Abstract
There is accumulating evidence that fibroblasts are target cells for steroids such as sex hormones and corticoids. The characteristics of fibroblasts vary among tissues and organs. Our aim in this study is to examine differences in responses to steroid hormones among fibroblasts from different cervicothoracic regions. We compared the actions of steroid hormones on cultured fibroblasts from the vocal folds, which are considered to be the primary target of steroid hormones, and the trachea and esophagus in adult male rats. Expression of steroid hormone receptors (androgen receptor, estrogen receptor α, and glucocorticoid receptor) was identified by immunofluorescence histochemistry. Androgen receptor was much more frequently expressed in fibroblasts from the vocal fold than in those from the trachea and esophagus. Cell proliferation analysis showed that administration of testosterone, estradiol, or corticosterone suppressed growth of all 3 types of fibroblasts. However, mRNA expression for extracellular matrix–associated genes, including procollagen I and III and elastin, and hyaluronic acid synthase I was elevated only by addition of testosterone to fibroblasts from the vocal fold. These results indicate that each steroid hormone exerts region-specific effects on cervicothoracic fibroblasts with different properties through binding to specific receptors.
Fibroblasts in connective tissues secrete extracellular matrix (ECM) components such as collagen, elastin, and glycosaminoglycans that establish the structural framework of most tissues. Fibroblasts in many regions are targets of steroid hormones including androgen, estrogen, and glucocorticoids (1–17) and express receptors for these steroid hormones (3, 11, 18–22). Fibroblasts exhibit different characteristics depending on the tissue or organ (4) and may have different gene expression programs depending on their anatomic site of origin (23, 24). However, to date, evaluation of the action of steroid hormones on fibroblasts from specific tissues or organs has been limited (4, 24, 25).
The vocal folds are part of the phonatory system and a primary target of steroid hormones. In humans, the male vocal folds lengthen and thicken during puberty when blood levels of androgens increase, causing a deepening of the voice (26). Prevention of pubertal development of the voice by castrating young male singers was a well-known practice, especially in Italy beginning in the 16th century. The “castrati” had a small larynx and vocal folds and were well known for their clear, high-pitched voices (27). In women, fluctuations in estrogen and progesterone levels during the menstrual cycle are accompanied by variations in the pitch of the voice (28, 29). Administration of laryngeal corticosteroids is used for treatment of vocal fold scars (30), whereas inhalation of corticosteroids for treatment of bronchial asthma occasionally induces vocal fold atrophy (31). However, the detailed mechanisms of steroidal effects on fibroblasts in the vocal folds remain to be determined.
The vocal folds of rats are structurally similar to those of humans (32). Rats emit ultrasonic vocalizations and ultrasonic vocalizations in adult rats are regarded as a form of communication between animals (33, 34). In this study, we compared the action of an androgen, an estrogen, and a glucocorticoid on fibroblasts cultured from the vocal folds and from the trachea and esophagus (which are adjacent to the vocal folds) of 10-week-old male rats. The expression patterns and intracellular distributions of 3 steroid hormone receptors, the androgen receptor (AR), estrogen receptor α (ERα), and glucocorticoid receptor (GR) in fibroblasts from the 3 regions were analyzed in response to treatment with their specific ligands, testosterone, estradiol, and corticosterone, the major glucocorticoids in rats, respectively. Cellular proliferation and mRNA expression for major ECM components (procollagen type I α1-chain, procollagen type III α1-chain, and elastin) and hyaluronic acid synthase (HAS) I were also compared among the fibroblasts after exposure to steroids. To examine the androgenic effect in vivo, immunohistochemical evaluation of AR and the ECM was performed in the vocal fold of gonadectomized rat.
Materials and Methods
Experimental animals
Male Sprague-Dawley rats aged 10 weeks were purchased from Shimizu Laboratory Supplies Co and housed in plastic cages with standard bedding and continuous access to food and water. The temperature was maintained at 22°C with a 12-hour light/dark cycle. Reproducibility of results was confirmed by repeating the experiment using at least 3 animals. The Committee for Animal Research of Kyoto Prefectural University of Medicine authorized all experimental procedures, and the study conformed to international guidelines on the ethical use of animals.
Surgery
Rats were anesthetized with ip injections of 50 mg/kg body weight sodium pentobarbital and bilaterally gonadectomized (GDX). Sham surgeries (sham) were also performed under sodium pentobarbital anesthesia. Twenty-eight days after surgery, rats were euthanized by perfusion under deep anesthesia with sodium pentobarbital and fixed for immunohistochemical and histologic analysis.
Tissue harvest and slide preparation
Rats were deeply anesthetized with ip injections of sodium pentobarbital and perfused via the left ventricle with 100 mL of physiological saline followed by 200 mL of 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) (pH 7.4). The larynx, trachea, and esophagus were immediately removed and immersed in 4% PFA in 0.1 M PB for 3 hours, followed by immersion in 25% sucrose in 0.1 M PB for 48 hours at 4°C. For preparation of cryosections, the larynx, trachea, and esophagus were soaked in an embedding medium (Tissue-Tek O.C.T., Sakura), quickly frozen using powdered dry ice, and cut into 10-μm axial sections using a cryostat (CM3050 S; Leica). The frozen sections were mounted on glass slides. Sections were air-dried and stored at −20°C before staining.
Immunohistochemistry and histology
AR expression levels in the vocal fold, tracheal, and esophageal fibroblasts of 10-week-old male rats were compared immunohistochemically. Mounted sections were washed with PBS and blocked for 1 hour at room temperature with 2% BSA containing 0.3% Triton X-100 in PBS. They were then incubated for 72 hours at 4°C with the following primary antibodies: mouse anti-HSP47 (1:500; Enzo Life Sciences, Inc) and rabbit anti-AR (1:200; Epitomics). After the primary antibody was removed by rinsing, the sections were incubated with secondary antibodies for 1 hour at room temperature. The following secondary antibodies were used for double-staining: anti-rabbit Alexa Fluor (AF) 488 (1:1000; Invitrogen) and anti-mouse AF 546 (1:1000; Invitrogen). AR expression levels in the sham and GDX groups were compared using the same method.
Deposition of collagen type I, type III, elastin, and hyaluronic acid (HA) in the vocal fold lamina propria were also compared between the sham and GDX groups. Deposition of collagen type I, type III, and elastin was detected by immunohistochemistry using the following antibodies: rabbit anti-type I collagen (1:20; AbD Serotec), mouse anti-type III collagen (1:4000; Sigma-Aldrich), and rabbit anti-elastin (1:400; Millipore Corp). Alexa Fluor 488 (1:1000; anti-rabbit or anti-mouse; Invitrogen) was used as the secondary antibody.
Slides were stained with propidium iodide (Nacalai Tesque) for nuclear staining. The slides were subsequently coverslipped with Gelvatol (13% polyvinyl alcohol and 33% glycerol in 0.2 M Tris HCl), and then images were acquired using an LSM-510 META laser scanning microscope (Carl Zeiss). Deposition of HA was detected using Alcian blue staining. The hyaluronidase (HAase) digestion technique was used to distinguish HA content from that of other glycosaminoglycans. For the HAase digestion procedure, 50 mg of bovine testes HAase (Sigma-Aldrich) was diluted in 100 mL of PBS, and slides were incubated in this solution for 1 hour at 37°C. Slides were then stained with Alcian blue (pH 2.5). HA deposition was detected by comparing the slides with HAase treatment with those without HAase treatment.
Culture of fibroblasts
Animals were euthanized by excess administration of sodium pentobarbital, and the larynx, trachea, and esophagus were removed. Fibroblasts from each organ were isolated as described by Goto et al (35) with minor modifications. The mucosa of the vocal folds, trachea, and esophagus were denuded of surface epithelium and minced into small pieces. The tissues were incubated in 0.1% collagenase (Wako) at 37°C for 1 hour. After centrifugation, the pelleted cells were plated on 35-mm culture dishes (Falcon; Becton-Dickinson) in phenol red–free DMEM (Gibco) containing 10% charcoal-dextran–treated fetal bovine serum and 1% penicillin-streptomycin (Invitrogen) and incubated for 12 hours in a CO2 incubator at 37°C with 5% CO2-95% air. Fibroblasts migrating from tissues were harvested using a trypsin-EDTA solution (Nacalai Tesque) and cultured in phenol red–free DMEM containing 10% charcoal-dextran–treated fetal bovine serum and 1% penicillin-streptomycin. Epithelial debris contaminating the fibroblasts was mechanically removed using a Pasteur pipette while viewing under a microscope. Confluent fibroblasts were harvested using trypsin-EDTA solution. Three types of fibroblasts of different origin from the same rats were used for each experiment at the same passage.
Immunocytochemistry
Fibroblasts (4 × 104 cells/dish) were seeded on poly-l-lysine–coated glass-bottom dishes (Matsunami Glass) and examined after 1 hour of incubation at 37°C in the absence or presence of 10−8 M testosterone (Nacalai Tesque), 10−8 M estradiol (Sigma-Aldrich), or 10−8 M corticosterone (Sigma-Aldrich). All fibroblasts were fixed with 4% PFA in 0.1 M PB (pH 7.4) at 37°C for 10 minutes, washed with PBS, and blocked for 1 hour at room temperature with 2% BSA containing 0.3% Triton X-100 in PBS (36). These cells were then incubated with the following antibodies for 72 hours at 4°C: mouse anti-heat shock protein (HSP) 47 (1:500; Enzo Life Sciences) and rabbit anti-AR (1:200; Epitomics), rabbit anti-ERα (1:5000; Millipore Corp), or rabbit anti-GR (1:1000; Morimoto et al, 1996; 37). HSP47 is a collagen-specific molecular chaperone that is used as a marker of collagen-secreting cells (38). In this study, HSP47 immunoreactivity was used as a marker for fibroblast cells. Anti-rabbit Alexa Fluor 488 (1:1000; Invitrogen) and anti-mouse Alexa Fluor 546 (1:1000; Invitrogen) were used as secondary antibodies for double staining. The glass coverslips were sealed with Gelvatol, and the immunocytochemical preparations were observed using a LSM-510 META laser scanning microscope (Carl Zeiss) (39). The immunoreactive (ir) cells were counted in the presence of steroid hormones at high magnification by an investigator in a blinded fashion to determine the percentages of AR-, ERα- or GR-ir cells in the HSP47-ir cells.
Hormone treatment
Fibroblasts from the 3 regions were treated with testosterone, estradiol, or corticosterone at 10−8 or 10−9 M for 48 hours. Fibroblasts without hormone treatment were used as controls. Each group underwent a cell proliferation assay and a quantitative real-time PCR.
Cell proliferation assay
Fibroblasts (5 × 103 cells/well) from the vocal fold, trachea, and esophagus were cultured in 96-well plates, and cell proliferation assays were performed in triplicate using a Cell Counting Kit-8 (Dojindo). WST-8 reagent was added to each well, and the absorbance at 450 nm (OD450) was measured using a GENios microplate fluorescence reader (Tecan) after incubation with the reagent for 2 hours at 37°C. The OD450 was normalized to that of the control in each trial.
Quantitative real-time PCR
Fibroblasts (5 × 105 cells/dish) from the 3 regions were treated with each hormone and collected for quantitative real-time PCR. Total RNA was extracted with a RNeasy Micro Kit (QIAGEN) and reverse transcribed using ReverTra Ace (Toyobo) and random hexamers. Gene expression was assessed by quantitative real-time PCR using the Light Cycler 480 II with Universal ProbeLibrary assays (Roche Diagnostics). Reactions were performed in triplicate in 96-well plates for 40 amplification cycles (95°C for 10 seconds, 60°C for 10 seconds, and 72°C for 10 seconds) in a 10-μL reaction volume. Primers were designed using the Roche Universal ProbeLibrary assay design center (www.universalprobelibrary.com): AR forward primer, 5′-GGCGCTTCTACCAGCTCA; AR reverse primer, 3′-GAATTGATGCAGCTCTCTTGC; procollagen type I α1-chain forward primer, 5′-TCCTGGCAAGAACGGAGAT; procollagen type I α1-chain reverse primer, 3′-CAGGAGGTCCACGCTCAC; procollagen type III α1-chain forward primer, 5′-TGGACCTCAGGGTATCAAGG; procollagen type III α1-chain reverse primer, 3′-AGGACCACGTTCCCCATTAT; elastin forward primer, 5′-TTCTGGGAGCGTTTGGAG; elastin reverse primer, 3′-CCTTGAAGCATAGGAGAGACCT; HAS I forward primer, 5′-CATGGGCTACGCTACCAAGT; HAS I reverse primer, 3′-GAGGAGGGCGTCTCTGAATA; GAPDH forward primer, 5′-ACAACTTTGGCATCGTGGA; and GAPDH reverse primer, 3′-CTTCTGAGTGGCAGTGATGG. The carboxyfluorescein-labeled probe (Universal ProbeLibrary) numbers were 128 for AR, 60 for procollagen type I α1-chain, 92 for procollagen type III α1-chain, 65 for elastin, 50 for HAS I, and 114 for GAPDH. For quantification of changes in gene expression, the comparative Cp (threshold cycle number) method was used to calculate relative fold changes normalized against GAPDH.
Statistical analyses
Data are expressed as means ± SEM. One-way ANOVA was performed after normal distribution was proved using the Kolmogorov-Smirnov test. For interpretation of the results, the Tukey or Games-Howell post hoc test was used, depending on the results of a Levene test for homogeneity of variances. All statistical analyses were performed using IBM SPSS Statistics 18 for Windows (IBM Deutschland GmbH). A value of P < .05 was considered to be statistically significant.
Results
Steroid receptor expression and intracellular localization in fibroblasts from the vocal fold
Immunofluorescent labeling showed expression of AR, ERα, and GR in cultured fibroblasts from the vocal fold (Figure 1, A–C), but the steroid hormone receptors had different intracellular distribution patterns. In the absence of testosterone, AR immunoreactivity was detected in the nucleus and cytoplasm, but it occurred predominantly in the nucleus after testosterone treatment (Figure 1A). ERα was localized predominantly in the nucleus in the absence and presence of estradiol (Figure 1B). GR was found predominantly in the nucleus but slightly in the cytoplasm in the absence of corticosterone and only in the nucleus after corticosterone treatment (Figure 1C).
Figure 1.
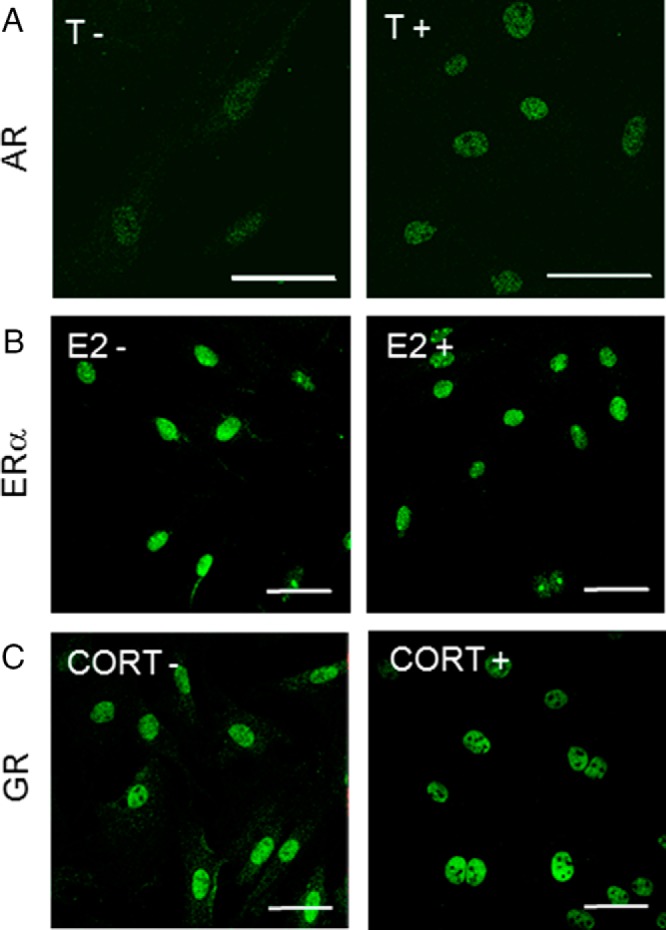
Steroid receptor expression and intracellular localization in cultured fibroblasts from the vocal fold. A, AR immunostaining in the absence (left) or presence (right) of 10−8 M testosterone (T). B, ERα immunostaining in the absence (left) or presence (right) of 10−8 M estradiol (E2). C, GR immunostaining in the absence (left) or presence (right) of 10−8 M corticosterone (CORT). Scale bar corresponds to 50 μm.
Effects of castration on AR expression in vocal fold fibroblasts
To examine the androgenic effect in vivo, immunohistochemical evaluation of AR was performed in the vocal fold of GDX rats. In the sham group, AR was localized in the nuclei of vocal fold fibroblasts, whereas in the GDX group AR was expressed throughout the cell body (Supplemental Figure 1).
AR expression in the vocal fold, tracheal, and esophageal fibroblasts
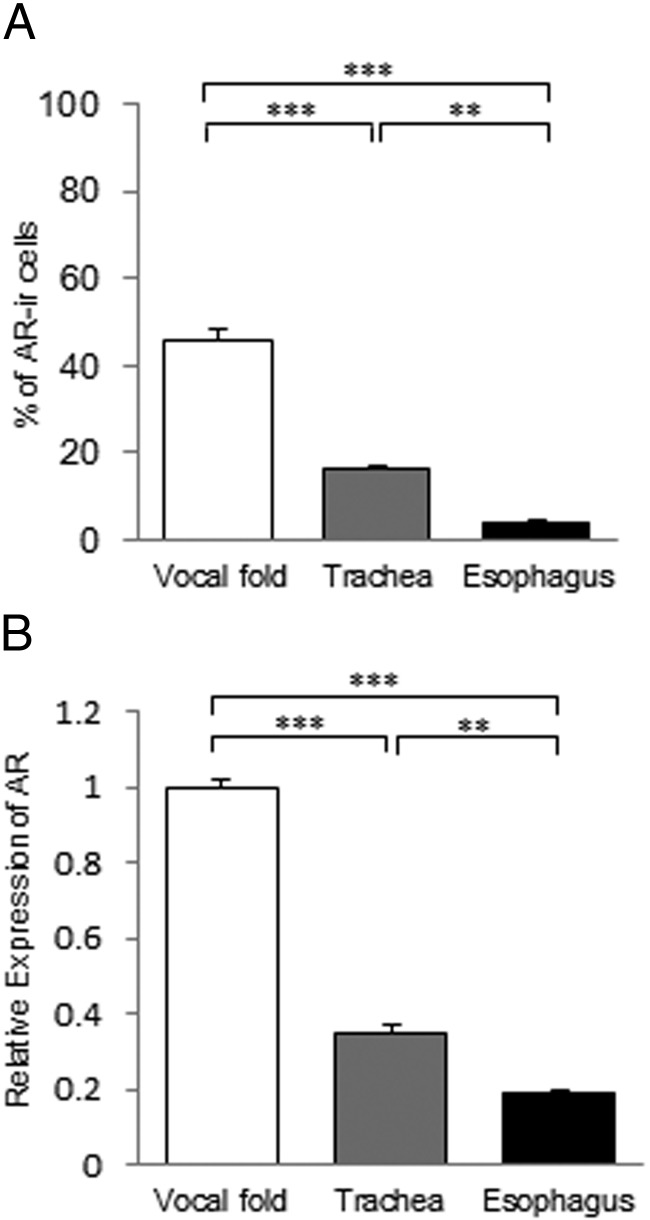
At least some of the cultured cells from all 3 types of fibroblasts expressed AR (Supplemental Figure 2A). The percentage of AR-ir cells in HSP47-positive cells was much higher in fibroblasts from the vocal fold than in those from the trachea and esophagus, and there was also a significant difference between the trachea and esophagus (Figure 2A). In parallel with the immunofluorescence data, AR mRNA expression levels showed a similar pattern with significant differences among the 3 types of fibroblasts (Figure 2B). AR was expressed in approximately 37.5% of the vocal fold fibroblasts but was not expressed in esophageal or tracheal fibroblasts (Supplemental Figure 3). These results show that AR expression in the vocal fold fibroblasts is significantly higher than that in the tracheal and esophageal fibroblasts. In contrast, double immunofluorescence staining revealed that all HSP47-positive cells coexpressed ERα and GR in all 3 types of fibroblasts (Supplemental Figure 2, B and C).
Figure 2.
Comparative analysis of AR expression in fibroblasts from the vocal fold, trachea, and esophagus. A, The percentage of AR-ir cells in HSP47-positive cells. B, AR mRNA levels normalized to those in fibroblasts from the vocal fold. Values are means ± SEM. **, P < .01; ***, P < .001.
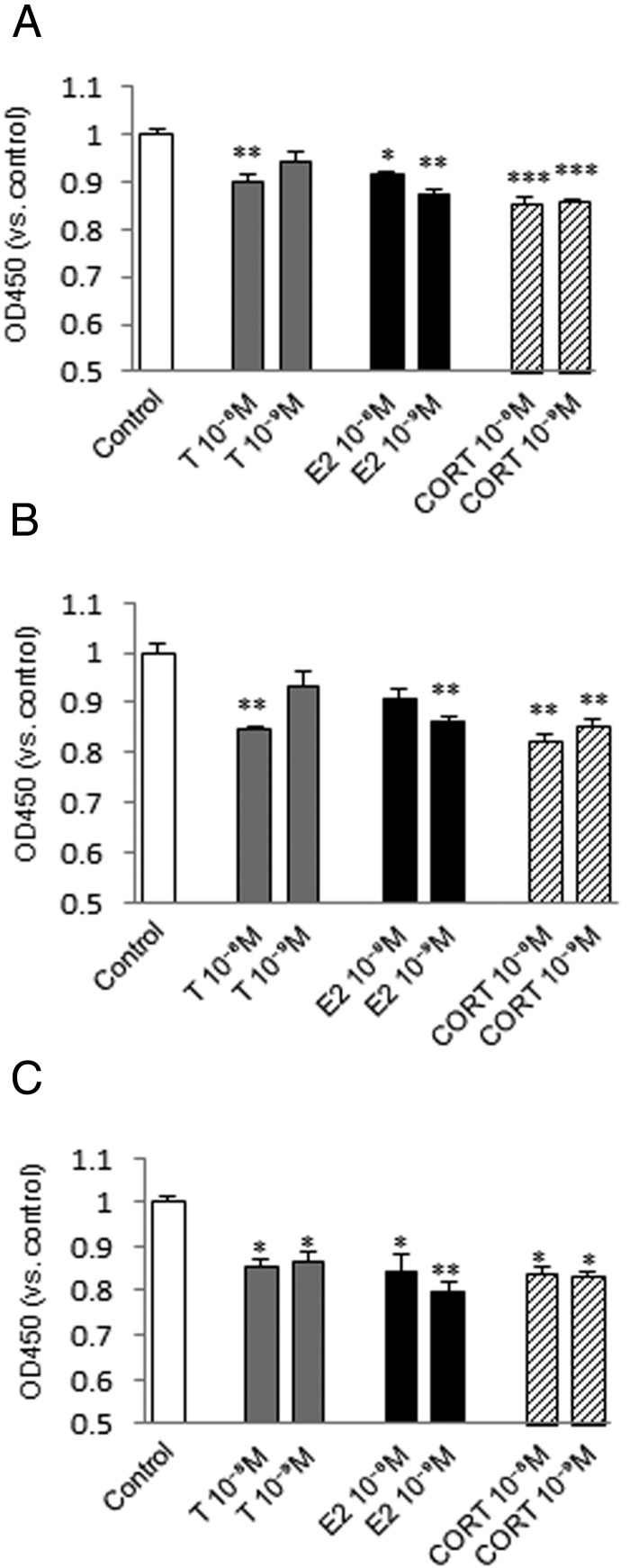
Suppression of cell proliferation of fibroblasts by steroid hormones
Fibroblasts from the vocal fold, trachea, and esophagus showed significantly lower proliferation upon treatment with most concentrations of testosterone, estradiol, or corticosterone (Figure 3, A–C). Changes in proliferation of fibroblasts from the vocal fold at 10−9 M testosterone and of those from the trachea at 10−9 M testosterone and 10−8 M estradiol were not significant, but still showed a trend for reduced growth.
Figure 3.
Effects of steroid hormones on cell proliferation of fibroblasts from the vocal fold (A), trachea (B), and esophagus (C) estimated from the absorbance (OD450) 48 hours after testosterone (T), estradiol (E2), or corticosterone (CORT) administration at 10−8 or 10−9 M. The OD450 was normalized to that of the control in each trial. Values are expressed as means ± SEM. *, P < .05 vs control; **, P < .01 vs control; ***, P < .001 vs control.
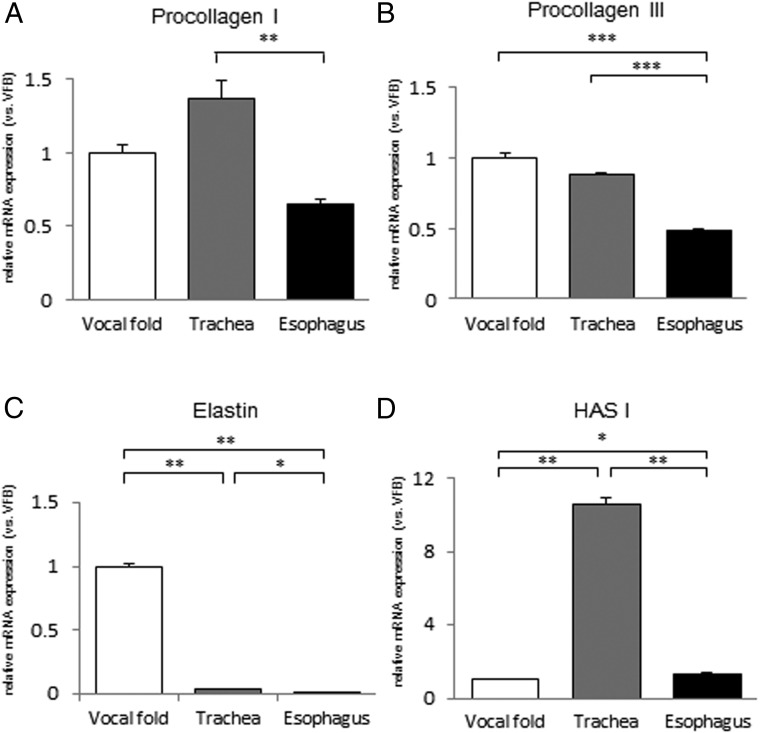
Differences in ECM gene expression among fibroblasts from the vocal fold, trachea, and esophagus
Expression of ECM genes in the cervicothoracic fibroblasts was examined by quantitative real-time PCR. The mRNA level for procollagen I was significantly higher in fibroblasts from the trachea than in those from the esophagus (Figure 4A). The mRNA level for procollagen III was significantly lower in fibroblasts from the esophagus than in those from the vocal fold and trachea (Figure 4B). Significant differences in expression of elastin genes were detected among the 3 types of fibroblasts (Figures 4C). The HAS I mRNA level was much higher in fibroblasts from the trachea than in those from the vocal fold and esophagus (Figure 4D).
Figure 4.
Expression levels of ECM genes in fibroblasts from the vocal fold, trachea, and esophagus. mRNA levels for procollagen I (A), procollagen III (B), elastin (C), and HAS I (D) were examined by quantitative real-time PCR and are normalized to those of fibroblasts from the vocal fold. Values are expressed as means ± SEM. *, P < .05; **, P < .01; ***, P < .001.
Effects of steroid hormones on ECM gene expression in cervicothoracic fibroblasts
Effects of testosterone
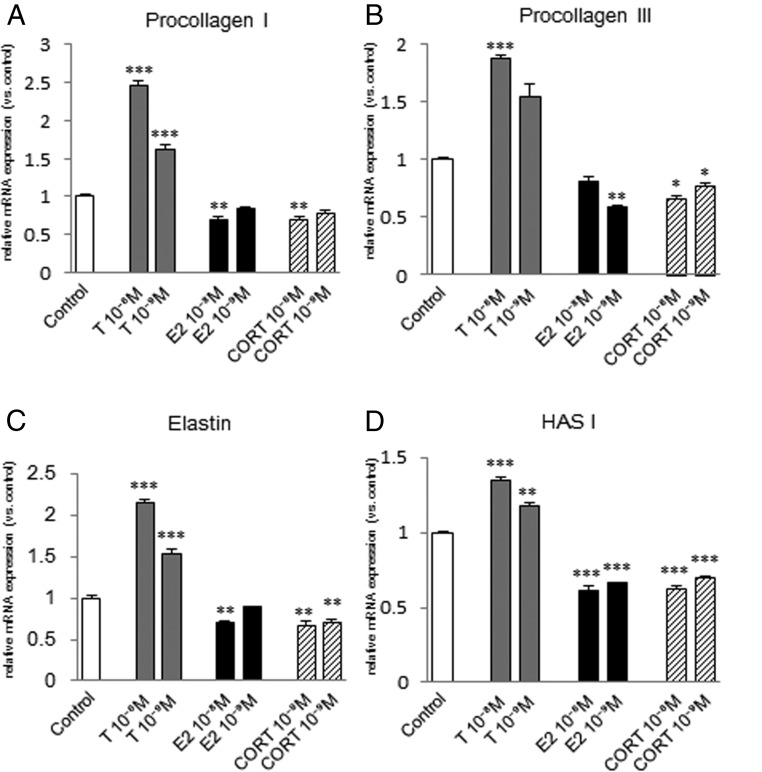
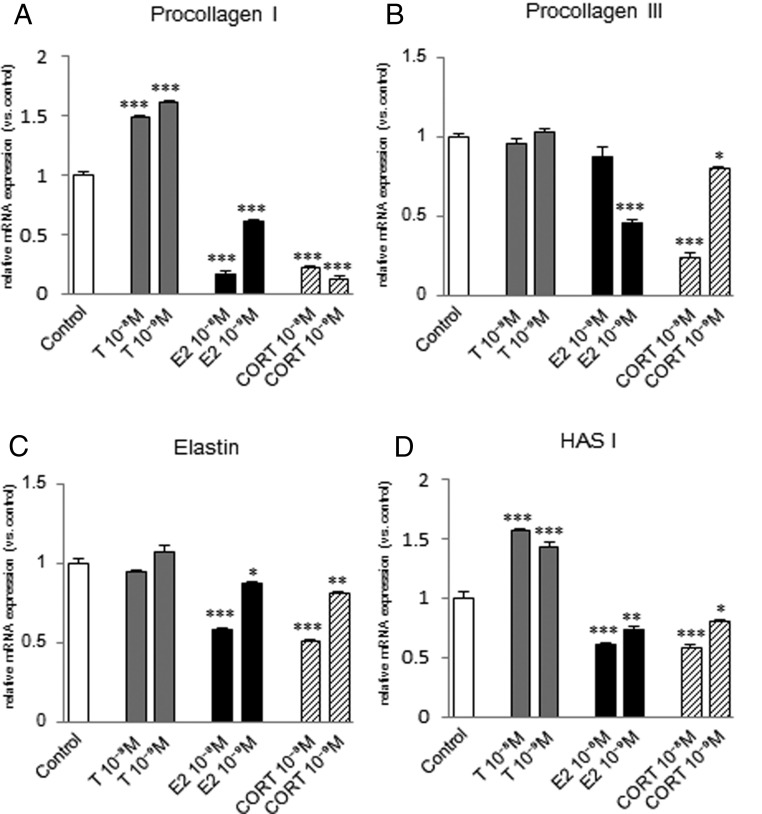
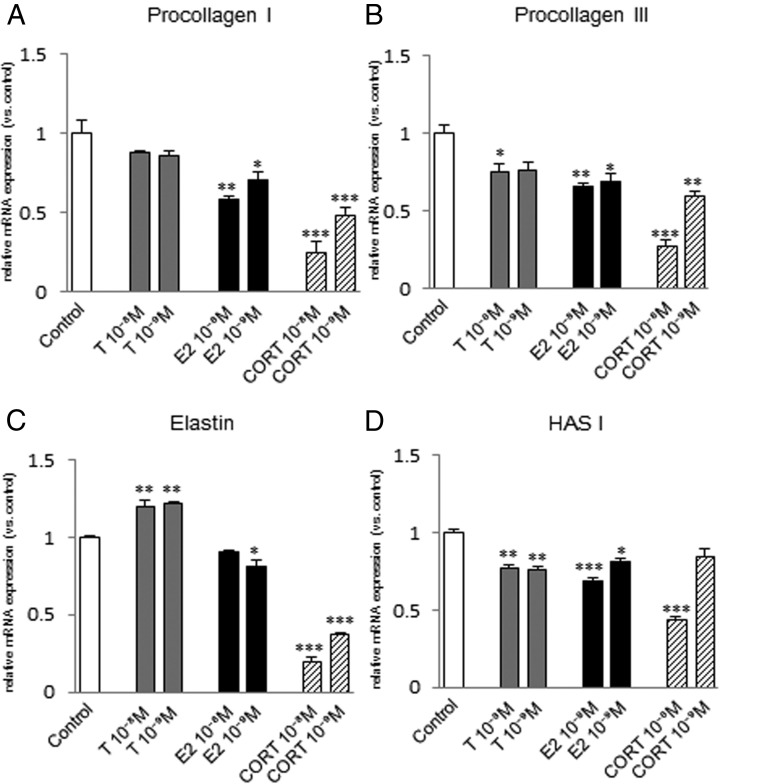
Procollagen I mRNA expression was significantly increased by 10−8 M or 10−9 M testosterone in fibroblasts from the vocal fold (Figure 5A) and trachea (Figure 6A), but not in those from the esophagus (Figure 7A). Procollagen III mRNA expression was significantly increased in fibroblasts from the vocal fold by 10−8 M testosterone (Figure 5B), but not in those from the trachea (Figure 6B), and was significantly decreased by 10−8 M testosterone in fibroblasts from the esophagus (Figure 7B). Elastin mRNA expression was induced by 10−8 M or 10−9 M testosterone in fibroblasts from the vocal fold (Figure 5C) and esophagus (Figure 7C), but not in those from the trachea (Figure 6C). HAS I mRNA expression was significantly increased by 10−8 M or 10−9 M testosterone in fibroblasts from the vocal fold (Figure 5D) and trachea (Figure 6D), but decreased in those from the esophagus (Figure 7D).
Figure 5.
Expression levels of ECM genes in fibroblasts from the vocal fold after steroid hormone administration. mRNA levels for procollagen I (A), procollagen III (B), elastin (C), and HAS I (D) were examined by quantitative real-time PCR 48 hours after testosterone (T), estradiol (E2), or corticosterone (CORT) administration at 10−8 or 10−9 M. mRNA levels of ECM components were normalized to those of the control in each trial. Values are expressed as means ± SEM. *, P < .05 vs control; **, P < .01 vs control; ***, P < .001 vs control.
Figure 6.
Expression levels of ECM genes in fibroblasts from the trachea after steroid hormone administration. mRNA levels for procollagen I (A), procollagen III (B), elastin (C), and HAS I (D) were examined by quantitative real-time PCR 48 hours after testosterone (T), estradiol (E2), or corticosterone (CORT) administration at 10−8 or 10−9 M. mRNA levels of ECM components were normalized to those of the control in each trial. Values are expressed as means ± SEM. *, P < .05 vs control; **, P < .01 vs control; ***, P < .001 vs control.
Figure 7.
Quantitative expression levels of ECM genes in fibroblasts from the esophagus after steroid hormone administration. mRNA levels for procollagen I (A), procollagen III (B), elastin (C), and HAS I (D) were examined by quantitative real-time PCR 48 hours after testosterone (T), estradiol (E2), or corticosterone (CORT) administration at 10−8 or 10−9 M. mRNA levels for ECM components were normalized to those of the control in each trial. Values are expressed as means ± SEM. *, P < .05 vs control; **, P < .01 vs control; ***, P < .001 vs control.
Effects of estradiol
Procollagen I mRNA was significantly reduced in fibroblasts from the vocal fold at 10−8 M estradiol (Figure 5A), trachea at 10−8 M or 10−9 M (Figure 6A), and esophagus at 10−8 M or 10−9 M (Figure 7A). Procollagen III mRNA was significantly lowered in fibroblasts from the vocal fold at 10−9 M estradiol (Figure 5B), trachea at 10−9 M (Figure 6B), and esophagus at 10−8 M or 10−9 M (Figure 7B). Either 10−8 M or 10−9 M estradiol significantly reduced elastin mRNA (Figures 5C, 6C, and 7C) and HAS I mRNA (Figures 5D, 6D, and 7D) in all 3 types of fibroblasts.
Effects of corticosterone
Corticosterone treatment significantly reduced procollagen I, procollagen III, elastin, and HAS I mRNA levels in all 3 types of fibroblasts, except for procollagen I mRNA in fibroblasts from the vocal fold at 10−9 M corticosterone and HAS I mRNA in those from the esophagus at 10−9 M corticosterone (Figures 5, A–D, 6, A–D, and 7, A–D).
Effects of castration on ECM protein expression in the vocal fold
Collagen type I, collagen type III, and elastin were particularly strongly expressed in the superficial layer of the lamina propria (Supplemental Figure 4, A–C), whereas HA was strongly expressed in the middle and deep layers (Supplemental Figure 4D). There was no marked difference between the distribution of ECM protein in the sham and GDX groups; however, the ECM protein level tended to be lower in the GDX group.
Discussion
The results of this study show that testosterone, estradiol, and corticosterone have different effects on cell proliferation and mRNA expression of ECM-associated genes in cervicothoracic fibroblasts. Fibroblasts from the 3 cervicothoracic regions showed variable ECM synthesis properties and responses to the steroid hormones. To the best of our knowledge, this is the first evidence that testosterone causes increased expression of ECM-associated genes and proteins, and this may occur because vocal fold fibroblasts have a higher level of AR expression than tracheal and esophageal fibroblasts.
Steroid hormones such as sex hormones and glucocorticoids are produced mainly in the gonads and adrenal glands and regulate various physiological functions by acting on different tissues. Steroid hormones pass through the cell membrane and form complexes by binding with nuclear receptors as ligands. The resulting complex binds to the DNA of specific genes and regulates the transcription of the genes. Steroid hormone receptors can be divided into 3 categories based on their unliganded distribution in cells: those that are primarily located in the cytoplasm, those that are located in the nucleus, and those with a mixed cytoplasmic and nuclear distribution (40, 41). However, in all cases, binding of ligands to their receptors leads to almost complete nuclear translocation of the receptors and recruitment of proteins to form large complexes with cofactors that induce or repress gene transcription (42). Similarly, in this study, the subcellular distribution of AR, ERα, and GR in cultured fibroblasts from different cervicothoracic regions indicated that ligand binding led to almost complete nuclear localization of the receptors in all cases, whereas there were slight differences in the intracellular distribution of AR, ERα, and GR in the absence of ligands. In particular, castration caused changes in the AR distribution in vocal fold lamina propria fibroblasts. This finding was consistent with the results of experiments conducted on cultured vocal fold fibroblasts and indicates that responses of fibroblasts from each region to steroid hormones are mediated by intracellular trafficking of receptors and are not the same for androgens, estrogens, and glucocorticoids. Expression of AR also varied according to the origin of the fibroblasts, whereas ERα and GR were 100% immunopositive in all fibroblasts from the 3 cervicothoracic regions. Thus, the responses of cervicothoracic fibroblasts to androgens might be determined by their expression levels of AR.
Steroid hormones also inhibited the growth of fibroblasts from the vocal fold, trachea, and esophagus. Estrogens and glucocorticoids have previously been found to inhibit the growth of fibroblasts (1, 12, 16, 43–45), whereas there have been various reports on the impact of androgens on the proliferative capacity of fibroblasts. Testosterone or dihydrotestosterone, a more potent androgen, inhibited proliferation of human skin fibroblasts and hair follicle papilla cells (7, 46), which is similar to the androgen effect on cervicothoracic fibroblasts in the present study, but promoted growth of human gingival fibroblasts (10, 17). The discrepancies are probably due to species differences, different dosages of androgen, or different sites and locations of the fibroblasts.
We also found that steroid hormones affect mRNA expression of ECM-associated genes in cervicothoracic fibroblasts. Previous reports on the impact of steroid hormones on ECM synthesis by fibroblasts have shown that testosterone promotes synthesis of collagen in rat coronary artery adventitial fibroblasts (13) and that estrogen inhibits synthesis of collagen in these cells (13) and decreases glycosaminoglycan levels in the uterus of rats (47). Glucocorticoids inhibit the synthesis of collagen (16), elastin (8, 9), and glycosaminoglycans (2) in human fibroblasts. The findings were essentially consistent with the results of our study. Estrogens also promote synthesis of collagen in mouse dermal fibroblasts (4, 11). The discrepancies are probably due to species differences or different sites and locations of the fibroblasts.
Evaluation of the actions of steroid hormones on fibroblasts from different tissues is limited (4, 24, 25). Our data reveal that fibroblasts from 3 cervicothoracic areas have different ECM synthesis capacities and different responses to steroid hormones. This may be due to differentiation of each fibroblast in accordance with the properties required by each organ or tissue. The particular ability of vocal fold fibroblasts to synthesize elastin might be present because the vocal folds must maintain their elasticity as phonatory organs. In addition, the marked synthesis of HAS I by tracheal fibroblasts may be attributable to the water retention required to maintain the function of the trachea. Hosokawa et al (4) found that different responses of fibroblasts were probably related to a specific stage of development and that the specificities of fibroblasts resulted in organ-specific responses of connective tissues. In the current study, the changes in the capacity of cervicothoracic fibroblasts to synthesize ECM caused by addition of testosterone varied with the site and location of the fibroblasts. Testosterone increased expression of procollagen I, procollagen III, elastin, and HAS I mRNAs in fibroblasts from the vocal fold, but did not consistently increase expression of all of these mRNAs in fibroblasts from the trachea and esophagus. This result might be due to the differences in the levels of AR among the 3 types of fibroblasts. Experiments using cultured cells and tissue sections showed that vocal fold fibroblasts expressed AR more strongly than tracheal and esophageal fibroblasts. Experiments using tissue sections showed no AR expression in tracheal and esophageal fibroblasts, in contrast to the results in cultured cells perhaps because the AR expression levels in tissues were below the limit of immunohistochemical detection. Brown and Migeon (3) found that the AR content of genital skin fibroblasts is higher than that in fibroblasts from nongenital skin sites. There was no apparent difference among the sites and locations in terms of the changes in the capacity of fibroblasts to synthesize ECM in response to estradiol or corticosterone, which might reflect the absence of differences in the levels of ERα and GR among cervicothoracic fibroblasts. Experiments in cultured cells and tissue sections showed the presence of both AR-positive and AR-negative cells in the fibroblasts. This may have been due to differences between the fibroblasts in terms of degrees of differentiation, differences in cell cycle stage (resting phase/active phase), and differences in terms of localization.
The vocal fold is believed to be an important target for steroid hormones and particularly for sex steroids. Administration of anabolic-androgenic steroids to female rats results in thickening of the vocal folds, including the lamina propria (48). This was in agreement with our experiment results, in which castration caused a decrease in vocal cord ECM expression in the vocal fold lamina propria. Ovariectomy in rats causes histopathological changes in larynx mucosa, with subepithelial edema, inflammation, and cilia and goblet cell loss (49), whereas corticosteroid injection in rabbit vocal fold injury was found to reduce collagen deposition during acute wound healing (50). However, the details of the underlying mechanisms of these steroidal actions remain to be determined, and even the effects of steroid hormones on fibroblasts in the lamina propria of the vocal fold are not yet well understood. The results of this study provide a new avenue to explain the mechanism of action of the effects of steroid hormones on the vocal folds. Testosterone administration to vocal fold fibroblasts promoted ECM gene expression, and this may be associated with male vocal changes with increases in the size and thickness of the vocal folds at puberty. Moreover, testosterone increased ECM gene expression more strongly in fibroblasts from the vocal folds than in those from the trachea and esophagus, which may be associated with greater changes in the vocal folds at puberty compared with those in the trachea and esophagus. Estradiol administration to fibroblasts from the vocal fold suppressed ECM gene expression, which may be related to the rise in voice pitch in females before ovulation, when serum estradiol levels are highest during the menstrual cycle. Corticosterone also suppressed ECM gene expression in fibroblasts from the vocal fold, consistent with the efficacy of laryngeal corticosteroid injection for treatment of vocal fold scars and the induction of vocal fold atrophy caused by inhalation of corticosteroids.
In summary, the results of this study show that steroid hormones exert regionally specific effects on cervicothoracic fibroblasts with different properties due to binding to a specific receptor. These findings suggest that the cervicothoracic fibroblast response to testosterone is determined by the expression level of AR. The data may also explain the mechanism underlying the effects of steroid hormones on the vocal folds. In particular, the finding that fibroblasts from the vocal fold show a higher level of AR expression than those from the trachea and esophagus may be related to the androgenic effects specific to the vocal fold. These findings may help to clarify the fundamental mechanisms of voice change and mutational voice disorder during puberty.
Acknowledgments
This work was supported by the Ministry of Education, Science, Sports, Culture and Technology, Japan (Grants-in-Aid for Scientific Research KAKENHI 24300128 to M.K., KAKENHI 25293350 to Y.H., and KAKENHI 23500396 to K.I.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- androgen receptor
- ECM
- extracellular matrix
- ERα
- estrogen receptor α
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GDX
- gonadectomized
- HA
- hyaluronic acid
- HAase
- hyaluronidase
- HAS
- hyaluronic acid synthase
- HSP
- heat shock protein
- ir
- immunoreactive
- PB
- phosphate buffer
- PFA
- paraformaldehyde.
References
- 1. Grossfeld H. Action of adrenalcortical steroids on cultured cells. Endocrinology. 1959;65:777–784. [DOI] [PubMed] [Google Scholar]
- 2. Castor CW. Adrenocorticoid suppression of mucopoly-saccharide formation in human connective tissue cell cultures. J Lab Clin Med. 1962;60:788–798. [PubMed] [Google Scholar]
- 3. Brown TR, Migeon CJ. Cultured human skin fibroblasts: a model for the study of androgen action. Mol Cell Biochem. 1981;36:3–22. [DOI] [PubMed] [Google Scholar]
- 4. Hosokawa M, Ishii M, Inoue K, Yao CS, Takeda T. Estrogen induces different responses in dermal and lung fibroblasts: special reference to collagen. Connect Tissue Res. 1981;9:115–120. [DOI] [PubMed] [Google Scholar]
- 5. Mecham RP, Morris SL, Levy BD, Wrenn DS. Glucocorticoids stimulate elastin production in differentiated bovine ligament fibroblasts but do not induce elastin synthesis in undifferentiated cells. J Biol Chem. 1984;259:12414–12418. [PubMed] [Google Scholar]
- 6. Smith TJ. Dexamethasone regulation of glycosaminoglycan synthesis in cultured human skin fibroblasts. Similar effects of glucocorticoid and thyroid hormones. J Clin Invest. 1984;74:2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kiesewetter F, Arai A, Schell H. Sex hormones and antiandrogens influence in vitro growth of dermal papilla cells and outer root sheath keratinocytes of human hair follicles. J Invest Dermatol. 1993;101(1 Suppl):98S–105S. [DOI] [PubMed] [Google Scholar]
- 8. Kähäri VM. Dexamethasone suppresses elastin gene expression in human skin fibroblasts in culture. Biochem Biophys Res Commun. 1994;201:1189–1196. [DOI] [PubMed] [Google Scholar]
- 9. Russell SB, Trupin JS, Kennedy RZ, Russell JD, Davidson JM. Glucocorticoid regulation of elastin synthesis in human fibroblasts: down-regulation in fibroblasts from normal dermis but not from keloids. J Invest Dermatol. 1995;104:241–245. [DOI] [PubMed] [Google Scholar]
- 10. Coletta RD, Reynolds MA, Martelli-Junior H, Graner E, Almeida OP, Sauk JJ. Testosterone stimulates proliferation and inhibits interleukin-6 production of normal and hereditary gingival fibromatosis fibroblasts. Oral Microbiol Immunol. 2002;17:186–192. [DOI] [PubMed] [Google Scholar]
- 11. Thornton MJ. The biological actions of estrogens on skin. Exp Dermatol. 2002;11(6):487–502. [DOI] [PubMed] [Google Scholar]
- 12. Liu YM, Choy KW, Lui WT, Pang MW, Wong YF, Yip SK. 17β-Estradiol suppresses proliferation of fibroblasts derived from cardinal ligaments in patients with or without pelvic organ prolapse. Hum Reprod. 2006;21:303–308. [DOI] [PubMed] [Google Scholar]
- 13. Jenkins C, Milsted A, Doane K, Meszaros G, Toot J, Ely D. A cell culture model using rat coronary artery adventitial fibroblasts to measure collagen production. BMC Cardiovasc Disord. 2007;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soldano S, Montagna P, Brizzolara R, et al. Effects of estrogens on extracellular matrix synthesis in cultures of human normal and scleroderma skin fibroblasts. Ann NY Acad Sci. 2010;1193:25–29. [DOI] [PubMed] [Google Scholar]
- 15. Zhou H, Kimura K, Orita T, Nishida T, Sonoda KH. Inhibition by female sex hormones of collagen degradation by corneal fibroblasts. Mol Vis. 2011;17:3415–3422. [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou H, Sivasankar M, Kraus DH, Sandulache VC, Amin M, Branski RC. Glucocorticoids regulate extracellular matrix metabolism in human vocal fold fibroblasts. Laryngoscope. 2011;121:1915–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohno H, Kowatari Y, Owaki M, et al. Effects of androgens on cultured cells derived from canine anterior cruciate ligament. Okajimas Folia Anat Jpn. 2012;89:35–38. [DOI] [PubMed] [Google Scholar]
- 18. Gadson PF, Russell JD, Russell SB. Glucocorticoid receptors in human fibroblasts derived from normal dermis and keloid tissue. J Biol Chem. 1984;259:11236–11241. [PubMed] [Google Scholar]
- 19. Parkar MH, Newman HN, Olsen I. Polymerase chain reaction analysis of oestrogen and androgen receptor expression in human gingival and periodontal tissue. Arch Oral Biol. 1996;41:979–983. [DOI] [PubMed] [Google Scholar]
- 20. Grohé C, Kahlert S, Löbbert K, et al. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett. 1997;416:107–112. [DOI] [PubMed] [Google Scholar]
- 21. Haczynski J, Tarkowski R, Jarzabek K, et al. Human cultured skin fibroblasts express estrogen receptor alpha and beta. Int J Mol Med. 2002;10:149–153. [PubMed] [Google Scholar]
- 22. Pelletier G, Ren L. Localization of sex steroid receptors in human skin. Histol Histopathol. 2004;19:629–636. [DOI] [PubMed] [Google Scholar]
- 23. Chang HY, Chi JT, Dudoit S, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877–12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holterhus PM, Deppe U, Werner R, et al. Intrinsic androgen-dependent gene expression patterns revealed by comparison of genital fibroblasts from normal males and individuals with complete and partial androgen insensitivity syndrome. BMC Genomics. 2007;8:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takeda T, Suzuki Y, Yao CS. Experimental studies on the effect of aging and endocrine control on collagen formation in various organs. Acta Pathol Jpn. 1975;25:135–151. [DOI] [PubMed] [Google Scholar]
- 26. Harries M, Hawkins S, Hacking J, Hughes I. Changes in the male voice at puberty: vocal fold length and its relationship to the fundamental frequency of the voice. J Laryngol Otol. 1998;112:451–454. [DOI] [PubMed] [Google Scholar]
- 27. King A, Ashby J, Nelson C. Effects of testosterone replacement on a male professional singer. J Voice. 2001;15:553–557. [DOI] [PubMed] [Google Scholar]
- 28. Raj A, Gupta B, Chowdhury A, Chadha S. A study of voice changes in various phases of menstrual cycle and in postmenopausal women. J Voice. 2010;24:363–368. [DOI] [PubMed] [Google Scholar]
- 29. Fischer J, Semple S, Fickenscher G, et al. Do women's voices provide cues of the likelihood of ovulation? The importance of sampling regime. PLoS One. 2011;6:e24490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mortensen M. Laryngeal steroid injection for vocal fold scar. Curr Opin Otolaryngol Head Neck Surg. 2010;18:487–491. [DOI] [PubMed] [Google Scholar]
- 31. Krecicki T, Liebhart J, Morawska-Kochman M, Liebhart E, Zatonski M, Zalesska-Krecicka M. Corticosteroid-induced laryngeal disorders in asthma. Med Sci Monit. 2006;12:CR351–CR354. [PubMed] [Google Scholar]
- 32. Tateya T, Tateya I, Sohn JH, Bless DM. Histologic characterization of rat vocal fold scarring. Ann Otol Rhinol Laryngol. 2005;114:183–191. [DOI] [PubMed] [Google Scholar]
- 33. Brudzynski SM. Communication of adult rats by ultrasonic vocalization: biological, sociobiological, and neuroscience approaches. ILAR J. 2009;50:43–50. [DOI] [PubMed] [Google Scholar]
- 34. Peterson JR, Watts CR, Morris JA, Shelton JM, Cooper BG. Laryngeal aging and acoustic changes in male rat ultrasonic vocalizations. Dev Psychobiol. 2013;55:818–828. [DOI] [PubMed] [Google Scholar]
- 35. Goto Y, Noguchi Y, Nomura A, et al. In vitro reconstitution of the tracheal epithelium. Am J Respir Cell Mol Biol. 1999;20:312–318. [DOI] [PubMed] [Google Scholar]
- 36. Matsuda K, Sakamoto H, Mori H, et al. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett. 2008;441:94–99. [DOI] [PubMed] [Google Scholar]
- 37. Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26:235–269. [DOI] [PubMed] [Google Scholar]
- 38. Masuda H, Hosokawa N, Nagata K. Expression and localization of collagen-binding stress protein Hsp47 in mouse embryo development: comparison with types I and II collagen. Cell Stress Chaperones. 1998;3:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matsuda K, Nishi M, Takaya H, Kaku N, Kawata M. Intranuclear mobility of estrogen receptor α and progesterone receptors in association with nuclear matrix dynamics. J Cell Biochem. 2008;103:136–148. [DOI] [PubMed] [Google Scholar]
- 40. Kawata M, Matsuda K, Nishi M, Ogawa H, Ochiai I. Intracellular dynamics of steroid hormone receptor. Neurosci Res. 2001;40:197–203. [DOI] [PubMed] [Google Scholar]
- 41. Kawata M. Subcellular steroid/nuclear receptor dynamics. Arch Histol Cytol. 2001;64:353–368. [DOI] [PubMed] [Google Scholar]
- 42. Kawata M, Nishi M, Matsuda K, et al. Steroid receptor signalling in the brain—lessons learned from molecular imaging. J Neuroendocrinol. 2008;20:673–676. [DOI] [PubMed] [Google Scholar]
- 43. Harvey W, Grahame R, Panayi GS. Effects of steroid hormones on human fibroblasts in vitro. I. Glucocorticoid action on cell growth and collagen synthesis. Ann Rheum Dis. 1974;33:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pratt WB. The mechanism of glucocorticoid effects in fibroblasts. J Invest Dermatol. 1978;71:24–35. [DOI] [PubMed] [Google Scholar]
- 45. Philips N, Devaney J. Beneficial regulation of type I collagen and matrixmetalloproteinase-1 expression by estrogen, progesterone, and its combination in skin fibroblasts. J Am Aging Assoc. 2003;26:59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kitagawa T, Matsuda K, Inui S, et al. Keratinocyte growth inhibition through the modification of Wnt signaling by androgen in balding dermal papilla cells. J Clin Endocrinol Metab. 2009;94:1288–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kofoed JA, Houssay AB, Tocci AA, Curbelo HM. Effects of oestrogens upon glycosaminoglycans in the uterus of rats. Acta Endocrinol. 1972;69:87–94. [DOI] [PubMed] [Google Scholar]
- 48. Amer HE, Asker SA, Mazroa SA. Structural changes and immunohistochemical localisation of epidermal growth factor receptor in the true vocal fold of female albino rats administered anabolic, androgenic steroids, and effects of anti-androgen therapy. J Laryngol Otol. 2011;125:829–836. [DOI] [PubMed] [Google Scholar]
- 49. Surmeli M, Habesoglu TE, Habesoglu M, et al. Histopathological effects of estrogen deficiency on larynx mucosa in ovariectomised rats. Eur Arch Otorhinolaryngol. 2011;268:261–266. [DOI] [PubMed] [Google Scholar]
- 50. Campagnolo AM, Tsuji DH, Sennes LU, Imamura R, Saldiva PH. Histologic study of acute vocal fold wound healing after corticosteroid injection in a rabbit model. Ann Otol Rhinol Laryngol. 2010;119:133–139. [DOI] [PubMed] [Google Scholar]