of AVPV kisspeptin neurons (44, 45). These actions of E2 would allow for an increased responsiveness of these neurons to synaptic signals, such as those from the SCN that convey the neural signal for the initiation of the GnRH surge, and a maximal release of kisspeptin at a time when stimulation of GnRH and LH secretion needs to be greatest.
Positive feedback actions of E2 in adulthood may also depend upon the activation of ERα in kisspeptin neurons during development. Previously, using immunohistochemistry, we reported that kisspeptin protein expression is diminished in the AVPV of adult KERαKO mice when compared with their WT littermates (17). Here, using ISH, we confirm that Kiss1 mRNA in the AVPV is also significantly reduced in KERαKO mice, even in the presence of E2. Studies of various transgenic mouse models, such as the aromatase knockout (31, 46), hypogonadal (47), and ERαKO (13, 23) mice, along with hormone replacement in prepubertal mice (31, 48), have shown that both ovarian E2 and neuronal ERα are necessary for the normal development of kisspeptin expression in the AVPV. More specifically, the maturation process occurs during a critical period, which precedes postnatal day 9 (P9), to allow for maximal kisspeptin expression in the AVPV of adult female mice (48). Our results suggest that E2 acts directly via ERα in kisspeptin neurons to allow for normal development of Kiss1 mRNA and protein expression in the AVPV. Thus, the absence of ERα in kisspeptin neurons during development may prevent the acquisition of a kisspeptin cell phenotype that can sufficiently produce and release the peptide in response to afferent signals in adulthood. We speculate that ERα figures importantly in both the organizational effects of E2 on kisspeptin neuronal development and the activational effects of E2 on mature kisspeptin neurons that evoke release of GnRH surges in adult animals. Discernment of the relative importance of these effects in conferring responsiveness to the positive feedback actions of E2 will require further experiments in animals sustaining temporally controlled kisspeptin cell-specific ERα deletions.
Although KERαKO mice failed to mount LH surges in response to an E2 challenge, they retained responsiveness to the negative feedback effects of E2, albeit against the backdrop of diminished LH levels following OVX. These results demonstrate that negative feedback effects of E2 can be mediated in their entirety by mechanisms that operate independently of ERα in kisspeptin neurons. These other pathways may normally function as part of the negative feedback mechanism in rodents or they may be recruited in a compensatory manner in the absence of ERα activation in kisspeptin neurons during development in KERαKO mice. It is possible that other E2-sensitive receptors that are coexpressed with kisspeptin neurons, such as ERβ (13), have been recruited to regulate E2-mediated negative feedback in KERαKO mice. However, the absence of E2-mediated negative feedback in complete ERαKO mice suggests that this may not be the case. It is also possible that negative feedback effects of E2 at the level of the gonadotrope are enhanced in KERαKO mice; however, this does not seem likely as pituitary responsiveness to GnRH stimulation was not found to be altered in the E2-treated KERαKO vs WT mice.
Most E2-mediated negative feedback regulation of GnRH/LH secretion is exerted through neurons located in the ARC as a recent study demonstrated that LH secretion is unaffected by chronic (5 days) E2 treatment in mice sustaining viral vector-mediated ERα knockdown in the ARC (49). Our finding that E2 inhibits GnRH/LH secretion in KERαKO female mice despite its inability to suppress Kiss1 mRNA expression in the ARC suggest that E2 acts through ERα on a pathway(s) independent of kisspeptin neurons in the ARC to inhibit GnRH secretion. These data further support recent electrophysiological evidence showing that ARC kisspeptin neurons exhibit reduced, rather than increased firing rates following OVX, findings that are also inconsistent with the involvement of ERα in ARC kisspeptin cells in E2-mediated negative feedback (50).
E2 may exert its negative feedback actions on other ERα-expressing neurons in the ARC, which then relay the information transynaptically to ARC kisspeptin neurons. However, ablation of almost all kisspeptin neurons in the ARC of adult female rats resulted in a blunted increase in LH levels after OVX that was suppressed to ovary-intact levels after E2 treatment (51). Together with our findings, these results suggest that although under normal circumstances ERα signaling in kisspeptin neurons may be sufficient for E2-mediated negative feedback and that redundant negative feedback pathways may exist, E2 primarily exerts its negative feedback actions in the ARC through a pathway(s) independent of kisspeptin cells. Evidence from electrophysiological studies of GFP-identified GnRH neurons have implicated γ-aminobutyric acid (GABA)ergic afferents to GnRH neurons in negative feedback (52), whereas others have suggested a role for proopiomelanocortin (POMC)-expressing neurons as targets of E2 negative feedback actions (53, 54).
It remains unclear why adult KERαKO mice exhibit a diminished LH response to OVX. Other models in which kisspeptin, neurokinin B, and/or dynorphin signaling is abrogated also render animals unable to fully respond to a release from negative feedback following OVX (49, 51, 55–57). We speculate, as others have, that kisspeptin neurons may be involved in GnRH pulse generation and that the lack of E2-mediated ERα activation in kisspeptin neurons during development may limit the acquisition of a full pulse-generating cell phenotype. It is not clear why this phenomenon has not been observed in complete ERαKO mice, but may be due to opposing neuronal influences on GnRH/LH secretion that may be eliminated during development in ERαKO mice.
Our findings that basal LH secretions are not different among ovary-intact WT and KERαKO mice stand in contrast with our previous findings that LH levels are elevated in P15 and P25 female KERαKO mice compared with their age-matched WT counterparts (17). In that study, we noted that these prematurely elevated LH secretions were associated with precocious vaginal opening, as well as increased kisspeptin gene expression in the medial basal hypothalamus at P25. These data suggested that a prepubertal negative feedback loop mediated by ERα in ARC kisspeptin neurons may normally function to restrain GnRH and LH secretion prior to the initiation of puberty. In the previous report (17) and in the current study, however, we also noted that LH levels in peri-pubertal and adult KERαKO mice are no different from those in WT mice, suggesting a developmental process in which E2-mediated negative feedback via ERα in kisspeptin cells in prepubertal animals is supplanted by a mechanism independent of kisspeptin neurons in adulthood. These postpubertal mechanisms, which may include GABAergic and/or POMC-expressing neurons, may be engaged through pubertal alterations in cell groups and synaptic circuitries that govern GnRH neuronal activity.
In summary, we show that ERα expression in kisspeptin neurons is required for both stimulation (AVPV) and inhibition (ARC) of Kiss1 mRNA expression and the positive, but not negative, feedback regulation of GnRH/LH secretion. These studies clearly demonstrate that the activation of ERα in kisspeptin neurons is an obligatory step in the neural mechanisms mediating release of E2-induced GnRH and LH surges. Our findings, taken together with those of many previous studies, are consistent with a model for a core neural circuit for the generation of GnRH surges, in which E2 activates ERα in AVPV kisspeptin neurons and thereby confers maximal responsiveness to circadian signals arriving from the SCN for release of the surge; the net result is an appropriately timed activation of AVPV kisspeptin neurons, enhanced release of kisspeptin from afferents to GnRH neurons, and GnRH surge initiation. This model incorporates physiological elements that are known to be essential for release of GnRH surges and does not preclude the serial or parallel modulatory influences shown to be mediated by other ERα-expressing cell groups, such as the catecholaminergic, GABAergic, glutamatergic, neuropeptide Y-expressing, and POMC-expressing neurons, or the role of ERα in kisspeptin neuronal development. Future studies will more precisely define the subcellular pathways by which ERα evokes these critically important events in kisspeptin neurons, and thereby integrates neuroendocrine signals that ultimately trigger ovulation.
Acknowledgments
We gratefully acknowledge the technical expertise of Ms. Brigitte Mann and the University of Virginia's Center for Research in Reproduction Ligand Assay and Analysis Core.
This work was supported by the National Institutes of Health Grants P01 HD21291, R01 HD68777, P50 HD44405, and 5R00 HD055446-04.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- CV
- coefficient of variation
- DTT
- dithiothreitol
- E2
- estradiol
- EB
- E2 benzoate
- ERα
- estrogen receptor α
- ERE
- estrogen response element
- Esr1
- ERα gene
- GABA
- γ-aminobutyric acid
- ISH
- in situ hybridization
- KERαKO
- kisspeptin cell–specific ERα knockout
- Kiss1
- kisspeptin gene
- OVX
- ovariectomized/ovariectomy
- P
- postnatal day
- POMC
- proopiomelanocortin
- ROI
- region of interest
- SCN
- suprachiasmatic nucleus
- SSC
- standard saline citrate
- WT
- wild-type.
References
- 1. Lindzey J, Jayes FL, Yates MM, Couse JF, Korach KS. The bi-modal effects of estradiol on gonadotropin synthesis and secretion in female mice are dependent on estrogen receptor-alpha. J Endocrinol. 2006;191:309–317. [DOI] [PubMed] [Google Scholar]
- 2. Hewitt SC, Korach KS. Estrogen receptors: structure, mechanisms and function. Rev Endocr Metab Disord. 2002;3:193–200. [DOI] [PubMed] [Google Scholar]
- 3. Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78:204–209. [DOI] [PubMed] [Google Scholar]
- 4. Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039–1053. [DOI] [PubMed] [Google Scholar]
- 5. Hrabovszky E, Shughrue PJ, Merchenthaler I, et al. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. [DOI] [PubMed] [Google Scholar]
- 6. Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304:345–347. [DOI] [PubMed] [Google Scholar]
- 7. Blake CA. A medial basal hypothalamic site of synergistic action of estrogen and progesterone on the inhibition of pituitary luteinizing hormone release. Endocrinology. 1977;101:1130–1134. [DOI] [PubMed] [Google Scholar]
- 8. Blake CA, Norman RL, Sawyer CH. Localization of inhibitory actions of estrogen and nicotine on release of luteinizing hormone in rats. Neuroendocrinology. 1974;16:22–35. [DOI] [PubMed] [Google Scholar]
- 9. Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. [DOI] [PubMed] [Google Scholar]
- 10. Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. [DOI] [PubMed] [Google Scholar]
- 11. Ronnekleiv OK, Kelly MJ. Plasma prolactin and luteinizing hormone profiles during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology. 1988;47:133–141. [DOI] [PubMed] [Google Scholar]
- 12. Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. [DOI] [PubMed] [Google Scholar]
- 14. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci U S A. 2007;104:8173–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. [DOI] [PubMed] [Google Scholar]
- 17. Mayer C, Acosta-Martinez M, Dubois SL, et al. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci U S A. 2010;107:22693–22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramirez VD, Sawyer CH. Differential dynamic responses of plasma LH and FSH to ovariectomy and to a single injection of estrogen in the rat. Endocrinology. 1974;94:987–993. [DOI] [PubMed] [Google Scholar]
- 19. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chappell PE, Schneider JS, Kim P, et al. Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology. 1999;140:3653–3658. [DOI] [PubMed] [Google Scholar]
- 21. Paxinos GAF. The Mouse Brain in Stereotaxic Coordinates. 2nd ed San Diego: Academic Press; 2001. [Google Scholar]
- 22. Gottsch ML, Navarro VM, Zhao Z, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wintermantel TM, Campbell RE, Porteous R, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adachi S, Yamada S, Takatsu Y, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. [DOI] [PubMed] [Google Scholar]
- 26. Kinoshita M, Tsukamura H, Adachi S, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. [DOI] [PubMed] [Google Scholar]
- 27. Pineda R, Garcia-Galiano D, Roseweir A, et al. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;151:722–730. [DOI] [PubMed] [Google Scholar]
- 28. Simerly RB. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res. 1998;92:195–203. [DOI] [PubMed] [Google Scholar]
- 29. Kauffman AS, Gottsch ML, Roa J, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. [DOI] [PubMed] [Google Scholar]
- 30. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology. 2009;150:3214–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. [DOI] [PubMed] [Google Scholar]
- 34. Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152:2387–2399. [DOI] [PubMed] [Google Scholar]
- 35. Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47:198–218. [DOI] [PubMed] [Google Scholar]
- 36. Williams WP, 3rd, Kriegsfeld LJ. Circadian control of neuroendocrine circuits regulating female reproductive function. Front Endocrinol (Lausanne). 2012;3:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod. 2006;75:778–784. [DOI] [PubMed] [Google Scholar]
- 38. Funabashi T, Aiba S, Sano A, Shinohara K, Kimura F. Intracerebroventricular injection of arginine-vasopressin V1 receptor antagonist attenuates the surge of luteinizing hormone and prolactin secretion in proestrous rats. Neurosci Lett. 1999;260:37–40. [DOI] [PubMed] [Google Scholar]
- 39. Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res. 2001;901:109–116. [DOI] [PubMed] [Google Scholar]
- 40. Vida B, Deli L, Hrabovszky E, et al. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol. 2010;22:1032–1039. [DOI] [PubMed] [Google Scholar]
- 41. Williams WP, 3rd, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology. 2011;152:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150:3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tomikawa J, Uenoyama Y, Ozawa M, et al. Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc Natl Acad Sci U S A. 2012;109:E1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piet R, Boehm U, Herbison AE. Estrous cycle plasticity in the hyperpolarization-activated current ih is mediated by circulating 17β-estradiol in preoptic area kisspeptin neurons. J Neurosci. 2013;33:10828–10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang C, Tonsfeldt KJ, Qiu J, et al. Molecular mechanisms that drive estradiol-dependent burst firing of Kiss1 neurons in the rostral periventricular preoptic area. Am J Physiol Endocrinol Metab. 2013;305:E1384–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bakker J, Pierman S, Gonzalez-Martinez D. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm Behav. 2010;57:390–395. [DOI] [PubMed] [Google Scholar]
- 47. Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One. 2010;5:e11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim J, Tolson KP, Dhamija S, Kauffman AS. Developmental GnRH signaling is not required for sexual differentiation of kisspeptin neurons but is needed for maximal Kiss1 gene expression in adult females. Endocrinology. 2013;154:3273–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yeo SH, Herbison AE. Estrogen-negative feedback and estrous cyclicity are critically dependent upon estrogen receptor-α expression in the arcuate nucleus of adult female mice. Endocrinology. 2014;155:2986–2995. [DOI] [PubMed] [Google Scholar]
- 50. de Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology. 2012;153:5384–5393. [DOI] [PubMed] [Google Scholar]
- 51. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27:1913–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kelly MJ, Loose MD, Ronnekleiv OK. Estrogen suppresses mu-opioid- and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Novaira HJ, Sonko ML, Hoffman G, et al. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol. 2014;28:225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol. 2009;21:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]