Abstract
Environmental endocrine disruptors are implicated as putative contributors to the burgeoning metabolic disease epidemic. Tolylfluanid (TF) is a commonly detected fungicide in Europe, and previous in vitro and ex vivo work has identified it as a potent endocrine disruptor with the capacity to promote adipocyte differentiation and induce adipocytic insulin resistance, effects likely resulting from activation of glucocorticoid receptor signaling. The present study extends these findings to an in vivo mouse model of dietary TF exposure. After 12 weeks of consumption of a normal chow diet supplemented with 100 parts per million TF, mice exhibited increased body weight gain and an increase in total fat mass, with a specific augmentation in visceral adipose depots. This increased adipose accumulation is proposed to occur through a reduction in lipolytic and fatty acid oxidation gene expression. Dietary TF exposure induced glucose intolerance, insulin resistance, and metabolic inflexibility, while also disrupting diurnal rhythms of energy expenditure and food consumption. Adipose tissue endocrine function was also impaired with a reduction in serum adiponectin levels. Moreover, adipocytes from TF-exposed mice exhibited reduced insulin sensitivity, an effect likely mediated through a specific down-regulation of insulin receptor substrate-1 expression, mirroring effects of ex vivo TF exposure. Finally, gene set enrichment analysis revealed an increase in adipose glucocorticoid receptor signaling with TF treatment. Taken together, these findings identify TF as a novel in vivo endocrine disruptor and obesogen in mice, with dietary exposure leading to alterations in energy homeostasis that recapitulate many features of the metabolic syndrome.
Obesity and diabetes rates across the globe have increased dramatically over the last 3 decades (1, 2). Current estimates place the worldwide burden of diabetes at 382 million individuals, with projections that this number will increase to a staggering 592 million people by 2035 (3). The individual and societal consequences of this deterioration in metabolic health are enormous. In the United States, diabetes is the leading cause of adult blindness, kidney failure, and nontraumatic amputations while contributing $245 billion annually to healthcare costs (4, 5). Thus, understanding the pathogenic factors that contribute to metabolic dysregulation is critical for alleviating individual suffering and improving public health.
Physical inactivity and excess caloric consumption are central drivers of the obesity and diabetes epidemics; however, increasing evidence suggests that additional factors promote metabolic dysfunction. Emerging data implicate environmental pollutants in the development of obesity, insulin resistance, and diabetes (6–8). Over the last several decades, obesity and diabetes trends mirror synthetic chemical production in the United States (8, 9), and similar increases in metabolic disease are occurring in Europe and across the globe, suggesting that similar relationships are active worldwide (3, 10). Moreover, epidemiological studies support associations between certain pollutants and various measures of metabolic dysregulation (8, 11, 12). Finally, basic science data show that a host of environmental toxicants can disrupt energy metabolism in model organisms through perturbations of multiple molecular pathways, including insulin and thyroid hormone action as well as glucocorticoid receptor (GR) and peroxisome proliferator-activated receptor (PPAR)γ signaling (13).
Given the close relationship between obesity and diabetes, endocrine-disrupting chemicals (EDCs) that lead to adipose tissue dysregulation may contribute to the pathogenesis of diabetes. Adipose tissue plays a critical role in maintaining energy homeostasis through the storage of excess calories during feeding and the mobilization of those stores upon fasting. In addition to its role as a dynamically regulated energy depot, adipose tissue also regulates global metabolism through the secretion of several hormones, ie, adipokines, that influence food intake/satiety, insulin sensitivity, and insulin secretion (14). Moreover, because many metabolic disruptors are lipophilic, they are expected to bioaccumulate in lipid-rich tissues, such as adipose. Thus, disruption of adipose tissue function may be a central mechanism by which EDCs promote the development of metabolic disease (15, 16). Importantly, GR signaling plays a critical and complex role in regulating multiple aspects of adipose function, including adipogenesis, insulin action, and lipid storage (17). Taken together, this suggests that lipophilic EDCs that modulate GR action may have particularly deleterious effects on adipose biology and global energy regulation.
Tolylfluanid (TF) (Euparen Multi) is a phenylsulfamide fungicide used in agriculture and as a booster biocide in marine paints (18, 19). It is one of the most commonly found pesticides in agricultural goods in Europe (20–22) and has been detected in groundwater in agricultural regions (23). The extent of exposure may be more global, however, because TF and another structurally similar compound, dichlofluanid, have been detected in the coastal waters of India (24). In addition to exposure through contaminated food and water, occupational exposure is expected among those employed in its application in agriculture and the shipping industry (25, 26). Importantly, TF is highly lipophilic (log octanol to water partition coefficient of 3.9) (18), suggesting that it may accumulate in lipid-rich tissues such as adipose. In previous studies, TF has been shown to promote preadipocyte to adipocyte differentiation in the 3T3-L1 cell line (27) and insulin resistance in primary murine and human adipose tissue exposed ex vivo (28). Furthermore, work has shown that the metabolic disruption induced by TF is likely mediated via inappropriate activation of GR signaling (27, 29). The present study extends this previous work to explore TF effects on adipose tissue mass and function as well as systemic energy metabolism after chronic dietary exposure to this novel metabolic disruptor.
Materials and Methods
Animals, exposure, and tissue processing
Eight-week-old male C57BL/6 mice were obtained from The Jackson Laboratory and housed in groups of 2–4 at 22.2 ± 1.1°C under a 12-hour light, 12-hour dark cycle. Control animals received a standard chow diet ad libitum (Teklad Global Diet 2018; Harlan Laboratories) containing 23.0% protein, 60.6% carbohydrate, and 16.5% fat (by kcal). Animals exposed to TF received the identical diet supplemented with 100 parts per million (ppm) TF added at the time of manufacturing (Harlan Laboratories). Animals were handled and weighed biweekly, and food was weighed and replaced weekly. Animals were treated humanely in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Chicago. At the conclusion of the 12-week exposure period, animals were euthanized with isoflurane followed by exsanguination via cardiac puncture. Relevant metabolic tissues were dissected, weighed, and flash frozen in liquid nitrogen followed by storage at −80°C before processing. Liver samples for histological analysis were frozen in Tissue-Tek O.C.T. Compound on dry ice before cryosectioning and staining with hematoxylin and eosin (H&E) or oil red O. Adipose samples for histology were fixed in 10% formalin for 24 hours before paraffin embedding and staining with H&E.
Intraperitoneal-glucose tolerance test (GTT)
At 4 and 9 weeks of exposure, mice were fasted for 6 hours beginning at 7:30 am. Fasting blood glucose levels were measured using a Freestyle Lite glucometer (Abbott Laboratories). Mice then received an ip injection of dextrose (2 g/kg body weight) with blood glucose concentrations measured serially for 120 minutes by tail snip. Blood samples (20–25 μL) for insulin levels were obtained using heparinized tubes at 0, 10, 30, and 60 minutes, placed immediately on ice, centrifuged at 1500g for 15 minutes at 4°C, and concentrations determined using an insulin ELISA kit per the manufacturer's instructions (Millipore).
Intraperitoneal-insulin tolerance test (ITT)
At 5 and 10 weeks of exposure, mice were fasted at 9 am for 3 hours. Fasting blood glucose levels were measured, followed by ip injection of Humalog insulin (0.4 U/kg body weight at 5 wk and 0.5 U/kg body weight at 10 wk; Eli Lilly). At 5 weeks, 2 out of 3 control mice experienced blood glucose drops below the limits of detection for the glucometer (20 mg/dL per the manufacturer) when 0.5 U/kg body weight was administered, whereas 0 out of 3 TF-treated mice exhibited this extreme drop; therefore, the dose was reduced at this time point for the remaining mice (8 control and 7 TF treated). At 10 weeks, mice with blood glucose readings that dropped below the limits of detection for the glucometer (20 mg/dL per the manufacturer) were confirmed on repeat testing and, if confirmed, recorded as having blood glucose levels of 20 mg/dL (2 of 9 controls, 0 of 12 TF treated). These mice subsequently received an ip injection of dextrose, and future data points were excluded from the analysis.
Measurement of body composition by dual energy x-ray absorptiometry (DEXA)
Assessment was performed at 0, 8, and 12 weeks of exposure with the assistance of the Metabolic Testing Facility of the Diabetes Research and Training Center at the University of Chicago. Mice were anesthetized before imaging by injection of ketamine (80 mg/kg) and xylazine (5 mg/kg) into the intrascapular region. Body composition was measured by DEXA (Lunar PIXImus densitometer system; GE Healthcare) using PIXImus 2 software after system calibration according to the manufacturer's instructions. Body weight and body length were also assessed after sedation.
Assessment of metabolic rate and fuel use by indirect calorimetry
After 12 weeks of dietary exposure, indirect calorimetric measurements were carried out using the LabMaster System (TSE Systems) maintained under standard housing conditions (22.2 ± 1.1°C; 12-h light, 12-h dark cycle). Mice were provided with ad libitum access to their treatment diets. After a 4-day acclimation period, oxygen consumption, carbon dioxide production, energy expenditure, activity, as well as food and water consumption were monitored for 2.5 days. The respiratory exchange ratio (RER) was measured as the ratio of O2 inhalation to CO2 production over a 30-minute period. RER values for light/dark phases represent the average RER of all data points collected every 30 minutes over that respective 12-hour period.
Adipocyte insulin signaling and immunoblotting
At harvesting, perigonadal fat was assessed for insulin sensitivity by the ratio of phosphorylated to total Akt (protein kinase B) as previously described (28). After membrane development with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific), densitometry was measured using ImageJ version 1.47 (National Institutes of Health). Insulin sensitivity was assessed as the ratio of the total area of bands corresponding to phosphorylated Akt (S473) over total Akt, normalized within each animal to the basal condition (0nM insulin) to assess relative insulin responsiveness.
Gene expression analyses
RNA extraction and quantitative RT-PCR were performed as previously described with 260/280-nm values of approximately 2.0 to ensure RNA purity (28). Primers were generated by Primer-BLAST (National Center for Biotechnology Information) and confirmed to have amplification efficiency of 90%–110%. Primer sequences (Integrated DNA Technologies) can be found in Supplemental Table 1. Gene expression levels were evaluated by the Δ-Ct method (30), with β-actin used to control for total mRNA recovery, and control values normalized to a group average of 1.0.
Serum analysis of adipokines and metabolites
At the time of euthanization, blood was obtained via cardiac puncture. Blood was allowed to clot at room temperature for 30 minutes, followed by centrifugation at 1500g for 15 minutes at 4°C. Sera were then collected and stored at −80°C before analysis. Serum parameters were quantified using commercially available ELISA kits (leptin [Crystal Chem, Inc] and adiponectin [Millipore]) or a colorimetric assay kit (free fatty acids [Gentaur]) according to the manufacturers' instructions.
Microarray analysis of gene expression
The quality of total RNA was checked using the Agilent Bio-Analyzer. Total RNA was processed into biotinylated cRNA using the Ambion TotalPrep-96 RNA Amplification kit (Life Technologies). Microarray hybridization, processing, and scanning were performed using standard Illumina-provided protocols. A total of 12 samples (6 biological replicates each from control and TF-exposed perigonadal adipose tissue after 4 wk of treatment) were profiled by Illumina MouseRef-8 v2.0 Expression BeadChip array using the Illumina HiScan Array Scanner. The raw probe average signal intensities were background subtracted, log2 transformed, and quantile normalized using Illumina Genome Studio v2011.1. The normalized expression data were further processed by retaining probes with the detection P ≤ .01, followed by batch effects removal using the ComBat program (31). Processed average signal intensities of 10 149 probes were used for further statistical analysis. ANOVA and multiple testing corrections to detect differentially expressed genes between TF and control groups were performed. Differentially expressed genes with different cut-off criteria were generated for each pairwise comparison. To investigate whether GR and PPARγ pathway genes were overrepresented in the expression data from 4-week treatment and control groups, probes without Entrez gene identifiers were removed followed by collapsing multiple probes that are mapped to the same Entrez gene by only retaining the probe with largest variance across the samples. Gene Set Enrichment Analysis (GSEA) was performed on the 7580 genes using the GSEA program (32).
Statistical analysis
Adiposity was defined as adipose tissue weight divided by total body weight. Three independent 12-week exposure studies were performed using separately prepared diets (n for each group of 8 in trials 1 and 2, and 12 in trial 3). Relative to control mice, TF exposure consistently increased adiposity, our primary outcome measure, with no differences in the magnitude of increase across the 3 cohorts (P = .61); therefore, data were pooled from all studies. Weight gain was assessed as percent body weight gain for 12 weeks of exposure (P < .05), followed by a breakdown of weight gain into 2 segments (wk 0–4 and wk 5–12), and assessed by two-way ANOVA. Weekly food consumption was assessed by two-way ANOVA. Glucose and insulin tolerance were measured as the area under the curve (AUC) of glucose over time by the trapezoidal method. Data are presented as mean ± SEM. Control and TF treatment groups were compared by F testing to determine differences in variance; for F < 0.05, t tests were performed with Welch's correction, whereas when F > 0.05, standard Student's t tests were performed. No correction for multiple comparisons was made to reduce type 2 error as has been suggested (33). All analyses were performed using GraphPad Prism version 6.0. P < .05 was considered statistically significant.
Results
TF promotes weight gain and increases adiposity
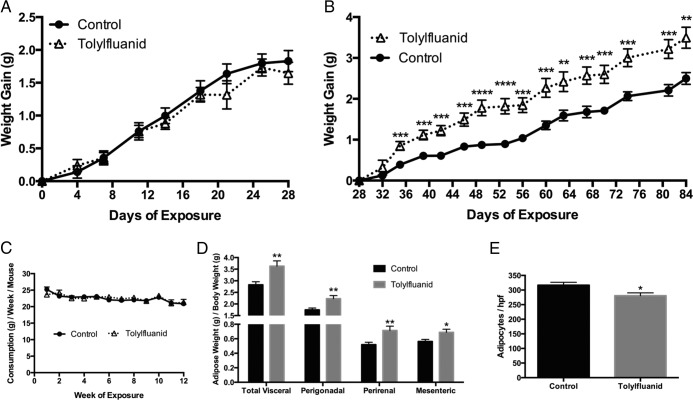
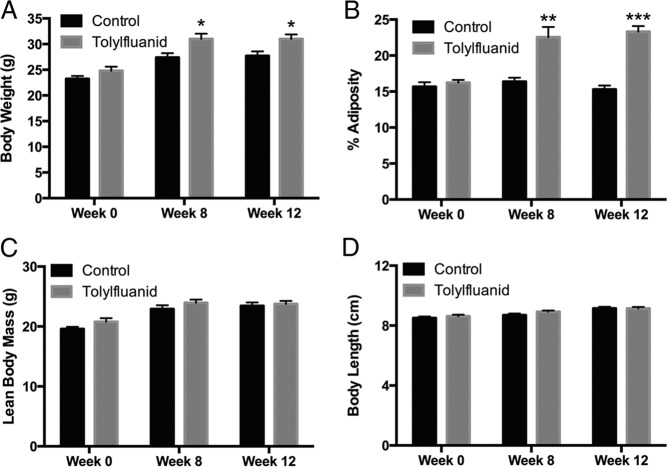
Previously, TF was shown to promote preadipocyte to adipocyte differentiation (27) as well as adipocytic insulin resistance in an ex vivo exposure model (28) through activation of GR signaling (27, 29). However, in vivo energy homeostasis requires the coordinate action of multiple tissues, and human exposure to TF likely occurs through contaminated food and water; therefore, studies were conducted to assess the metabolic impact of chronic dietary TF exposure in intact organisms. Toward this goal, 8-week-old male mice were fed a standard chow diet (control) or the same diet supplemented with 100 ppm TF ad libitum for 12 weeks. This concentration was estimated to provide exposure at or below reported no observed adverse effect levels (34). Although no differences in body weight were observed for the first month of treatment (P = .59) (Figure 1A), TF-exposed mice exhibited augmented weight gain by 35 days of exposure, an effect that persisted until the end of the study, ultimately resulting in a 19% increase in weight gain (P = .0001) (Figure 1B). This augmented weight gain occurred without a change in weekly food consumption (P = .89) (Figure 1C). Because TF promotes adipocyte differentiation, it was hypothesized that this weight gain might result from increased adipose mass. At the conclusion of the study, mice were euthanized and 3 adipose depots (perigonadal, perirenal, and mesenteric) quantitatively harvested and weighed. TF-exposed mice exhibited a 28% increase in overall adiposity (P < .01), and increased depot-specific adiposity in all 3 depots measured (P < .05) (Figure 1D). Histological analysis of perigonadal adipose tissue revealed adipocyte hypertrophy with a reduction in adipocyte number per high-power field (P < .05) (Figure 1E). To assess the possibility that these changes in adiposity occurred at earlier time points of exposure, serial DEXA scans were performed in a separate cohort of mice at 0, 8, and 12 weeks of exposure. These scans identified a TF-induced augmentation of body weight (P < .05) (Figure 2A) and adiposity (P < .01) (Figure 2B) by 8 weeks of exposure. Importantly, this increase in body weight and adiposity occurred without concurrent increases in lean body mass (P = .24) or body length (P = .14) (Figure 2, C and D, respectively). These increases in body weight and adiposity persisted at 12 weeks of TF exposure, with exposed mice ultimately exhibiting a 52% increase in fat content by DEXA (P < .001). To assess for lipid accumulation in other metabolic tissues, histological analysis of liver sections was performed; however, no difference in hepatic steatosis was observed (data not shown). These studies indicate that dietary TF exposure promotes the accretion of fat mass; moreover, the DEXA scanning data suggest that this increase occurs by 8 weeks of exposure and includes fat expansion that extends beyond the directly measured depots.
Figure 1.
Dietary consumption of TF promotes increased body weight gain and adiposity. Mice were provided a chow diet or the same diet fortified with 100 ppm TF for 12 weeks (n = 28 per group), and body weight was measured biweekly, revealing a 19% increase in body weight (data not shown; P < .05). Weight gain was plotted over time from baseline to 28 days (RM two-way ANOVA; P = .59) (A), and from 28 to 84 days (RM two-way ANOVA; P = .0001) (B). Weekly food consumption by cage was measured and is presented as food consumption in grams per mouse per week (RM two-way ANOVA; P = .89) (C). At killing, 3 visceral adipose depots (perigonadal, perirenal, and mesenteric) were harvested and weighed followed by correction for body weight to provide a measure of adiposity (D). At harvest, a portion of the perigonadal adipose depot was fixed and stained with H&E followed by counting of adipocytes per high-power field (8 mice per group with 3 separate high-power fields measured per mouse). Data are presented as mean ± SEM. *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001.
Figure 2.
TF-induced augmentation of adiposity by DEXA scanning. Serial DEXA scanning was performed at baseline (wk 0) as well as after 8 and 12 weeks of exposure to a chow diet or the same diet fortified with 100 ppm TF (n = 4 per group with the same mice measured at each time point). Body weight (A), adiposity (B), lean body mass (C), and body length (D) were all assessed at these time points. Data are presented as mean ± SEM. *, P < .05; **, P < .01; ***, P < .001.
TF exposure promotes glucose intolerance and insulin resistance
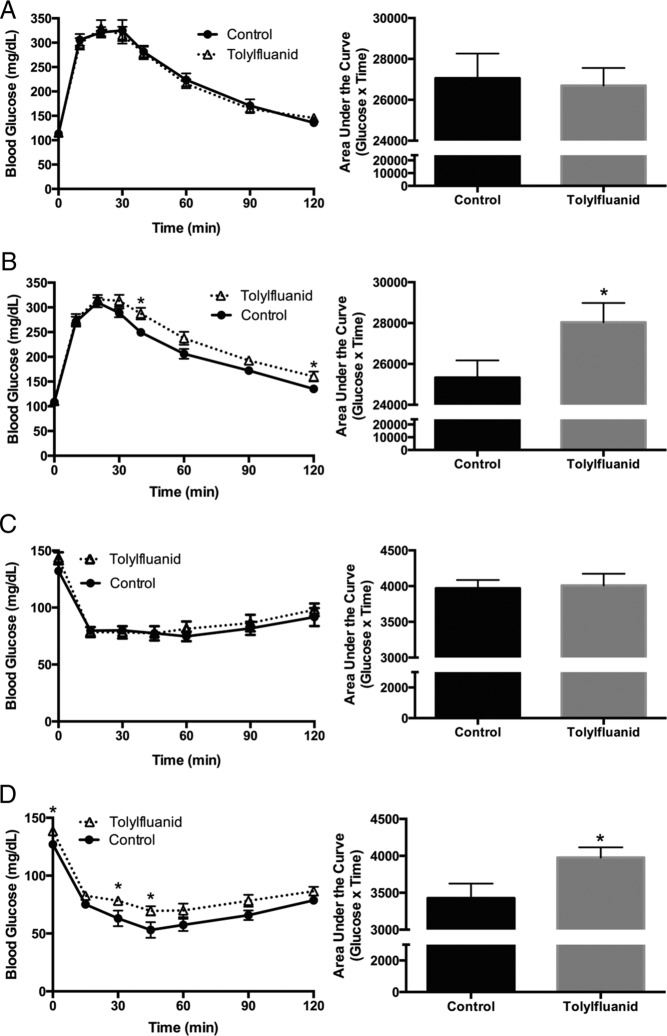
To determine whether TF-induced adipose expansion promoted global alterations in glucose homeostasis and insulin sensitivity, glucose and insulin tolerance tests were performed. After 4 and 9 weeks of exposure, an ip-GTT was performed after a 6-hour fast. At 4 weeks of exposure, no significant differences between treated and control mice were identified (P = .81) (Figure 3A). However, after 9 weeks on their respective diets, TF-exposed mice exhibited relative glucose intolerance as evidenced by a higher AUC during the ip-GTT (P < .05) (Figure 3B). Insulin levels at 0, 10, 30, and 60 minutes revealed TF mice had higher average insulin levels at all time points examined, although differences were not significant (P = .41) (data not shown), suggesting that the observed glucose intolerance was not due to overt β-cell failure, but rather resulted from an impairment in the response of peripheral tissues to insulin stimulation. To assess whether TF altered global insulin sensitivity, insulin tolerance tests were performed after 5 and 10 weeks of exposure after a 3-hour fast. At 5 weeks of exposure, no significant differences between groups were identified (P = .84) (Figure 3C). However, after 10 weeks, TF-exposed mice demonstrated relative insulin resistance as assessed by the AUC through 45 minutes, as well as 3-hour fasting hyperglycemia (P < .05) (Figure 3D). These data demonstrate that dietary exposure to TF disrupts global energy metabolism, rendering the animals more glucose intolerant with reduced systemic insulin sensitivity.
Figure 3.
TF-exposed mice develop glucose intolerance and insulin resistance. An ip-GTT was performed at 4 weeks of exposure by ip injection of glucose (2 g/kg) with serial blood glucose measurements taken for 120 minutes (n = 9 per group) (A). An ip-GTT was also performed at 9 weeks of exposure (n = 9 control; 10 TF) (B). Glucose tolerance was measured as the AUC of glucose over time for 120 minutes by the trapezoidal method. An ip-ITT was performed at 5 weeks of exposure by ip injection of insulin (0.4 U/kg) and serial blood glucose measured for 120 minutes (n = 8 control; 7 TF) (C). An ITT was also performed at 10 weeks of exposure with a higher insulin dose (0.5 U/kg). Mice with blood glucose readings that dropped below the limits of detection for the glucometer (20 mg/dL per the manufacturer) and confirmed on repeat testing were recorded as having blood glucose levels of 20 mg/dL, and were immediately injected with a bolus of dextrose and future time points excluded (n = 9 controls through 45 min, 7 after 45 min; 12 TF throughout) (D). Insulin tolerance was measured as the AUC of glucose over time for the first 45 minutes of the study by the trapezoidal method. Data are presented as mean ± SEM. *, P < .05.
TF exposure impairs metabolic flexibility, promotes dark-phase lipid use, and alters the diurnal rhythm of energy expenditure and food intake
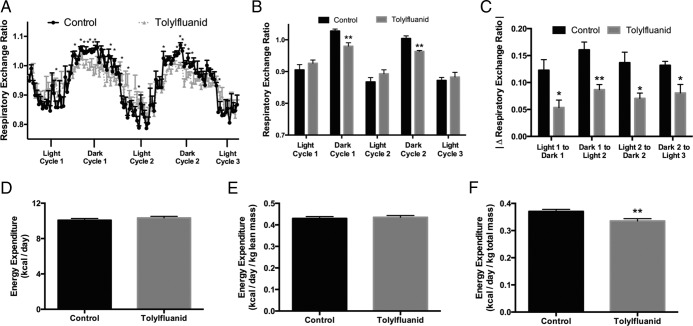
Disruptions in insulin sensitivity promote shifting of energy use from carbohydrate to fat while perturbing energy expenditure (reviewed in Ref. 35). To determine whether TF in the diet promoted shifts in fuel use and energy expenditure, mice were placed in metabolic cages after 12 weeks of exposure. The RER is a measure of relative nutrient use, with values near 0.8 suggesting fat as the primary energy source and values near 1.0 suggesting carbohydrates as the predominant energy source. In mice, RER values are typically higher during the dark phase, when mice consume a majority of their daily calories, and lower during the light phase (Figure 4A). During the dark phase, TF-exposed mice were found to have a reduced RER, demonstrating a relative preference for lipid use as an energy substrate compared with control mice (P < .01) (Figure 4B). In addition, examination of shifts in RER between fed and fasted states revealed that TF impaired the ability to shift between fuel sources, on average by 47%, demonstrating relative metabolic inflexibility upon exposure to this EDC (P < .05) (Figure 4C). Uncorrected and lean mass-corrected energy expenditure did not differ with TF treatment (Figure 4, D and E, respectively); however, total body mass-corrected energy expenditure was reduced in the TF-exposed group, likely reflecting the accumulation of less metabolically active adipose mass (P < .01) (Figure 4F).
Figure 4.
TF exposure promotes lipid use, impairs metabolic flexibility, and impairs body weight-adjusted daily energy expenditure. After 12 weeks of consumption of a chow diet or diet fortified with 100 ppm TF, mice were placed in metabolic cages for 1 week. Data were collected after a 4-day acclimation period. The RER was calculated as the ratio of carbon dioxide exhaled to oxygen inhaled and measured in 30-minute time increments. Of the 111 time points analyzed, 21 were significantly different between groups (n = 4 per group) (A). RER values for light/dark phases represent the average RER of all data points collected every 30 minutes over that respective 12-hour period (B). Metabolic flexibility was defined as the change in average RER between sequential light and dark states with the absolute value of the difference taken in order to yield consistent directionality (C). Daily energy expenditure (D), lean body mass-adjusted energy expenditure (E), and total body mass-adjusted energy expenditure (F) were calculated (4 mice per group with 2 data points taken from each mouse over 2 distinct, nonoverlapping 24-h periods). Data are presented as mean ± SEM. *, P < .05; **, P < .01.
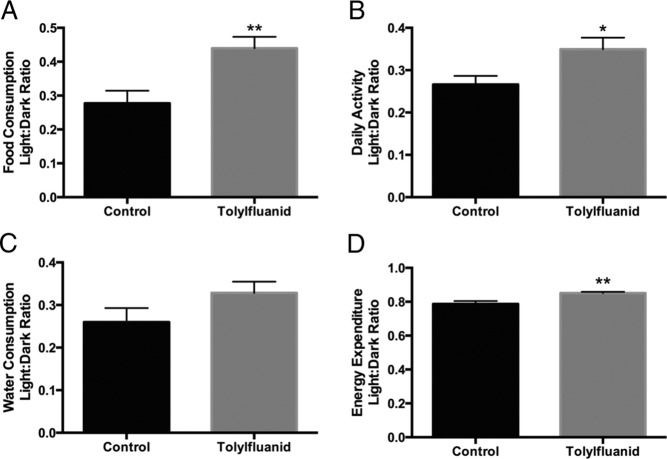
Although total food intake over the 12-week study was not altered by TF exposure, analysis in the metabolic cages demonstrated a difference in the diurnal rhythm of food consumption with TF-exposed mice consuming more of their daily calories during the light phase than control mice (P < .01) (Figure 5A). Relative light cycle water intake trended higher but did not reach statistical significance (P = .13) (Figure 5C). Although total activity was no different between groups, there was an increase in the fraction of activity occurring during the light phase with TF treatment (P < .05) (Figure 5B). There was also a modest increase in energy expenditure during the light phase that may reflect this increase in activity (P < .01) (Figure 5D). These data suggest that exposure to TF disrupts fuel use, resulting in a metabolic state that favors lipid utilization with a coordinate impairment in the ability to toggle between fat and carbohydrate use during fed-fasted transitions. Furthermore, these data suggest that dietary TF intake may perturb circadian rhythms.
Figure 5.
TF-exposed mice exhibit disruptions in the diurnal variation of food consumption, activity, and energy expenditure. After 12 weeks of consumption of a chow diet or the same diet fortified with 100 ppm TF, mice were placed in metabolic cages for 1 week. Data were collected after a 4-day acclimation period (4 mice per group with 2 data points collected for each mouse over 2 distinct, nonoverlapping 24-h periods). Daily food and water consumption, activity, and energy expenditure did not differ between groups (data not shown). To assess the diurnal distribution of these measures, the ratio of the light to dark phase was calculated for food consumption (A), activity (B), water consumption (C), and energy expenditure (D). Data are presented as mean ± SEM. *, P < .05; **, P < .01.
TF disrupts circulating adiponectin and leptin levels and induces tissue-level insulin resistance
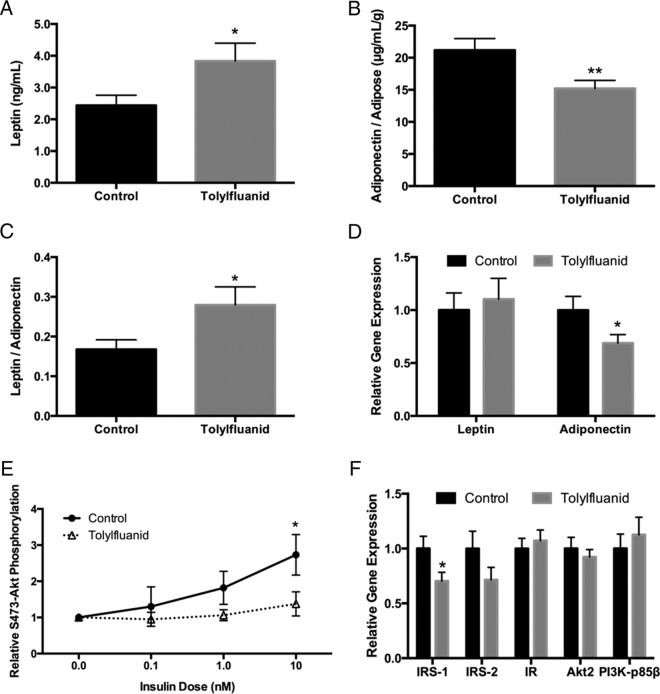
Adipose tissue is now a well-recognized endocrine organ that exerts effects on systemic energy metabolism through the secretion of various adipokines, including leptin and adiponectin (36). In ex vivo studies, 48-hour exposure of adipose tissue to TF decreased both basal and insulin-stimulated leptin secretion (28). To determine whether chronic dietary TF exposure similarly perturbed adipose endocrine function, fasting serum measurements of leptin and adiponectin were performed after 12 weeks. TF-exposed mice demonstrated increased serum leptin levels (P < .05) (Figure 6A). Because leptin is secreted in proportion to fat mass, this finding was consistent with data showing that TF exposure promotes accretion of adipose tissue. When corrected for the increased adiposity seen in exposed mice, TF reduced the relative production of the metabolically beneficial adipokine adiponectin (P < .01) (Figure 6B). This resulted in a 67% increase in the leptin to adiponectin ratio (LAR) (P < .05) (Figure 6C); importantly, an increase in LAR has been identified as a biomarker for insulin resistance (37, 38), the metabolic syndrome (39), and increased cardiovascular risk (40). Together, these data suggest that mice exposed to TF in the diet have a disrupted adipokine profile with a relative impairment in adiponectin secretion. These findings were confirmed by gene expression analysis, which revealed a 31% reduction in adiponectin gene expression in perigonadal fat (P < .05) (Figure 6D).
Figure 6.
TF exposure perturbs the balance in circulating adipokines and impairs adipose insulin sensitivity through a reduction in IRS-1 gene expression. At harvest, blood was collected via cardiac puncture and serum assessed by ELISA for levels of the circulating adipokines leptin (n = 18) (A) and adiponectin. Serum adiponectin levels were corrected for adipose mass (n = 19 control, 20 TF) (B). The LAR was also calculated based on serum levels (n = 17 control, 18 TF) (C). At euthanasia, perigonadal adipose tissue was stimulated with insulin for 10 minutes. Protein was collected, processed, and resolved by Western blotting. Insulin sensitivity was assessed as the ratio of the band sizes of phosphorylated to total Akt at the S473 site (n = 13 control, 15 TF; except n = 7 for 0.1nM insulin) (E). Gene expression of leptin and adiponectin (D) and insulin signaling intermediates (F) was interrogated in perigonadal adipose tissue, normalizing to β-actin as a reference gene to control for RNA recovery (n = 16 per group). Data are presented as mean ± SEM. For gene expression, values were normalized to a control group average of 1.0. For insulin signaling, values have been normalized to the basal condition within each mouse to identify relative insulin responsiveness. *, P < .05; **, P < .01. IR, insulin receptor; PI3K, phosphatidylinositol 3-kinase.
Previous work demonstrated that direct treatment of adipose tissue explants to TF promoted adipocytic insulin resistance through a specific reduction in levels of insulin receptor substrate (IRS)-1 (28). To determine whether in vivo exposure recapitulated the previous ex vivo model, the effects of dietary TF on adipocytic insulin action were assessed in perigonadal adipose tissue. Insulin-stimulated Akt phosphorylation serves as an assessment of upstream insulin signaling events, providing an integrative cellular marker of adipocytic insulin action from insulin binding to its receptor to Akt phosphorylation, including phosphorylation of IRS-1, activation of phosphatidylinositol 3-kinase, and Akt activation (28). The S473 phosphorylation site of Akt was investigated, with TF-exposed mice exhibiting an attenuation of insulin-stimulated Akt phosphorylation at an insulin concentration of 10nM (P < .05) (Figure 6E). To determine whether this reduction in insulin sensitivity was a result of changes in gene expression, multiple insulin signaling genes upstream of Akt were interrogated. In accord with ex vivo studies (28), a specific 30% reduction in IRS-1 was identified, without significant reductions in the other insulin signaling genes investigated (P < .05) (Figure 6F). These data demonstrate that systemic exposure to TF recapitulates changes in cellular physiology induced by direct effects of TF on adipose tissue and suggests one mechanism by which global insulin action may be attenuated in the presence of this novel metabolic disruptor.
TF exposure disrupts expression of genes regulating fatty acid oxidation (FAO) and lipolysis
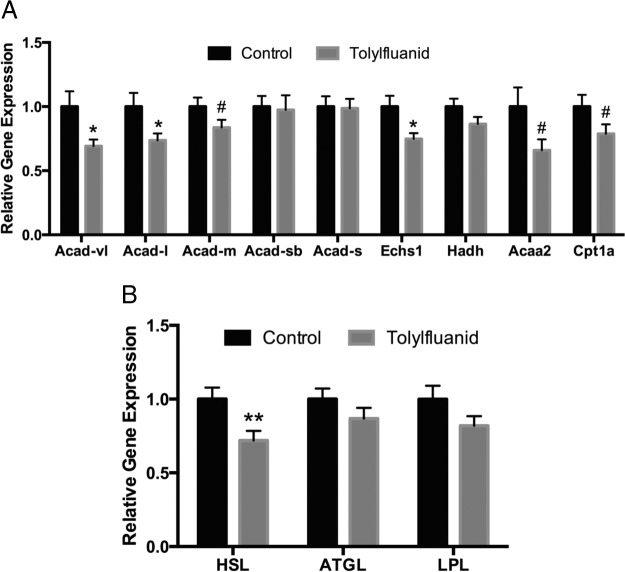
Adipose expansion can result from increased lipid synthesis and esterification or a reduction in lipolysis and FAO. To gain insights into the mechanisms by which TF modulates adipose tissue mass, perigonadal adipose expression of genes regulating lipid handling was investigated. Importantly, in contrast to ex vivo studies demonstrating that TF increases gene expression of the fatty acid synthesis genes acyl-coenzyme A (CoA) carboxylase and stearoyl-CoA desaturase-1 (29), expression of these genes was unaffected by in vivo exposure (data not shown). However, 3 genes in the FAO pathway were significantly reduced: 2 isoforms of acyl-CoA dehydrogenase (Acad), the Acad (very long chain) and Acad (long chain) forms, as well as enoyl-CoA hydratase 1 (P < .05) (Figure 7A). Importantly, 3 other FAO genes demonstrated a trend toward down-regulation in exposed mice at P < .10: the medium chain Acad isoform, carnitine palmitoyltransferase 1a, and acetyl-CoA acyltransferase 2. Finally, expression of hormone-sensitive lipase (HSL), a critical regulator of lipolysis, was significantly reduced (P < .01), whereas adipose triglyceride lipase (ATGL) was not altered (Figure 8B). Together, these findings support a model in which fat oxidation and lipolysis are impaired in adipose tissue after exposure to TF, thus supporting the accumulation of adipose mass.
Figure 7.
TF exposure promotes adipose accumulation through a reduction in FAO and lipolysis. At euthanasia, perigonadal fat was processed for RNA and pathways relevant to lipid metabolism were investigated, normalizing to β-actin as a reference gene to control for RNA recovery (n = 16 per group). Genes important in fatty acid synthesis (data not shown), FAO (A), and lipolysis (B) were interrogated. Data are presented as mean ± SEM normalized to a control group average of 1.0. #, P < .10; *, P < .05; **, P < .01. Acaa2, acetyl-CoA acyltransferase 2; Acad-l, acyl-CoA dehydrogenase (long chain); Acad-m, Acad (medium chain); Acad-s, Acad (short chain); Acad-sb, Acad (short/branched chain); Acad-vl, Acad (very long chain); Cpt1a, carnitine palmitoyltransferase 1a; Echs1, enoyl-CoA hydratase 1; Hadh, hydroxyacyl-CoA dehydrogenase; LPL, lipoprotein lipase.
Figure 8.
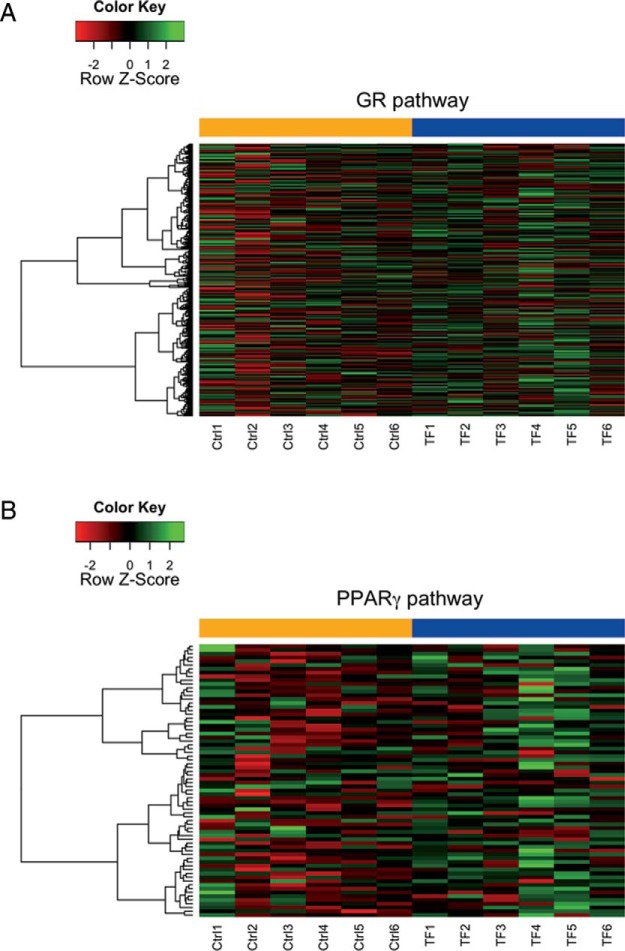
TF stimulates GR activity in the adipose tissue of exposed mice. After 4 weeks of consumption of a chow diet or the same diet fortified with 100 ppm TF, mice were harvested and perigonadal adipose depots collected and processed for RNA (n = 6 per group). Samples were profiled by Illumina MouseRef-8 v2 expression array and investigated for GR and PPARγ pathway enrichment. Mice exposed to TF showed significant enrichment in GR-dependent gene expression (nominal P < .001; 533 unique Entrez genes) (A), but not PPARγ-dependent gene expression, although TF mice did trend towards enrichment (nominal P = .08; 91 unique Entrez genes) (B).
Dietary TF exposure activates GR signaling
In previous work it was shown that TF promoted GR signaling in an ex vivo exposure model of primary adipose tissue (29); however, in vivo mechanisms may differ. To determine whether GR signaling was activated by dietary TF exposure, adipose tissue was harvested after 4 weeks of treatment, the time at which body weight begins to diverge between the control and TF-treated mice (Figure 1B). Based on published gene sets for ligand-mediated GR (41) and PPARγ (42) activation in murine 3T3-L1 adipocytes, GSEA was performed using microarray data to determine whether TF activated GR signaling, PPARγ signaling, or both. At 4 weeks of exposure, TF significantly up-regulated GR-dependent gene expression (nominal P < .001) (Figure 8A). Although PPARγ-dependent gene expression trended toward an overall increase (Figure 8B), this activation did not reach statistical significance (nominal P = .08), suggesting that TF may modulate global energy metabolism principally through activation of GR signaling.
Discussion
Exposure to synthetic chemicals has recently been implicated as a potential risk factor in the development of metabolic disease. The present study is the first to identify TF as an EDC with the capacity to promote the development of metabolic dysfunction with hallmarks of the metabolic syndrome using a paradigm that mimics human exposure. Dietary exposure to this novel metabolic disruptor for 12 weeks, at a concentration in the range of previously reported no observed adverse effect levels (34), promotes body weight gain (Figures 1B and 2A) and adipose accretion, increasing total fat content by as much as 52% in exposed mice (Figure 2B). This occurred without a concurrent increase in lean body mass (Figure 2C). Importantly, there was a marked expansion of depots analogous to visceral adipose tissue in humans (Figures 1D) (43). Visceral adipose tissue expansion appears to underlie the metabolic complications associated with obesity (44), including increased cardiometabolic disease risk (reviewed in Ref. 45). However, expansion of the 3 depots examined does not completely account for the total increase in body fat content detected by DEXA, suggesting a potential increase in sc adipose depots as well. Likewise, although we did not observe hepatic steatosis, ectopic lipid deposition in other metabolic tissues may contribute to increased total body fat content, a process linked to metabolic dysfunction (reviewed in Ref. 46).
Adipose lipid stores are dependent on de novo lipid synthesis and fatty acid esterification to increase levels, and lipolysis and FAO to lower them. Upon examination of gene expression in the perigonadal depot, there were no differences detected in the lipogenic genes investigated (data not shown); however, the relative expression of HSL was reduced in exposed mice, without a concordant reduction in the expression of ATGL (Figure 8B). Interestingly, it has been previously shown in human studies that adipose tissue of obese subjects exhibits reduced expression of HSL (47). Despite the reduction in HSL expression, serum non-esterified fatty acid levels were not different between groups (data not shown); it is possible that the reduction in HSL expression is offset by the increase in total adipose mass (48) or by increased mobilization from sc adipose tissue, which is thought to be the primary source of serum non-esterified fatty acids in humans (49). In addition to altered expression of the lipolytic pathway, expression of 3 FAO genes was reduced in TF-exposed mice: 2 Acad isoforms specific for long chain fatty acids (LCFAs) and very long chain fatty acids as well as a reduction in enoyl-CoA hydratase 1 (Figure 8A). Taken together, this suggests that impaired FAO and lipolysis may promote fat accretion; however, further work is needed using assessments of metabolic flux to establish this as the precise mechanism.
Interestingly, this specific reduction in long chain FAO gene expression would be predicted to increase relative levels of LCFAs. Recent evidence suggests LCFAs function as endogenous ligands of the PPARs (50). PPARγ is a central regulator of adipose tissue development; thus, TF exposure may also indirectly modulate adipose function by facilitating production of endogenous PPARγ ligands. Moreover, PPARγ plays a role in establishing circadian rhythms, with suppressed diurnal variations in metabolic parameters in mice with systemic PPARγ deletion (51). Because PPARγ is a target of a number of EDCs, eg, tributyltin (16), GSEA was performed to determine whether modulation of this pathway was involved in TF-mediated metabolic disruption. Using published gene sets for 3T3-L1 adipocytes stimulated with thiazolidinediones (42) or dexamethasone (41), gene expression differences in perigonadal adipose tissue were analyzed at the time at which body weight begins to diverge. These data demonstrated a clear activation of GR signaling (Figure 8A) with a nonsignificant trend toward PPARγ activation (Figure 8B). This suggests that TF principally modulates energy metabolism through activation of GR signaling; however, this analysis does not definitively exclude secondary or indirect PPARγ activation as a possible contributor to TF-induced metabolic disruption.
Adipose tissue also regulates global metabolism through the release of adipokines. Adiponectin functions globally to promote metabolic health by increasing peripheral insulin sensitivity (52) and inhibiting β-cell apoptosis (53). Controlling for total adipose mass, TF-exposed mice exhibited a reduction in relative adiponectin levels (Figure 6B), presumably through reduced adiponectin gene expression (Figure 6D). Other EDCs have previously been shown to inhibit the production and release of adiponectin, including the ubiquitous EDC bisphenol A (54, 55) and the classical obesogen tributyltin (56), suggesting that disruption of adiponectin action may be a common mechanism of environmentally mediated metabolic perturbations. Another critical adipokine is leptin, which is secreted in proportion to fat mass and signals the state of peripheral nutrient stores to the hypothalamus to regulate food intake (57). Importantly, obese individuals often exhibit increased leptin levels due to increased adipose tissue mass, yet also exhibit leptin resistance (reviewed in Ref. 58). Consistent with the increase in fat mass, mice exposed to TF also exhibited higher circulating leptin levels (Figure 6A). The LAR is one method for assessing the relative metabolic health of adipose tissue. In large-scale population studies the LAR significantly correlated with measures of insulin resistance, including the homeostatic model assessment of insulin resistance and the euglycemic hyperinsulinemic clamp (37, 38). TF-exposed mice exhibited a significantly increased LAR (Figure 6C), suggesting the presence of global insulin resistance. In addition, an increased LAR has been associated with augmented risk of cardiovascular disease in humans (40, 59), whereas some EDCs have been implicated as causal factors in the development of cardiovascular disease (60).
To further assess the metabolic health of adipose tissue, the insulin sensitivity of adipose tissue was investigated. Integrating all upstream events in insulin signal transduction, the ratio of phosphorylated to total Akt under insulin stimulation assesses insulin sensitivity, with increased relative phosphorylation indicative of increased insulin sensitivity (28). Although control mice exhibited the expected increase in the ratio of phosphorylated to total Akt with increasing insulin stimulation, TF-exposed mice showed a significantly blunted insulin response (Figure 6E). Consistent with ex vivo studies (28), a specific reduction in the expression of IRS-1 was identified, without significant changes in other genes of the insulin-signaling cascade (Figure 6F). These findings suggest that dietary TF exposure promotes the accretion of dysfunctional adipose tissue, specifically impairing insulin sensitivity and modulating the balance of adipokine hormones in a metabolically deleterious manner.
Global insulin-glucose homeostasis is dependent on multiple tissues, including adipose, pancreas, liver, muscle, and brain, and an increasing body of epidemiological literature links exposure to synthetic chemicals with disruptions in glucose homeostasis (8). Chronic exposure to TF induced both glucose intolerance and insulin resistance (Figure 3, B and D, respectively), findings consistent with alterations in insulin-glucose homeostasis seen in individuals with the metabolic syndrome and type 2 diabetes (61). One outcome of global insulin resistance is a shift in nutrient use from carbohydrates to lipids (35). Consistent with this, TF-exposed mice exhibited increased lipid use during the dark cycle (Figure 4B) and a blunting of metabolic flexibility during fed-fasted transitions (Figure 4C). These findings are directionally similar to those induced by high-fat feeding in mice (62) and obese human males (63), suggesting that supplementation of a normal chow diet with TF drives a phenotype analogous to that induced by high-fat feeding. Finally, a recent study demonstrated that both prediabetic and diabetic individuals had higher serum concentrations of persistent organic pollutants relative to weight-matched metabolically healthy individuals and also exhibited metabolic inflexibility with a preference for lipid use (64). To our knowledge, the present study is the first to directly implicate exposure to an environmental pollutant in metabolically deleterious alterations in metabolic flexibility and nutrient use.
In addition to alterations in lipid-carbohydrate use, the metabolic cage studies here reported suggest a potential disruption in the diurnal rhythm of energy intake, activity, and energy expenditure (Figure 5, A, B, and D, respectively). An increasing body of literature implicates alterations in circadian rhythms in the pathogenesis of metabolic disease (reviewed in Ref. 65). Disruptions of clock genes, both globally and peripherally, promote obesity in mouse models (66, 67). Specifically, disruption of circadian rhythms has been shown to alter feeding behavior (68), whereas light-cycle feeding alone has been shown to promote increased weight gain without increased caloric consumption (69). In another study, exposure to dim light at night was shown to alter circadian feeding behavior, promoting increased weight gain and impaired glucose tolerance (70), raising the possibility that exposure to EDCs like TF may exacerbate metabolic disruption induced by other environmental insults. Importantly, disruptions in sleep specifically induce adipocytic insulin resistance in humans (71). Although it is acknowledged that metabolic cage analyses have drawbacks with regard to monitoring food intake and circadian behavior (72), the present data provide intriguing evidence that EDC-induced alterations in circadian rhythmicity contribute to metabolic dysfunction that requires further investigation.
Previous work demonstrated that TF promotes GR signaling (27, 29), and it is intriguing that the metabolic phenotype identified in these studies mirrors those of clinical glucocorticoid excess, which is characterized metabolically by obesity, insulin resistance, and frank diabetes (73). Consistent with these phenotypic similarities, GSEA demonstrated a significant up-regulation of GR signaling after 4 weeks of dietary treatment (Figure 8A), the point at which weight gain between the groups began to diverge (Figure 1, A and B). Moreover, TF-induced changes in gene expression at study termination, such as the reduction in adipose expression of HSL but not ATGL (Figure 7B), mimic effects induced by glucocorticoid treatment (74). Finally, inappropriate GR signaling has been shown to perturb circadian rhythmicity (75, 76), whereas circadian genes may also regulate peripheral glucocorticoid sensitivity (77) and glucocorticoid-mediated glucose intolerance (78). In concert with ex vivo evidence, these data support a model in which the in vivo effects of TF result from activation of GR signaling, suggesting that TF may function as a novel environmental glucocorticoid.
Taken together, these findings strongly suggest that TF exposure may contribute to the development of metabolic disease, notably through an accumulation of dysfunctional adipose tissue, disruption of global insulin-glucose homeostasis, and diminution of metabolic flexibility, effects similar to those seen in the metabolic syndrome. However, many questions remain. The above study has solely investigated one concentration of TF; therefore, establishment of a dose-response relationship is warranted, especially given the issue of nonmonotonic effects of many EDCs (79). Moreover, because TF was added during diet production, it remains to be resolved whether TF or its metabolites are primarily responsible for the observed metabolic disruption. This may have important implications for agricultural policy regarding residues of this fungicide on produce. In addition, the consequences of shorter durations of exposure to TF or its metabolites on energy homeostasis need to be studied, especially given the interesting finding that weight gain differences in the present study were not observed until after 4 weeks of exposure. Importantly, because the endogenous hormonal milieu and fat deposition patterns differ between males and females (80), studies examining the effects of TF in females are needed. Moreover, given the linkage between early-life EDC exposure and altered metabolism later in life (81), determining how developmental TF exposure influences adult energy metabolism is critical for comprehensively assessing the risk associated with exposure to this EDC. Furthermore, the findings of altered circadian rhythmicity and adipokine levels raise important questions regarding the effects of TF on brain signaling that must be explored. Finally, it is paramount that human exposure to TF be characterized to determine whether humans are at risk for TF-induced metabolic complications. As the field of environmental metabolic disruption expands, it is hoped that these studies will provide further insights into the effects and mechanisms by which environmental contaminants contribute to the burgeoning metabolic disease epidemic in order to develop interventions to mitigate the deleterious impact of toxicant exposures on human health.
Acknowledgments
We thank Matthew J. Brady, PhD, for his constructive comments regarding this manuscript and Chuanhong Liao, PhD, for her biostatistical assistance.
This work was supported by a Pilot and Feasibility Grant from the National Institutes of Health-funded University of Chicago Diabetes Research and Training Center (P60-DK020595), a Junior Investigator Award from the Brinson Foundation, an Early Career Development Award from the Central Society for Clinical and Translational Research, and National Institutes of Health Grants K08-ES019176 and R21-ES021354 (to R.M.S.) as well as T32-HD007009 (to S.M.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Acad
- acyl-CoA dehydrogenase
- ATGL
- adipose triglyceride lipase
- AUC
- area under the curve
- CoA
- coenzyme A
- DEXA
- dual energy x-ray absorptiometry
- EDC
- endocrine-disrupting chemical
- FAO
- fatty acid oxidation
- GR
- glucocorticoid receptor
- GSEA
- Gene Set Enrichment Analysis
- GTT
- glucose tolerance test
- H&E
- hematoxylin and eosin
- HSL
- hormone-sensitive lipase
- IRS
- insulin receptor substrate
- ITT
- insulin tolerance test
- LAR
- leptin to adiponectin ratio
- LCFA
- long chain fatty acid
- PPAR
- peroxisome proliferator-activated receptor
- ppm
- parts per million
- RER
- respiratory exchange ratio
- TF
- tolylfluanid.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: United States Department of Health and Human Services; 2014. [Google Scholar]
- 2. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 3. International Diabetes Federation. IDF Diabetes Atlas 2013. Brussels, Belgium: International Diabetes Federation; 2013. Available at: www.idf.org/diabetesatlas Accessed January 29, 2014. [Google Scholar]
- 4. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 6. Grün F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009;304(1–2):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. [DOI] [PubMed] [Google Scholar]
- 8. Neel BA, Sargis RM. The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes. 2011;60(7):1838–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8(2):185–192. [DOI] [PubMed] [Google Scholar]
- 10. Von Ruesten A, Steffen A, Floegel A, et al. Trend in obesity prevalence in European adult cohort populations during follow-up since 1996 and their predictions to 2015. PLoS One. 2011;6(11):e27455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee D-H, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One. 2011;6(1):e15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sargis RM. The hijacking of cellular signaling and the diabetes epidemic: mechanisms of environmental disruption of insulin action and glucose homeostasis. Diabetes Metab J. 2014;38(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Regnier SM, Sargis RM. Adipocytes under assault: environmental disruption of adipose physiology. Biochim Biophys Acta. 2014;1842(3):520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grün F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 suppl):S50–S55. [DOI] [PubMed] [Google Scholar]
- 17. Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. 2014;1842(3):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Environmental Protection Agency (EPA). Pesticide Fact Sheet: Tolylfluanid. Washington, DC: United States Environmental Protection Agency; 2002. [Google Scholar]
- 19. Chemwatch. Global Chemicals Management. Glen Huntly, Victoria, Australia: Chemwatch; 2011. [Google Scholar]
- 20. Cesnik HB, Gregorcic A, Bolta SV, Kmecl V. Monitoring of pesticide residues in apples, lettuce and potato of the Slovene origin, 2001–04. Food Addit Contam. 2006;23(2):164–173. [DOI] [PubMed] [Google Scholar]
- 21. Stensvand A, Christiansen A. Investigation on fungicide residues in greenhouse-grown strawberries. J Agric Food Chem. 2000;48(3):917–920. [DOI] [PubMed] [Google Scholar]
- 22. Sadlo S, Szpyrka E, Jazwa A, Zawislak A. Pesticide residues in fruit and vegetables from Southeastern Poland, 2004–05. Pol J Environ Stud. 2007;16(2):313–319. [Google Scholar]
- 23. Reemtsma T, Alder L, Banasiak U. Emerging pesticide metabolites in groundwater and surface water as determined by the application of a multimethod for 150 pesticide metabolites. Water Res. 2013;47(15):5535–5545. [DOI] [PubMed] [Google Scholar]
- 24. Mukherjee A, Rao KVM, Ramesh US. Predicted concentrations of biocides from antifouling paints in Visakhapatnam Harbour. J Environ Manage. 2009;90(suppl 1):S51–S59. [DOI] [PubMed] [Google Scholar]
- 25. Tielemans E, Louwerse E, de Cock J, Brouwer D, Zielhuis G, Heederik D. Exposure to fungicides in fruit growing: re-entry time as a predictor for dermal exposure. Am Ind Hyg Assoc J. 1999;60(6):789–793. [DOI] [PubMed] [Google Scholar]
- 26. Links I, Van Der Jagt KE, Christopher Y, et al. Occupational exposure during application and removal of antifouling paints. Ann Occup Hyg. 2007;51(2):207–218. [DOI] [PubMed] [Google Scholar]
- 27. Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring). 2010;18(7):1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sargis RM, Neel BA, Brock CO, et al. The novel endocrine disruptor tolylfluanid impairs insulin signaling in primary rodent and human adipocytes through a reduction in insulin receptor substrate-1 levels. Biochim Biophys Acta. 2012;1822(6):952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neel BA, Brady MJ, Sargis RM. The endocrine disrupting chemical tolylfluanid alters adipocyte metabolism via glucocorticoid receptor activation. Mol Endocrinol. 2013;27(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 31. Chen C, Grennan K, Badner J, et al. Removing batch effects in analysis of expression microarray data: an evaluation of six batch adjustment methods. PLoS One. 2011;6(2):e17238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saville DJ. Multiple comparison procedures: the practical solution. Am Statistic. 1990;44(2):174–180. [Google Scholar]
- 34. Environmental Protection Agency (EPA). Notice of Filing of Pesticide Petitions. Washington, DC: United States Environmental Protection Agency; 1997:42980–42986. [Google Scholar]
- 35. Stump CS, Henriksen EJ, Wei Y, Sowers JR. The metabolic syndrome: role of skeletal muscle metabolism. Ann Med. 2006;38(6):389–402. [DOI] [PubMed] [Google Scholar]
- 36. Leal Vde O, Mafra D. Adipokines in obesity. Clin Chim Acta. 2013;419:87–94. [DOI] [PubMed] [Google Scholar]
- 37. Finucane FM, Luan J, Wareham NJ, et al. , European Group for the Study of Insulin Resistance: Relationship between Insulin Sensitivity and Cardiovascular Disease Risk Study Group), Savage DB. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia. 2009;52(11):2345–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inoue M, Maehata E, Yano M, Taniyama M, Suzuki S. Correlation between the adiponectin-leptin ratio and parameters of insulin resistance in patients with type 2 diabetes. Metab Clin Exp. 2005;54(3):281–286. [DOI] [PubMed] [Google Scholar]
- 39. Kotani K, Sakane N. Leptin:adiponectin ratio and metabolic syndrome in the general Japanese population. Korean J Lab Med. 2011;31(3):162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kappelle PJ, Dullaart RP, van Beek AP, Hillege HL, Wolffenbuttel BH. The plasma leptin/adiponectin ratio predicts first cardiovascular event in men: a prospective nested case-control study. Eur J Intern Med. 2012;23(8):755–759. [DOI] [PubMed] [Google Scholar]
- 41. Yu C-Y, Mayba O, Lee JV, et al. Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PLoS One 2010;5(12):e15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sears DD, Hsiao A, Ofrecio JM, Chapman J, He W, Olefsky JM. Selective modulation of promoter recruitment and transcriptional activity of PPARγ. Biochem Biophys Res Commun. 2007;364(3):515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development. 2013;140(19):3939–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–18. [DOI] [PubMed] [Google Scholar]
- 45. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. [DOI] [PubMed] [Google Scholar]
- 46. Snel M, Jonker JT, Schoones J, et al. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int J Endocrinol. 2012;2012:983814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Langin D, Dicker A, Tavernier G, et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes. 2005;54(11):3190–3197. [DOI] [PubMed] [Google Scholar]
- 48. Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60(10):2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113(11):1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res. 2014;53:124–144. [DOI] [PubMed] [Google Scholar]
- 51. Yang G, Jia Z, Aoyagi T, McClain D, Mortensen RM, Yang T. Systemic PPARγ deletion impairs circadian rhythms of behavior and metabolism. PLoS One. 2012;7(8):e38117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7(8):947–953. [DOI] [PubMed] [Google Scholar]
- 53. Brown JE, Conner AC, Digby JE, et al. Regulation of β-cell viability and gene expression by distinct agonist fragments of adiponectin. Peptides. 2010;31(5):944–949. [DOI] [PubMed] [Google Scholar]
- 54. Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116(12):1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: implications for the metabolic syndrome. Mol Cell Endocrinol. 2009;304(1–2):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zuo Z, Chen S, Wu T, et al. Tributyltin causes obesity and hepatic steatosis in male mice. Environ Toxicol. 2011;26(1):79–85. [DOI] [PubMed] [Google Scholar]
- 57. Leibel RL. The role of leptin in the control of body weight. Nutr Rev. 2002;60(10 pt 2):S15–S19; discussion S68–S84, 85–87. [DOI] [PubMed] [Google Scholar]
- 58. Kennedy AJ, Ellacott KLJ, King VL, Hasty AH. Mouse models of the metabolic syndrome. Dis Model Mech. 2010;3(3–4):156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kotani K, Sakane N, Saiga K, Kurozawa Y. Leptin : adiponectin ratio as an atherosclerotic index in patients with type 2 diabetes : relationship of the index to carotid intima-media thickness. Diabetologia. 2005;48(12):2684–2686. [DOI] [PubMed] [Google Scholar]
- 60. Kirkley AG, Sargis RM. Environmental endocrine disruption of energy metabolism and cardiovascular risk. Curr Diab Rep. 2014;14(6):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Lenfant C, American Heart Association, National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. [DOI] [PubMed] [Google Scholar]
- 62. Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. [DOI] [PubMed] [Google Scholar]
- 63. van Herpen NA, Schrauwen-Hinderling VB, Schaart G, Mensink RP, Schrauwen P. Three weeks on a high-fat diet increases intrahepatic lipid accumulation and decreases metabolic flexibility in healthy overweight men. J Clin Endocrinol Metab. 2011;96(4):E691–E695. [DOI] [PubMed] [Google Scholar]
- 64. Færch K, Højlund K, Vind BF, et al. Increased serum concentrations of persistent organic pollutants among prediabetic individuals: potential role of altered substrate oxidation patterns. J Clin Endocrinol Metab. 2012;97(9):E1705–E1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106(3):447–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Paschos GK, Ibrahim S, Song WL, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18(12):1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stucchi P, Gil-Ortega M, Merino B, et al. Circadian feeding drive of metabolic activity in adipose tissue and not hyperphagia triggers overweight in mice: is there a role of the pentose-phosphate pathway? Endocrinology. 2012;153(2):690–699. [DOI] [PubMed] [Google Scholar]
- 69. Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring). 2009;17(11):2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fonken LK, Workman JL, Walton JC, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107(43):18664–18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157(8):549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kalliokoski O, Jacobsen KR, Darusman HS, et al. Mice do not habituate to metabolism cage housing–a three week study of male BALB/c mice. PLoS One. 2013;8(3):e58460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol. Metab. 2003;88(12):5593–5602. [DOI] [PubMed] [Google Scholar]
- 74. Chimin P, Farias T da SM, Torres-Leal FL, et al. Chronic glucocorticoid treatment enhances lipogenic activity in visceral adipocytes of male Wistar rats. Acta Physiol (Oxf). 2014;211(2):409–420. [DOI] [PubMed] [Google Scholar]
- 75. Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. [DOI] [PubMed] [Google Scholar]
- 76. Le Minh N, Damiola F, Tronche F, Schütz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20(24):7128–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Charmandari E, Chrousos GP, Lambrou GI, et al. Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PLoS One. 2011;6(9):e25612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci USA. 2009;106(41):17582–17587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111(8):994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4(1):20–34. [DOI] [PubMed] [Google Scholar]
- 81. Newbold RR. Developmental exposure to endocrine-disrupting chemicals programs for reproductive tract alterations and obesity later in life. Am J Clin Nutr. 2011;94(6 suppl):1939S–1942S. [DOI] [PMC free article] [PubMed] [Google Scholar]