Abstract
The aim of this study was to determine whether bisphenol A (BPA) has adverse effects on cardiovascular functions in CD-1 mice and define sex-specific modes of BPA action in the heart. Dams and analyzed progeny were maintained on a defined diet containing BPA (0.03, 0.3, 3, 30, or 300 ppm) that resulted in BPA exposures from 4–5 to approximately 5000 μg/kg · d or a diet containing 17α-ethinyl estradiol (EE; ∼0.02, 0.2, and 0.15 μg/kg · d) as an oral bioavailable estrogen control. Assessment of electrocardiogram parameters using noninvasive methods found that ventricular functions in both male and female mice were not altered by either BPA or EE. However, exposure-related changes in the rates of ventricular contraction, suggestive of a shift in sympathovagal balance of heart rate control toward increased parasympathetic activity, were detected in males. Decreased systolic blood pressure was observed in males exposed to BPA above 5 μg/kg · d and in females from the highest BPA exposure group. Morphometric histological measures revealed sexually dimorphic changes in the composition of the cardiac collagen extracellular matrix, increases in fibrosis, and evidence of modest exposure-related remodeling. Experiments using the α-selective adrenergic agonist phenylephrine found that BPA enhanced reflex bradycardia in females, but not males, revealed that BPA and EE exposure sex specifically altered the sympathetic regulation of the baroreflex circuits. Increased sensitivity to the cardiotoxic effects of the β-adrenergic agonist isoproterenol was observed in BPA- and EE-exposed females. This effect was not observed in males, in which BPA or EE exposures were protective of isoproterenol-induced ischemic damage and hypertrophy. The results of RNA sequence analysis identified significant sex-specific changes in gene expression in response to BPA that were consistent with the observed exposure-related phenotypic changes in the collagenous and noncollagenous extracellular matrix, cardiac remodeling, altered autonomic responses, changes in ion channel and transporter functions, and altered glycolytic and lipid metabolism.
As a high-volume production, chemical bisphenol A (BPA) is used primarily as a monomer in the production of polycarbonate plastics and epoxy resins. Because of this widespread use and resulting environmental contamination, measurable levels of BPA were found in more than 93% of human urine samples in the 2003–2004 National Health and Nutrition Examination Survey (1). Much concern has been raised about possible adverse health consequences related to this evident and continuous exposure to BPA. Although results have been variable and dependent on specific study cohort and analysis models used, findings from epidemiological studies suggest a link between BPA exposures and increased risk for obesity (2), diabetes and insulin resistance (3–5), hypertension in adults and obese children (6, 7), and cardiovascular disease (CVD) (3, 8). After the initial suggestion that BPA was associated with CVD, we demonstrated that subnanomolar concentrations of BPA and 17β-estradiol could sex specifically alter rapid estrogen signaling in cultured adult rodent cardiomyocytes (9).
Those effects of BPA were mediated through estrogen receptor (ER)-α- and ERβ-dependent mechanisms that influenced Ca2+ handling to modify excitation-contraction coupling. These estrogen-like actions of BPA were shown to increase arrhythmia frequencies in response to β-adrenergic stress in isolated hearts from female, but not male, rats and mice (10). Subsequent in vitro studies showed that acute exposures to supraphysiological concentrations of BPA could also decrease the rate and force of contractility and cardiac conduction velocity in hearts from female rats (11, 12). These ER-mediated negative impacts of BPA on female heart rate control are seemingly at odds with the improved cardiovascular function and improved vascular homeostasis associated with estrogen in premenopausal women, which arise in large part from beneficial effects on cholesterol and lipid metabolism and decreased incidence of coronary artery disease (13, 14). However, in contrast to those generally beneficial effects of estrogen, levels of circulating estradiol are also positively correlated with arrhythmia incidence in women (15, 16). As a result, the in vitro experimental results demonstrating alterations in conduction, and the proarrhythmogenic actions of BPA in the female rodent heart suggest that BPA exposure may be adversely affecting women's heart health.
The current study has focused on determining whether BPA when administered by oral ingestion had adverse effects on end points related to cardiovascular functions in the CD1 mouse. The framework of this study was built on our previously published analysis that assessed the effects of BPA on reproductive and metabolic functions (17, 18). Here, as in our previous analysis, exposures occurred through dietary ingestion to mimic human-relevant routes of exposures. Exposures spanned a 5 order of magnitude dose range with the exposure [∼5000 μg/kg−1 · d−1 body weight (bw)] from high dose (300 ppm) approaching the established no observed adverse effect level. In the low-dose group (0.03 ppm), exposures (4–5 μg kg−1 · d−1 bw) may approach estimated levels of human oral exposure (17–20). The five doses of BPA used also allowed us to investigate effects in a dose range that spans the European Food Safety Authority's currently recommended tolerable daily intake of 50 μg/kg−1 · d−1 bw and the recommended provisional tolerable daily intake of 5 μg/kg−1 · d−1 bw (20). Three doses of 17α-ethinyl estradiol, resulting in exposures of approximately 0.02, 0.2, and 0.15 μg/kg−1 · d−1 bw, were included as comparative controls for effects of this orally bioavailable estrogen. Assessment of cardiovascular functions in exposed animals was done using noninvasive methods to collect electrocardiogram (ECG) and hemodynamic measures [blood pressure (BP)]. Pharmacological experiments using the α-selective adrenergic agonist phenylephrin and the nonselective β-adrenergic receptor agonist, isoproterenol (Iso), were performed to assess the sex-specific effects of exposure on control of baroreflex responses and the sensitivity to catecholamine-induced ischemia and cardiac hypertrophy. Mechanistic insights into the sex-specific modes of BPA action were established by identifying exposure-related changes in the cardiac transcriptome, and functional genomic analysis was used to identify functional networks of altered gene expression in left ventricular (LV) tissues for each sex.
Materials and Methods
Animal procedures
All animal procedures were performed to ensure barrier maintenance in accordance with protocols approved by the University of Cincinnati Institutional Animal Care and Use Committee. Six-week-old Hsd:ICR mice (CD1) breeders were from Harlan Laboratories, Inc. Study animals were housed in BPA-free polyethylene cages (Innovive) with Sani-chip bedding (Irradiated Aspen Sani-chip; P. J. Murphy Forest Products Corp). Sterile drinking water with oxidizable organics reduced to less than 1% of source was generated by a dedicated water purification system (Millipore Rios 16 with ELIX UV/Progard 2) and dispensed from BPA-free polyethylene water bottles. All details of animal husbandry, elimination of uncontrolled endocrine disruptor contamination, study animal randomization, breeding, test compound source and purity, test material exposure, necropsy, and tissue isolation were described previously (17, 18, 21). Animals selected for electrocardiographic (ECG) and hemodynamic measurements and pharmacological treatments were not included in the study cohort analysis reported previously (17). Comparison of functional and morphometric measures were made for identically manipulated unexposed, or sham-treated and unexposed controls from each exposure group. For end points analyzed in live animals males and females most closely representing the mean litter body weight for that sex were selected for analysis. Each postmortem tissue sample used for gene expression analysis was from a different litter, representative of mean litter brain to body weight ratio for each sex and was part of the cohort described by Kendig et al (17). Necropsy and experimental analysis of females was performed during estrus (17, 18). With the exception of animals used for baroreflex and Iso response analysis, euthanasia, necropsy, and tissue preparation was performed beginning on postnatal day (PND) 90. For baroreflex and Iso response analysis, males and females in estrus were euthanized the day after the final treatments/analysis; the remaining females were euthanized on the first estrous day after the final treatment.
Electrocardiographic and blood pressure analysis
ECGs were collected from conscious ambulatory mice using the ECGenie screening system (Mouse Specifics, Inc) (22) and LabChart 6 software (ADInstruments). Recordings with at least 30 consecutive signals were analyzed using e-Mouse (Mouse Specifics, Inc). Data were collected in a sterile laminar flow hood to maintain barrier. For PND7 pups (4–6 g), body temperatures was maintained at 30°C in a heated recording platform, and all other data were collected at ambient air temperatures of approximately 20°C. Study animals were acclimated for at least 5 minutes and allowed to passively enter the lead II recording platform. Except for PND7, the sympathetic impact of ambient temperatures below the thermoneutral zone of 30ºC of mice was accounted for by applying an adjustment factor of −14.4 beats/min−1ºC−1 to the calculated heart rates (23). Blood pressures and heart rates were determined by tail-cuff volume pressure recording (Kent Scientific) (24, 25). Animals were habituated to the procedure on 3 consecutive days, with data collection on consecutive study days 4 and 5. External body temperature was maintained at 30ºC.
Analysis of baroreflex control and sympathetic stimulation
Beginning on PND 77, the baseline ECG measures after the mock treatment were collected on 2 consecutive days, with all the study animals receiving an ip injection of 100 μL sterile saline (0.09% NaCl). On the third experimental day, a single 5.0 μg/kg bw dose of phenylephrine HCl (Sigma-Aldrich) in sterile saline was administered ip to each animal, and the ECG data were collected at multiple time intervals between 5 and 30 minutes after the injections.
Beginning on PND 80, study animals received twice-daily ip injections of either 0.09% NaCl (mock vehicle treatment) or 5 mg/kg body weight of the nonselective β-adrenergic agonist isoproterenol-HCl (Iso; Sigma-Aldrich). Treatment was repeated for 3 consecutive days with ECG data captured between 5 and 30 minutes after the treatment. Final ECG data were measured the day after the final treatment, and the animals were then euthanized and necropsy was performed with body weights and size measured. Hearts and associated cardiac fat external to the pericardium were dissected, weighed, and prepared for histology.
Cardiac histology
Quantitative analysis of LV free-wall thickness was determined for each animal from a single section at the level of the papillary muscle as described previously (26). Tissue pathology and fibrosis were assessed by Masson's trichrome staining with blue collagen-like staining for each section analyzed by an overlay of 0.25 mm2 grid mask of 0.0625-mm2 squares onto a 1× magnification digital image. Grid intersection points of free collagen staining were scored and normalized to the LV area (ImagePro; Media Cybernetics). The areas of evident tissue necrosis and lymphocyte invasion were considered separate pathological indices, and blood vessels were excluded from the volume calculations/analysis.
RNA isolation and quantitative RT-PCR analysis
LV tissue free of extracardial fat was dissected from frozen hearts of five male and five female non-littermates from the 0 ppm BPA (control) and 3.0 ppm BPA exposure groups. RNA was isolated from each replicate sample using RNeasy (74704; QIAGEN) according to the manufacturer's protocol. For quantitative RT-PCR analysis, RNA samples were treated with TurboDNAase (Applied Biosystems). cDNA was prepared from 1 μg of RNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems). For selected genes of interest, the differences in gene expression between control and 3.0 ppm BPA groups for each sex were determined by the ΔΔ cycle threshold method with gene-specific TaqMan assays (Applied Biosystems; Supplemental Table 1).
RNA sequence (RNAseq) library construction, cluster generation, and sequencing
Isolated RNA was submitted to the Genomics Sequencing Core (University of Cincinnati Department of Environmental Health, Cincinnati, Ohio) for quality control analysis using the Agilent 2100 bioanalyzer and sequencing using the Illumina Hiseq 1000. A TruSeq RNA sample preparation kit (Illumina) was used to purify poly-A RNA from 1 μg of total RNA. Purified mRNA with an RNA integrity number of 7.0 or greater was enzymatically fragmented and random hexamer primed for the first- and second-strand cDNA synthesis with the resulting cDNA products purified using Agencourt AMPure XP beads (Beckman Coulter). Purified double-strand cDNA fragments were ligated to sample-specific indexing adapters and enriched by 10 cycles of PCR. DNA size (∼260 bp) and yield of the purified PCR products were confirmed and quantified by quantitative PCR. Individually indexed cDNA libraries of equal amounts were pooled for clustering in the cBot system (Illumina) and clustered onto a flow cell at the concentration of 8 pM using Illumina's TruSeq SR Cluster kit version 3 and then sequenced for 50 cycles using TruSeq SBS on the Illumina HiSeq system. Each sample generated approximately 30 million reads.
RNAseq data analysis and bioinformatics
Sequence reads were aligned to the mouse genome using the standard Illumina sequence analysis pipeline by the University of Cincinnati Statistical Genomics and Systems Biology Core. Sequence reads were demultiplexed and exported to fastq files using Illumina's CASAVA 1.8 software and aligned using TopHat aligner (Trapnell et al, 2009). Counts of reads aligning to each gene's coding region were summarized using ShortRead (27), and associated Bioconductor packages (GenomicFeatures, IRanges, GenomicRanges, Biostrings, Rsamtools) and were used for initial analysis (28). Individually for males or females, statistical analysis to identify genes whose expression was significantly different (P < .01) between control and BPA exposure groups was performed using the negative-binomial model of read counts as implemented in DESeq Biocondoctor package (29). For each sex, GOTERMFINDER (30) was used to identify ontology and molecular pathways associated with response to treatment. Common functional network relationships between differentially expressed genes in the entire gene set were identified or inferred and visualized using Cytoscape version 3.1 (31) and the BINGO application (32). String version 9.1 (33) was used to identify and visualize interactions between individual differentially expressed genes. Functional and phenotypic associations between the differentially expressed transcripts and human gene functions were inferred using the Genecards database (www.genecards.org).
Statistical analysis
The statistical unit used was the litter for all analyses. With the exception of the RNAseq analysis, a minimal level of statistical significance for differences in values among or between groups was considered at P < .05. All statistical analyses for differences in values compared with control were made independently for BPA and 17α-ethinyl estradiol (EE) exposures (34). Variations due to litter size differences were accounted for by considering the number of pups in a litter as a covariate in analysis of covariance (ANCOVA) for body weight. Body weight was considered as a covariate for organ weights. In cases in which variances among groups were unequal, data were log (ln) transformed for analysis. For each experimental end point, exposure groups were analyzed for statistical outliers using a Grubb's test, which identifies only a single outlier. Identification of an outlier was a rare event. Analysis of ECG parameters, wall thickness, fibrosis, and BP data were performed using a one-way or two-way ANOVA, followed by Dunnett's or Bonferroni's multiple comparison tests. Differences in mean expression values for control and exposure groups analyzed by quantitative RT-PCR were identified using a Mann-Whitney test. Analysis was done using SyStat 13 (Systat Software, Inc IL) or Prism version 5 software (GraphPad Software, Inc).
Results and Discussion
Exposure induced effects on ECG parameters and heart rate
Analysis of ECGs collected from young adults on PND 56 and PND 90 indicated a modest lengthening of the RR interval in males exposed to either BPA or EE. Whereas variability related to ambient temperature control in these ambulatory mice limited sensitive quantitative detection of differences in RR interval, ANOVA did indicate a significant difference in the mean RR interval for EE exposed males [F(3,57) = 3.195 (P = .0291)], with a significant increase in the RR interval detected in the 0.0001 ppm (0.02 μg/kg−1 · d−1) EE group. Results of tail-cuff pressure/volume analysis of heart rates confirmed that the apparent sex-specific effects on RR interval resulted in decreases in heart rates of exposed males; an ANOVA [F(5,46) = 2.505 (P = .0435)] and Dunnett's multiple comparison test indicated significant decreases in mean heart rate for all BPA exposures except 0.3 ppm (44 μg/kg−1 · d−1) (Table 1). A decreased heart rate was also observed for males in the 0.0001 ppm (0.02 μg/kg−1 · d−1) and 0.001 ppm (0.13 μg/kg−1 · d−1) EE exposure groups [F(3,35) = 6.901 (P = .0009)]. No significant effects on rate were observed in females exposed to BPA [F(5,48) = 1.045 (P = .4023)] or EE [F(3,31) = 1.353 (P = .2753)]. The lengthening of the RR interval and significant decreases in heart rates observed in BPA and EE-exposed males suggest that BPA cause a sex-specific, exposure-related shift in the sympathovagal balance of heart rate control. The finding that low levels of EE exposure result in similar decreases in heart rate suggests that effects on autonomic regulation of heart rate may be mediated by an estrogen-like mode of action.
Table 1.
Heart Rate and BP for Control or BPA- or EE-Exposed Adult CD-1 Mice
| Age, wk | Sex | Control | BPA 0.03 | BPA 0.3 | BPA 3.0 | BPA 30 | BPA 300 | EE 0.0001 | EE 0.001 | EE 0.01 |
|---|---|---|---|---|---|---|---|---|---|---|
| 8–10 | M | |||||||||
| Rate | 651.1 ± 50.2 (10) | 580.6 ± 77.9 (8)a | 599.4 ± 37.8 (8) | 588.9 ± 46.8 (10)a | 579.5 ± 55.7 (8)a | 584.9 ± 44.4 (8)a | 567 ± 43.8 (9)a | 549.7 ± 79.3 (11)a | 622.9 ± 41.9 (9) | |
| Systolic | 120.1 ± 7.6 (8) | 117.8 ± 14.4 (8) | 103.8 ± 13.26 (8)a | 99.0 ± 8.74 (10)a | 103.6 ± 10.1 (8)a | 104.4 ± 8.9 (8)a | 105.5 ± 88.9 (8)a | 113.7 ± 16.0 (11) | 121.1 ± 15.3 (10) | |
| Diastolic | 83.4 ± 7.5 (9) | 85.2 ± 9.9 (9) | 73.8 ± 8.0 (8) | 76.1 ± 10.4 (10) | 78.5 ± 8.8 (8) | 78.8 ± 8.9 (8) | 77.3 ± 6.6 (8) | 82.6 ± 10.4 (10) | 83.7 ± 10.6 (9) | |
| 8–10 | F | |||||||||
| Rate | 628.4 ± 56.7 (8) | 594.2 ± 56.3 (9) | 622.3 ± 35.7 (9) | 608.9 ± 65.3 (10) | 638.4 ± 28.9 (9) | 591 ± 86.6 (9) | 609.1 ± 61.9 (9) | 606 ± 49.23 (9) | 655.1 ± 59.8 (9) | |
| Systolic | 112.5 ± 8.6 (8) | 102.1 ± 6.7 (8) | 105.8 ± 5.5 (9) | 105.8 ± 7.0 (10) | 106.9 ± 9.0 (9) | 98.7 ± 6.7 (9)a | 107.8 ± 14.8 (10) | 103.2 ± 9.6 (5b) | 104.0 ± 7.4 (10) | |
| Diastolic | 81.2 ± 8.2 (9) | 80.0 ± 7.7 (9) | 81.1 ± 5.0 (9) | 76.4 ± 6.8 (10) | 78.7 ± 8.2 (9) | 74.9 ± 6.3 (9) | 83.1 ± 11.0 (10) | 89.2 ± 10.3 (9) | 74.9 ± 10.1 (10) |
Abbreviations: F, female; M, male. Values listed are in beats per minute or millimeters of mercury and represent the group mean ± SD; n = is shown in parentheses. Exposures are described in parts per million of diet.
Dunnent's multipal comparison test found values were significantly different from control (P < .05).
Reduced sample size resulted from miscalibration of instrument.
No effects of BPA or EE exposure on depolarization of the right and left ventricles (QRS interval), or the onset of the ventricular depolarization to the end of repolarization (QTc) were observed in either males or females (Table 2). These findings suggest that ventricular functions in both male and female mice were not overtly altered by continuous dietary exposure to BPA and EE. The PQ interval (time between atrial depolarization and beginning of ventricular depolarization), and the PR interval (time the electrical signal travels from SA to AV node) were notably lengthened in males exposed to BPA or EE (Table 2). A modest increase in the ST interval, indicating a delay in ventricular repolarization, was also observed (Table 2). This effect reached significance in males from the lowest EE exposure group. These observations are consistent with in vitro findings showing that BPA can alter cardiomyocyte contractility (9, 10), influence ion conductance to decrease conduction velocity (11), and decrease the rate and force of atrial contraction (12).
Table 2.
ECG Parameters at PND56 Male and Female CD-1 Mice
| ECG Parameter | Sex | Control | BPA 0.03 | BPA 0.3 | BPA 3.0 | BPA 30 | BPA 300 | EE 0.0001 | EE 0.001 | EE 0.01 |
|---|---|---|---|---|---|---|---|---|---|---|
| RR | M | 71.9 ± 2.0 (14) | 74.4 ± 2.4 (14) | 74.2 ± 2.6 (14) | 73.1 ± 2.9 (15) | 73.9 ± 3.0 (14) | 74.2 ± 3.2 (16) | 74.5 ± 2.7 (15)a | 73.8 ± 2.3 (17) | 72.5 ± 3.1 (15) |
| F | 72.6 ± 3.0 (15) | 74.2 ± 3.2 (14) | 73.7 ± 2.6 (17) | 73.0 ± 3.0 (17) | 74.4 ± 2.8 (13) | 73.5 ± 3.2 (16) | 72.4 ± 1.4 (16) | 74.1 ± 2.8 (16) | 73.1 ± 2.5 (16) | |
| PQ | M | 17.6 ± 2.3 (15) | 19.6 ± 2.9 (14) | 19.2 ± 2.8 (14) | 19.7 ± 3.1 (15) | 19.3 ± 2.4 (14) | 19.3 ± 2.5 (16) | 20.2 ± 2.7 (16)a | 20.2 ± 1.8 (17)a | 19.1 ± 1.9 (15) |
| F | 18.9 ± 2.2 (15) | 17.8 ± 2.8 (14) | 20.6 ± 1.7 (17) | 18.1 ± 3.5 (17) | 18.9 ± 2.9 (14) | 19.1 ± 3.3 (17) | 19.1 ± 2.9 (16) | 19.2 ± 3.2 (16) | 20.0 ± 1.7 (14) | |
| PR | M | 24.3 ± 2.2 (15) | 26.4 ± 2.9 (14) | 25.8 ± 3.0 (14) | 26.0 ± 3.5 (15) | 26.2 ± 2.6 (14) | 26.0 ± 2.8 (16) | 26.8 ± 2.7 (16)a | 26.8 ± 1.8 (17)a | 25.8 ± 2.0 (15) |
| F | 25.5 ± 2.5 (15) | 24.6 ± 2.9 (14) | 27.5 ± 1.7 (17) | 25.5 ± 2.9 (16) | 25.9 ± 2.8 (14) | 25.8 ± 3.5 (17) | 26.2 ± 2.4 (15) | 26.1 ± 3.2 (16) | 26.7 ± 2.2 (14) | |
| QRS | M | 10.1 ± 0.4 (15) | 10.2 ± 0.4 (13) | 10.3 ± 0.8 (14) | 9.9 ± 0.6 (15) | 10.3 ± 0.5 (14) | 10.2 ± 0.6 (16) | 10.1 ± 0.5 (16) | 10.2 ± 0.7 (17) | 10.2 ± 0.7 (15) |
| F | 10.2 ± 0.4 (14) | 10.6 ± 0.6 (14) | 10.4 ± 0.4 (17) | 10.3 ± 0.6 (17) | 10.4 ± 0.7 (12) | 10.5 ± 0.7 (16) | 10.1 ± 0.7 (16) | 10.4 ± 0.8 (16) | 10.2 ± 0.7 (14) | |
| QTc | M | 44.1 ± 2.1 (15) | 43.4 ± 1.9 (13) | 44.9 ± 1.9 (14) | 44.7 ± 2.0 (15) | 45.1 ± 2.5 (14) | 44.5 ± 2.0 (16) | 45.2 ± 1.3 (16) | 44.1 ± 2.0 (17) | 43.9 ± 1.5 (15) |
| F | 43.6 ± 2.1 (15) | 44.2 ± 2.4 (14) | 45.0 ± 2.4 (17) | 44.3 ± 2.5 (17) | 44.6 ± 2.5 (14) | 44.0 ± 3.3 (17) | 44.3 ± 1.9 (16) | 44.7 ± 2.7 (16) | 44.1 ± 1.3 (14) | |
| ST | M | 27.9 ± 2.0 (15) | 28.1 ± 2.5 (14) | 28.9 ± 2.6 (14) | 28.8 ± 2.2 (15) | 29.0 ± 2.2 (14) | 28.6 ± 2.0 (16) | 29.5 ± 1.8 (16)a | 28.2 ± 1.6 (17) | 27.7 ± 1.6 (15) |
| F | 27.5 ± 2.1 (15) | 28.0 ± 2.2 (14) | 28.8 ± 2.3 (17) | 28.0 ± 2.2 (17) | 28.1 ± 1.9 (13) | 27.6 ± 2.6 (16) | 28.0 ± 1.4 (16) | 28.9 ± 2.1 (15) | 28.3 ± 1.3 (14) |
Abbreviations: F, female; M, male. Values listed are in milliseconds and represent the group mean ± SD; n = is shown in parentheses.
Dunnett's multiple comparison test found values were significantly different from control (P < .05). Exposures are described in parts per million of diet.
Exposure-induced effects on blood pressures
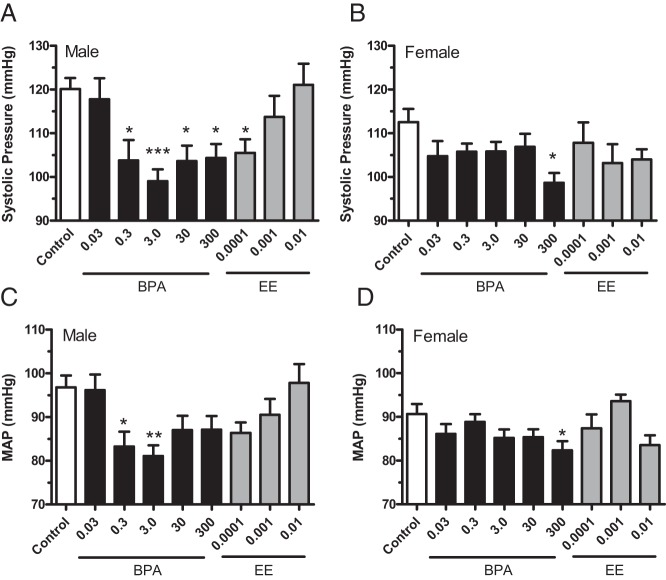
Exposure to BPA resulted in decreased systolic and mean atrial pressures (MAP) in both male and female CD1 mice (Figure 1 and Table 1). Results of ANOVA revealed significant systolic BP effects of BPA in males [F(5,46) = 5.859 (P = .0003)] and females [F(5,47) = 2.621 (P = .0357)]. In BPA-exposed males, systolic BP was significantly decreased in all but the lowest exposure group (Figure 1A). A significant reduction in systolic pressure was also observed for males in the lowest EE exposure group. In females, systolic pressures in the 300 ppm (42.3 mg/kg−1 · d−1) BPA exposure group were significantly reduced (Figure 1B), whereas EE exposures had no effect [F(3,29) = 1.187 (P = .3320)]. Exposure-induced effects of BPA were similar for MAP in males and females (Figure 1, C and D). The decreases in systolic blood pressures observed here in CD1 mice differ from results reporting no effects on systolic, but female-specific increases in diastolic pressure in C57Ll/6N mice at BPA exposures equal to and lower than the lowest dose of BPA used here (35). It is possible that lower doses of BPA have differential sex-specific effects in C57bL/6N mice or that effects noted in the older females may represent a progression of cardiovascular dysfunction, effects not yet detectable in these younger adult mice. There are, however, well-known strain-specific differences in the cardiovascular and hypertrophic responses of inbreed mice. Most notably, estrogen has opposite effects on the physiological hypertrophic growth in the hearts of C57bL/6N and the C57bL/6J substrains (36). The differences in BPA's effects on BP may arise from similar differences between inbred C57bL/6N and outbred CD1 mice.
Figure 1.
The BPA- and EE-induced changes in systolic BP and MAP of male and female CD-1 mice exposed to BPA or EE. Blood pressures of 8- to 10-week-old mice were determined by tail-cuff volume pressure recording for male (A and C) and female (B and D) CD-1 mice for each indicated BPA or EE exposure (parts per million). A significant decrease in systolic pressure for 0.3, 3.0, 30, and 300 ppm BPA-exposed groups was detected in males (A) and females (B) exposed to 300 ppm BPA. MAP was significantly reduced in 0.3 and 3.0 ppm BPA treatment groups of males (C) and in 300 ppm BPA in females (B). Data are presented as group mean ± SEM. The level of statistical significance is indicated with asterisks.
Exposure-related effects on autonomic regulation of baroreflex control
Estrogens, especially estradiol, have well-known effects on autonomic regulation of heart rate, baroreflex sensitivity, and responsiveness to β-adrenergic agonist Iso (36–38). Because estrogens increase sympathetic baroreflex sensitivity in humans and rodents (39–42), experiments to interrogate whether BPA or EE exposures resulted in modifications of sympathetic baroreceptor reflex control of heart rate were performed. The magnitude of the reflex bradycardia in response to phenylephrine in males was not significantly influenced by either BPA [F(5,44) = 0.1493 (P = .9792)] or EE [F(3,38) = 1.095 (P = .3630)] (Figure 2A). Consistent with an estrogenic mode of action, in females the magnitude of the reflex bradycardia response to phenylephrine was significantly greater for all EE [F(3,40) = 7.639 (P = .0004)] and all but the lowest BPA exposure group [F(5,58) = 4.804 (P = .0010)] (Figure 2B). The magnitude of the responses in exposed females was similar to the heightened responses of control males, suggesting a loss of the normal sex differences in baroreflex control. Because estrogen-mediated enhancement of baroreceptor-controlled reflex bradycardia is centrally mediated (39), the observed enhancement of reflex bradycardia in females, but not males, suggests that chronic BPA and EE exposure sex specifically alters central sympathetic regulation of the baroreflex circuits resulting in an estrogen-like enhancement of the baroreflex response in females (39, 41).
Figure 2.
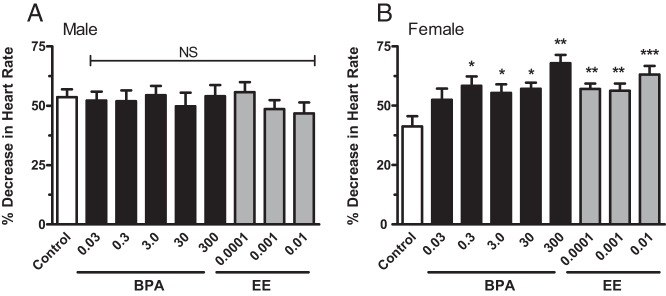
The impact of BPA and EE exposure on changes in heart rate in response to phenylephrine in male and female CD-1 mice exposed to BPA or EE. No significant effect of exposure was observed for any BPA or EE exposure in males (A). Both BPA and EE significantly increased the magnitude of the baroreflex responsive in females (B). Significant effects were observed in all exposure groups except the lowest BPA dose group. Data are presented as group mean of the percentage peak decrease in baseline HR ± SEM. The level of statistical significance is indicated with asterisks. NS, no significant difference compared with control.
Effects of exposures on heart weight, LV wall thickness, and collagen
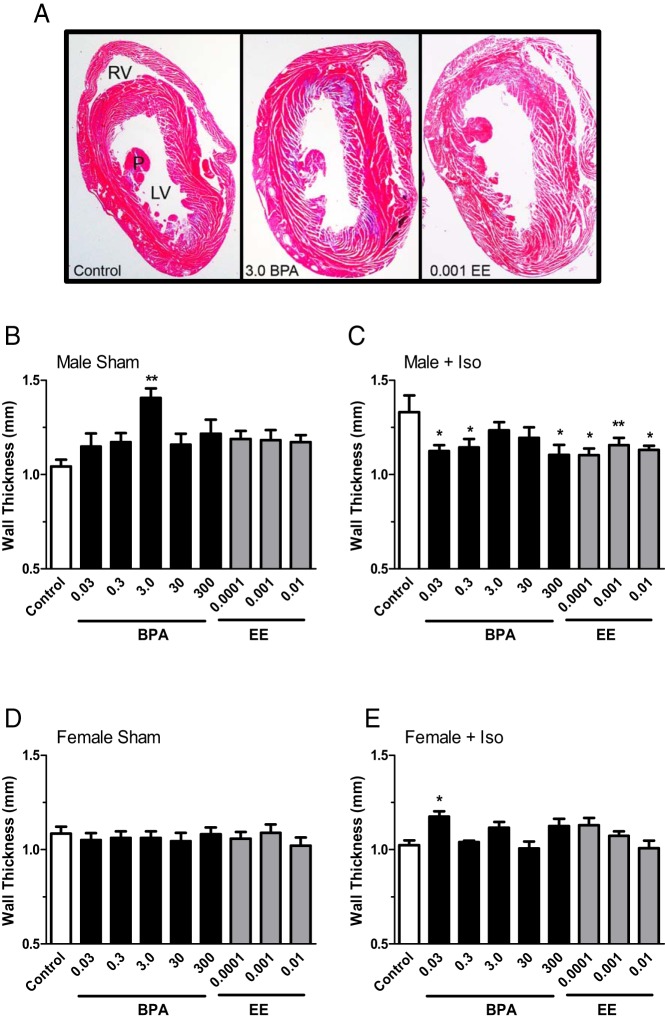
Mean heart weights (with body weight considered a covariant) of males exposed to BPA were significantly increased [F(5,62) = 3.609 (P = .0063)]. Dunnett's multiple comparison tests identified a significant increase in mean heart weights of males in the 3.0 ppm (430 μg/kg−1 · d−1) and 30 ppm (4.49 mg/kg−1 · d−1) exposure groups. Mean heart weights of EE-exposed males [F(3,53) = 2.122 (P = .1084)], and females exposed to BPA [F(5,86) = 1.666 (P = .1515)] or EE [F(3,53) = 0.7885 (P = .5057)] did not significantly differ from controls. In males LV wall thickness was also significantly [F(5,41) = 3.337 (P = .0128)] affected by BPA exposure; LV wall thickness of males in the 3.0 ppm BPA (430 μg/kg−1 · d−1) exposure group was significantly increased (Figure 3, A and B). In EE-exposed males, the mean LV wall thickness was greater than the control in each group, but the effects of exposure were not significant [F(3,28) = 2.445 (P = .0848)]. Shown in Figure 3A are representative Masson's trichrome-stained heart sections from control and 3.0-BPA, and 0.001-EE exposed males, demonstrating this modestly increased LV wall thickness. The LV wall thickness of females was not influenced by BPA [F(5,52) = 0.1806 (P = .9687)] or EE [F(3,36) = 0.6266 (P = .6025)] exposure (Figure 3D).
Figure 3.
The impact of BPA and EE on LV wall thickness and effects of exposures on the response to isoproterenol in male and female CD-1 mice exposed to BPA or EE. A, Representative photomicrographs of hematoxylin and eosin-stained sections of male hearts from the control, 3.0 ppm BPA and 0.001 EE exposure groups. LV, right ventricle (RV), and papillary muscle (P) are indicated. In sham BPA-exposed males (B), a significant increase in LV wall thickness was observed for the 3.0 ppm exposure group. Compared with sham control males, Iso increased LV wall thickness, an effect that was reversed in each BPA and EE exposure group except for the 3.0 and 30 ppm BPA exposure groups (C). In females exposure to BPA or EE did not affect LV wall thickness in sham-treated females (D). In females from the lowest BPA group (0.03), a significant increase in LV wall thickness was observed in response to Iso (E). Data are presented as group mean of wall thickness in millimeters ± SEM. The level of statistical significance determined from a two-way ANOVA, and Bonferroni's multiple comparison tests are indicated with asterisks.
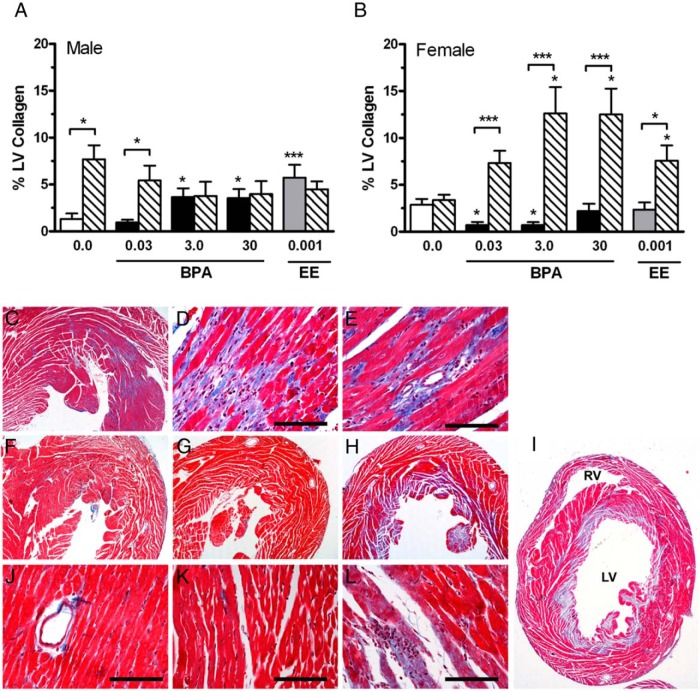
Hearts from the control, 0.03 ppm (4 μg/kg−1 · d−1), 3.0 (430 μg/kg−1 · d−1), and 30 ppm BPA (4.49 mg/kg−1 · d−1) and 0.001 ppm EE (0.13 μg/kg−1 · d−1) groups were stained with Masson's trichrome, and LV collagen content was quantified. Compared with control, a significant increase [F(4,37) = 6.820 (P = .0003)] in both interstitial and perivascular collagen was observed in the males from the 3.0 BPA, 30 BPA, and 0.001 EE groups (Figure 4A). In contrast to males, a significant decrease [F(3,34) = 3.844 (P = .0180)] in collagen was detected in females from the 0.03 and 3.0 BPA groups (Figure 4B). Shown in Figure 4C is an example of a Masson's trichrome-stained section of the male heart from the 30 ppm BPA group, demonstrating increased interstitial (Figure 4D) and perivascular (Figure 4E) collagen and fibrosis. Evident interstitial collagen was observed in control females (Figure 4F), which was significantly reduced [F(4,36) = 4.294 (P = .0109)] in female hearts from the 3.0 ppm and 30 ppm BPA groups (Figure 4, G, J, and K). The observed changes in collagen accumulation indicate that BPA has sex-specific effects on the composition of the collagen extracellular matrix of the heart.
Figure 4.
The impact of BPA and EE on LV collagen and effects of exposures on the hypertropic response to Iso ischemia in male and female CD-1 mice exposed to BPA or EE. A, In 3.0 and 30 BPA and 0.001 EE male exposure groups, a significant increase in LV collagen levels were observed compared with controls. The effects of Iso treatment on collagen accumulation in the LV for each exposure group is indicated by hatched bars. Iso increased collagen in the control and 0.03 ppm exposure groups but had no effect in the 3.0 and 30 BPA and 0.001 EE groups. B, In the female 0.03 and 3.0 BPA exposure groups, a significant decrease in LV collagen was observed compared with controls. Control females were protected from Iso-induced increases in collagen accumulation. Iso significantly increase collagen accumulation in each of the BPA and EE exposures groups. Representative photomicrographs of Masson's trichrome-stained sections of male hearts from the control (C) and 30 ppm BPA exposure groups demonstrate increased interstitial (D) perivascular fibrosis (E). Compared with female controls (F), in the 3.0 BPA exposure group (G), decreased levels of collagen were detected in Masson's trichrome-stained sections of female hearts. In female hearts of the 3.0 BPA exposure group, Iso treatment resulted in a dramatic increase collagen staining (H), which often involved the entire LV endocardium and papillary muscle (I). Areas of infarction-like necrosis with evident myocardial necrosis and numerous infiltrates were common in sections from the 3.0 BPA exposure group (L). RV, right ventricle. Scale bar, 50 μm.
Effects of exposures on cardiac adiposity and body weights
At the time of necropsy (PND90), a notable increase in the extrapericardial visceral fat pad of BPA-exposed male mice was observed. Compared with controls, the weight of this cardiac fat was significantly increased only in BPA-exposed males [F(5,63) = 3.912 (P = .0038)], with significant increases found in the 0.03 ppm (4.0 μg/kg−1 · d−1) and 0.3 BPA (44 μg/kg−1 · d−1) exposure groups. No significant differences in body weight [F(5,65) = 0.705 (P = .621)] or the weight of the perigonadal fat depot [F(5,64) = 0.1.244 (P = .299)] in the BPA-exposed males were observed (Table 3). In BPA-exposed females, a significant increase in body weight was detected [F(5,85) = 2.592 (P = .031)] in the 3 ppm (560 μg/kg−1 · d−1), 30 ppm (5.52 mg/kg−1 · d−1), and 300 ppm (423 mg/kg−1 · d−1) exposure groups and in the low-dose EE group. There were no effects on cardiac fat [F(5,85) = 1.430 (P = .222)] or perigonadal fat [F(5,85) = 0.415 (P = .837)] weight in BPA-exposed females (Table 3). Significant increases in mean body weights were not observed in the larger cohort of litter mates from this study (17); the apparent discrepancy between those data and the results presented here likely arise from the selection of animals representing the litter mean and the influence of decreased variability in the study population. In light of a failure to detect evidence of increased adiposity, the changes in weight noted here should be interpreted with caution due to these experimental influences, potential bias, and possible false-positive identification.
Table 3.
Effects of Exposures and Iso Treatment
| Treatment | Control | BPA 0.03 | BPA 0.3 | BPA 3.0 | BPA 30 | BPA 300 | EE 0.0001 | EE 0.001 | EE 0.01 |
|---|---|---|---|---|---|---|---|---|---|
| Male | |||||||||
| Pretreatment body weight, g | |||||||||
| Sham | 39.3 ± 3.4 | 42.2 ± 5.3 | 40.0 ± 3.8 | 41.8 ± 2.9 | 41.0 ± 4.5 | 41.3 ± 3.9 | 42.1 ± 4.4 | 41.8 ± 4.9 | 38.2 ± 3.9 |
| Iso | 38.1 ± 3.5 | 42.8 ± 5.9 | 39.3 ± 5.7 | 42.4 ± 1.7 | 40.8 ± 4.5 | 41.5 ± 2.4 | 40.6 ± 6.0 | 40.3 ± 4.1 | 39.4 ± 3.3 |
| Necropsy body weight, g | |||||||||
| Sham | 37.8 ± 3.9 | 39.0 ± 4.7 | 39.8 ± 1.8 | 40.5 ± 3.2 | 39.2 ± 3.9 | 39.5 ± 4.9 | 41.2 ± 4.9 | 40.4 ± 3.8 | 37.8 ± 3.8 |
| Iso | 36.4 ± 4.1 | 41.1 ± 4.4a | 38.7 ± 4.5 | 39.6 ± 4.0 | 38.3 ± 4.5 | 39.9 ± 2.8 | 39.9 ± 4.7 | 38.8 ± 4.3 | 37.6 ± 3.5 |
| Heart weight, g | |||||||||
| Sham | 0.208 ± 0.014 | 0.213 ± 0.021 | 0.225 ± 0.019 | 0.244 ± 0.026a | 0.304 ± 0.058a | 0.209 ± 0.017 | 0.241 ± 0.034 | 0.240 ± 0.028 | 0.233 ± 0.051 |
| Iso | 0.231 ± 0.021b | 0.257 ± 0.027b | 0.224 ± 0.027 | 0.258 ± 0.031 | 0.262 ± 0.048 | 0.304 ± 0.058a | 0.264 ± 0.026 | 0.263 ± 0.054b | 0.247 ± 0.013b |
| LV wall thickness, mm | |||||||||
| Sham | 1.043 ± 0.096 | 1.127 ± 0.186 | 1.136 ± 0.167 | 1.349 ± 0.161a | 1.192 ± 0.149 | 1.216 ± 0.210 | 1.188 ± 0.128 | 1.116 ± 0.153 | 1.172 ± 0.110 |
| Iso | 1.398 ± 0.262b | 1.126 ± 0.081a | 1.126 ± 0.115a | 1.234 ± 0.148 | 1.194 ± 0.162 | 1.104 ± 0.173a | 1.073 ± 0.077a | 1.124 ± 0.093a | 1.113 ± 0.033a |
| Pericardial fat, g | |||||||||
| Sham | 0.047 ± 0.016 | 0.078 ± 0.022a | 0.068 ± 0.018a | 0.064 ± 0.016a | 0.066 ± 0.018 | 0.054 ± 0.022 | 0.061 ± 0.014 | 0.063 ± 0.015 | 0.056 ± 0.021 |
| Iso | 0.050 ± 0.025 | 0.059 ± 0.018b | 0.053 ± 0.023b | 0.046 ± 0.014b | 0.053 ± 0.018b | 0.052 ± 0.015 | 0.056 ± 0.015 | 0.052 ± 0.016 | 0.047 ± 0.015 |
| Female | |||||||||
| Pretreatment body weight, g | |||||||||
| Sham | 28.3 ± 2.5 | 30.2 ± 3.8 | 30.7 ± 4.7 | 32.6 ± 3.4a | 33.0 ± 3.6a | 33.1 ± 4.8a | 35.7 ± 6.9a | 33.3 ± 1.9a | 30.2 ± 3.6 |
| Iso | 28.6 ± 1.7 | 30.3 ± 3.6 | 30.8 ± 3.8 | 32.3 ± 2.6a | 31.4 ± 2.9 | 32.2 ± 2.8a | 35.5 ± 5.4a | 32.7 ± 5.7 | 29.9 ± 4.0 |
| Necropsy body weight, g | |||||||||
| Sham | 28.0 ± 3.2 | 29.6 ± 3.9 | 29.8 ± 3.1 | 31.4 ± 3.1a | 31.7 ± 3.9a | 31.5 ± 3.5a | 33.3 ± 6.3a | 31.5 ± 3.3 | 28.7 ± 2.2 |
| Iso | 28.8 ± 2.0 | 30.0 ± 3.3 | 28.7 ± 2.9 | 31.2 ± 3.0 | 29.8 ± 3.9 | 30.5 ± 3.6 | 31.3 ± 5.0 | 29.5 ± 4.6 | 26.4 ± 2.8 |
| Heart weight, g | |||||||||
| Sham | 0.176 ± 0.013 | 0.180 ± 0.017 | 0.180 ± 0.029 | 0.185 ± 0.037 | 0.188 ± 0.027 | 0.199 ± 0.036 | 0.183 ± 0.030 | 0.170 ± 0.026 | 0.145 ± 0.016 |
| Iso | 0.204 ± 0.015b | 0.215 ± 0.040b | 0.229 ± 0.026b | 0.216 ± 0.025b | 0.221 ± 0.035 | 0.211 ± 0.029 | 0.218 ± 0.028b | 0.225 ± 0.030b | 0.179 ± 0.001 |
| LV wall thickness, mm | |||||||||
| Sham | 1.110 ± 0.126 | 1.047 ± 0.115 | 1.062 ± 0.110 | 1.062 ± 1.115 | 1.085 ± 0.164 | 1.082 ± 0.116 | 1.058 ± 0.112 | 1.088 ± 0.141 | 1.020 ± 0.149 |
| Iso | 1.024 ± 0.076 | 1.175 ± 0.078a | 1.039 ± 0.023 | 1.115 ± 0.086 | 1.006 ± 0.101 | 1.125 ± 0.121 | 1.092 ± 0.141 | 1.073 ± 0.078 | 1.008 ± 0.110 |
| Pericardial fat, g | |||||||||
| Sham | 0.069 ± 0.016 | 0.063 ± 0.019 | 0.072 ± 0.015 | 0.079 ± 0.014 | 0.079 ± 0.014 | 0.073 ± 0.020 | 0.077 ± 0.024 | 0.079 ± 0.032 | 0.086 ± 0.032 |
| Iso | 0.044 ± 0.017b | 0.057 ± 0.016a,b | 0.062 ± 0.016a,b | 0.064 ± 0.017a,b | 0.052 ± 0.008a,b | 0.061 ± 0.018a | 0.054 ± 0.017a,b | 0.063 ± 0.022a,b | 0.047 ± 0.011b |
Values represent group mean ± SD.
Significantly different from control within treatment.
Significantly different from sham group in the same diet (P < .05 as assessed by ANCOVA or ANOVA where appropriate).
Effects of exposures on β-adrenergic receptor-stimulated hypertrophy
To investigate in more detail the impacts of BPA or EE exposure on sympathetic drive, fibrosis, and pathological hypertrophy in the heart, 8- to 10-week-old males and females were treated with Iso twice daily for 3 consecutive days. Monitoring of ECG data in males and females immediately after Iso treatment found significant S-T depression indicative of Iso-induced ischemia. The impacts of BPA or EE exposure on the structural and functional response of the heart to treatment were compared with sham-treated animals from each exposure group. In males, a two-way ANCOVA indicated significant effects of both BPA exposure [F(5,39) = 3.733 (P = .0074)] and Iso treatment [F(5,39) = 6.309 (P = .0163)], and increases in mean heart weight after the Iso treatment were significant in the 0.03 ppm BPA and 300 ppm BPA, 0.001 EE, and 0.01 EE exposure groups (Table 3). In BPA-exposed males LV wall thickness was also increased [F(5,89) = 2.474 (P = .038)], but the effect of Iso treatment was not significant [F(1,89) = 0.0049 (P = .944)]. Hypertrophic effects were evident in control males in which Iso treatment significantly increased LV wall thickness compared with sham-treated control (Table 3 and Figure 3, B and C). Compared with their intragroup sham controls, a significant reduction in LV wall thickness was evident in the 0.03, 0.3, and 300 ppm BPA and each EE group. This effect is similar to the normal protection against the ischemic effects of Iso observed in unexposed females (Table 3).
Unlike their male counterparts, neither BPA [F(5,164) = 1.500 (P = .1924)] nor EE [F(3,106) = 1.537 (P < .2093)] exposures had a significant effect on female heart weights. The loss of normal female-specific protection from Iso treatment was evident in the significantly increased mean heart weights of both BPA- [F(1,164) = 24.44 (P < .0001)] and EE-exposed [F(1,106) = 20.52 (P < .0001)] females (Table 3). Compared with sham-treated females from the same exposure group, ANCOVA and post hoc analysis indicated that Iso treatment increased the mean heart weight in all groups except the 30 and 300 ppm BPA and high-dose EE groups (Table 3). Treatment with Iso resulted in a small but significant increase in LV wall thickness in the 0.03 BPA female exposure groups (Figure 3, D and E).
Iso treatments also resulted in significant [F(1,77) = 8.182 (P = .0054)] increases in LV collagen deposition in males, with significant increases compared with sham detected for control (0 BPA) and lowest-dose BPA groups only; the lack of effects observed with increasing exposures of BPA and EE are also reminiscent of estrogen-like protection from Iso-induced pathology typical of unexposed females (Figure 4A). In BPA- and EE-exposed females, a remarkable increase in the sensitivity to Iso induced ischemic damage were observed. Although Iso treatment did not affect collagen in the female control group (Figure 4B and Table 3), Iso significantly [(1,91) = 75.84 (P < .0001)] increased collagen accumulation in all of the BPA and EE exposure groups analyzed (Figure 4B). Histological analysis of hematoxylin and eosin- and Masson's trichrome (Figure 4, H and I)-stained female hearts also identified areas of infarction-like necrosis, with evident myocardial necrosis and numerous infiltrates (Figure 4L). In some female hearts from the 3.0 and 30 ppm groups, this damage involved the entire LV endocardium and papillary muscle (Figure 4, H and I), demonstrating that BPA results in a harmful increase in sensitivity of the female heart to ischemia in response to β-adrenergic stress.
The amount of pericardial fat was also differentially affected by Iso treatment and exposure in males and females (Table 3). In males, both BPA exposure [F(5,60) = 4.175 (P = .0025)] and Iso treatment [F(1,130) = 11.69 (P = .0008)] significantly affected cardiac fat mass. For EE-exposed males, neither exposure [F(3,94) = 1.727 (P = .1646)] nor Iso treatment [F(1,94) = 2.484 (P = .1154)] significantly affected pericardial fat mass. Compared with sham-treated controls, the amount of pericardial fat was significantly greater in sham-treated males exposed to 0.03, 0.3, and 3.0 BPA. Compared with sham-treated males from the same exposure group, Iso treatment caused a significant decrease in pericardial fat mass in males exposed to 0.03, 0.3, 3.0, and 30 BPA. In sham-treated females, neither BPA [F(5,85) = 1.965 (P = .0921)] nor EE [F(3,54) = 0.9602 (P = .4182)] exposure significantly changed pericardial fat mass compared with sham-treated control, whereas Iso treatment significantly [F(1,160) = 36.86 (P < .0001)] decreased pericardial fat in all exposure groups. These differential and sex-specific effects of β-adrenergic stimulation on pericardial fat, a fat depot that plays a critical role in myocardial metabolism (43), suggests that BPA exposure also affects the autonomic regulation of cardiac fat metabolism in males, with the male phenotypes shifting toward increased sympathetic responsiveness characteristic of unexposed females.
Effects of BPA exposure on the transcriptome of LV cardiac tissue
To determine the nature of the effects of BPA exposure on gene regulation in the heart, RNA isolated from LV cardiac tissues from control and 3.0 ppm BPA-exposed males (430 μg/kg−1 · d−1) and females (530 μg/kg−1 · d−1) was used for RNAseq analysis. For males, 144 genes were identified as differentially expressed compared with control (P < .01); the expression of 117 of those transcripts (82%) was reduced by exposure. In BPA-exposed females, 193 genes were identified as differentially expressed in LV cardiac tissue. In contrast to males, expression in the BPA-exposed females was increased for 187 (of the differentially expressed transcripts 96%) compared with control (Supplemental Data). In males, gene ontology (GO) group analysis of the differentially expressed gene set identified significant (P < .01) enrichment for 151 GO molecular processes and seven GO biological functions related to extracellular matrix and collagen (Supplemental Table 2). For females, GO analysis identified significant biological functions related to glycosaminoglycan and heparin binding and significant association with cytoplasmic and ribosomal cellular components (Supplemental Table 2). Additional analysis of network interactions and functional associations for the differentially regulated gene sets suggested that BPA exposure had important sex-specific effects related to changes in extracellular matrix (ECM) structure and signaling functions, ion channel activity, mitochondrial function, and altered lipid and glucose metabolism consistent with observed phenotypic changes (Supplemental Table 3).
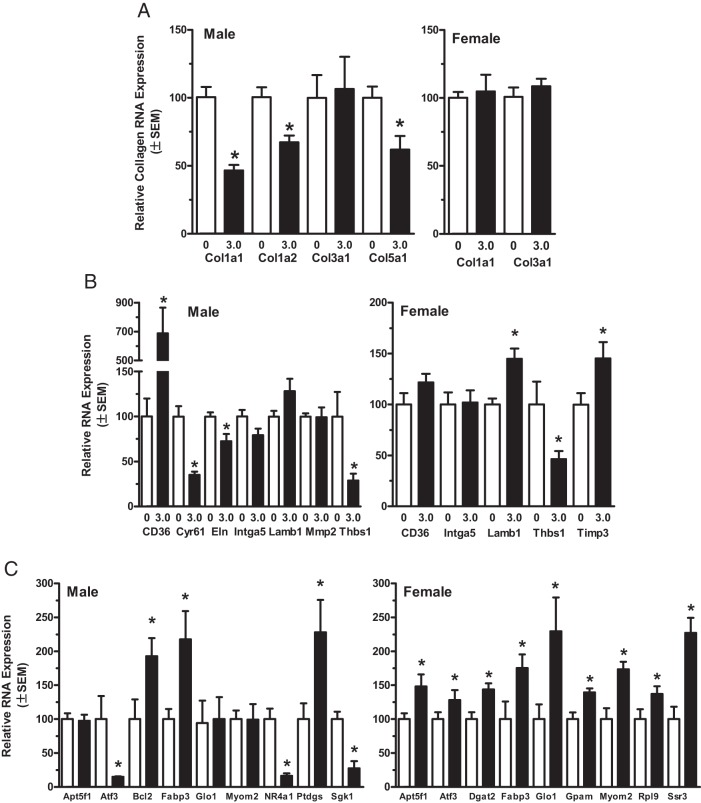
Quantitative RT-PCR was used to validate identified differential expression of selected genes implicated in observed alterations in ECM and cardiac remodeling, and to investigate possible sex-specific effects resulting from exposures (Figure 5 and Supplemental Table 3). This analysis confirmed BPA-induced changes in collagen gene expression with the levels of Col1a1, Col1a2, and Col5a1 significantly reduced in BPA-exposed males, whereas Col3a1 was unaffected (Figure 5A). The levels of Col1a1 and Col3a1 mRNA were unchanged in females. The expression of a number of proteases and regulators of protease activity that establish and maintain ECM composition were also coordinately altered in the BPA exposure groups, and these include tissue inhibitor of metalloproteinase 3 (Timp3), Serpine2, and Plod2 in females (Supplemental Table 3 and Figure 5D). In males, the expression of Adam8, Adamts1, and Mmp8 was significantly decreased, supporting the morphological finding of a relative increase of collagen deposition in exposed males (Supplemental Table 3).
Figure 5.
Quantitative RT-PCR analysis of selected in LV genes identified as differentially expressed in BPA-exposed male and female CD-1 mice. For selected genes of interest, the relative differences in gene expression between control and 3.0 ppm BPA groups for each sex were determined by the ΔΔ cycle threshold method with gene-specific TaqMan assays. Genes selected for RT-PCR analysis were chosen to confirm findings from RNAseq analysis demonstrating effects of BPA exposure on collagen gene expression (A), functional alterations in ECM and matricellular interactions and signaling (B), and genes to confirm effects on specific GO functions/processes and to confirm detection of both increases and decreases in expression identified by RNAseq analysis (C). The level of statistical significance for differences in mean values between the control and exposure groups was determined by a Mann-Whitney test and is indicated with asterisks.
For both males and females, thrombospondin 1 (Tsp1) expression in the BPA groups was decreased compared with control (Figure 5B). Although not identified as differentially expressed by RNAseq, RT-PCR analysis found that expression of the Tsp1 receptor and long-chain fatty acid transporter CD36 was increased nearly 8-fold in males exposed to BPA but not females (Figure 5B and Supplemental Table 3). Further supporting functional alterations in ECM and matricellular interactions resulting from BPA exposures, Cyr61, a member of the CCN (CYR61/CTGF/NOV) family of matricellular proteins that acts through integrin binding to regulate cardiac development, cardiac remodeling, and fibrosis (44), was confirmed as being markedly down-regulated in males (Figure 5B and Supplemental Table 3).
The expression of the ECM constituent of elastic fibers elastin (Eln; Figure 5B), neutrophil cytosolic factor 1 (Ncf1), and Ncf2 was significantly decreased in LV cardiac tissue of BPA-exposed males (Supplemental Table 3). Each of these genes are located in a 28-gene-containing region of human chromosome 7q11.23 from which small (1.5–1.8 Mbp) hemizygous deletions cause the Williams-Beuren syndrome (WBS) (45). Expression of Cldn5, a paralog of the two Claudin genes (Cldn3, Cldn4) deleted in WBS, which function as components of tight junctions, was also significantly down-regulated in males. In BPA-exposed female tissues, expression of WBS chromosome region 17 homolog (Wbscr17) and fibrillin 2 (Fbn2), which associates with elastin to form elastic fibers in the ECM, was increased. The most frequent pattern of symptoms characteristic of the WBS in humans and in Eln−/+ mouse models of WBS include supravalvular stenosis and peripheral artery stenosis, abnormal cardiac wall dynamics, decreased vascular compliance, and hypertension resulting from haplioinsufficiency of Eln and the resulting alteration in cardiac and vascular ECM (45–47). The coordinated changes in WBS genes expressed from 7q11.23 and WBS paralogs suggest a shared etiology between WBS and some of the observed effects of BPA exposure. Further study is warranted to investigate additional possible mechanistic connections between common clinical features of WBS, which include altered social behaviors, early puberty, and abnormal weight gain, and the phenotypes associated with BPA exposures (47).
In BPA-exposed female hearts, there was a general increase in the expression of genes associated with mitochondrial activities and altered glycolytic and fatty acid metabolism, effects that in combination with deceased accumulation of fibril collagen may contribute to the increased sensitivity and severity of Iso-induced ischemic damage in the hearts of BPA-exposed females (48). The mitochondrial ATP synthase subunit Atp5f1 and the cytoplasmic enzyme glyoxalase 1 (Glo1) were confirmed by RT-PCR as up-regulated mRNAs involved in glucose metabolism and mitochondrial activity (Supplemental Table 3 and Figure 5C). Further supporting the GO analysis finding that differentially expressed genes in females were significantly associated with cytoplasmic and ribosomal cellular components, expression of signal sequence receptor-γ (Ssr3), which is required for the uptake and processing of proteins in the endoplasmic reticulum, and the 60S ribosomal protein subunit Rpl9 were confirmed up-regulated by BPA exposure (Figure 5C).
In humans an increased accumulation of abdominal visceral adipose tissue and cardiac fat, especially epicardial adipose, are associated with cardiac dysfunction, insulin resistance, and metabolic syndrome (43, 49, 50). Many of the changes in gene expression in males are related to changes in fatty acid metabolism and modified accumulation of white and brown adipocytes within the epicardial adipose of the LV tissues (51). Along with a marked up-regulation of CD36 expression in males, BPA exposure in both males and females modified the expression of a number of other genes involved with lipid metabolism (Supplemental Table 3). Exposure-related changes in cardiac lipid metabolism were also supported by increased fatty acid binding protein 4 (Fabp4) expression in both sexes and the up-regulation of Ucp1 and Plin 4 in females, suggesting that BPA influences epicardial adiposity and cardiac brown adipocyte content.
Consistent with the observed functional alterations in the autonomic regulation and responsiveness of ECG and hemodynamic parameters in females, α1a adrenergic receptor (Adra1a) and the muscarinic type 2 cholinergic receptor (Chrm2) expression was identified as significantly increased by BPA exposures (Supplemental Table 3). Exposure-related alterations in expression of genes involved with ion channel function were also significantly impacted by BPA exposures in LV tissue from both males and females (Supplemental Table 3). Along with changes in expression of mRNA encoding channel proteins, changes in the expression of molecular scaffold proteins involved in receptor localization and protein kinases that modify the functional properties of the channels were also identified. In most cases the effects were sex specific, suggesting differential functional consequences in males and females. For example, the expression of serum/glucocorticoid-regulated kinase 1 (Sgk1) was markedly down-regulated in males but not females (Figure 5C and Supplemental Table 3). A general decrease in expression of transcripts related to potassium channel functions were noted in BPA-exposed males. The finding that expression of the voltage-gated potassium channels, Kcna1 and Kcna6, which are coordinately expressed from a multigene K-channel cluster on mouse chromosome 6 (52), were reduced by the same extent lends further confidence for validity of the effects identified by RNAseq analysis (Supplemental Table 3). In BPA-exposed females, a general increase in expression of sodium channel and solute carrier family members was observed. These findings suggest that small shifts in body weight and changes in heart weights noted here could be related to BPA exposures altering fluid balance. Expression of Slc9a4, a proton antiporter involved with regulation of intracellular pH and cell volume, was significantly increased in both males and females exposed to BPA (Supplemental Table 3).
Summary and Conclusions
In addition to regulating sexual development and reproductive functions, estrogens can have wide-ranging affects, impacting every tissue and organ system. Estrogen regulates energy balance and metabolism; reduces food intake in females; controls the health and aging of skin; regulates development, growth, and homeostasis of bone and cartilage; and influences innate and adaptive immunity (53–56). In the cardiovascular system, estrogens regulate heart function and vascular tone through direct actions on cardiac and vascular tissues and through central effects on autonomic nervous system activity. Based on a number of epidemiological studies suggesting a link between BPA exposures and cardiovascular disease (3, 8), the annual costs attributable to BPA-associated childhood obesity and adult CVD has been estimated to be nearly 3 billion dollars per year (56). The results of current study demonstrate that the CD1 mouse myocardium and cardiovascular functions are sensitive to BPA administered by oral ingestion. The resulting phenotypic changes observed are in general agreement with findings from previous in vitro and in vivo experimental results demonstrating potentially adverse actions of BPA in the rodent heart and suggest that BPA exposure may have especially harmful effects on heart health in females.
Results from experiments comparing the effects of oral BPA or EE exposure on ECG parameters found no overt changes indicative of cardiac contractile dysfunction and were in line with analysis of cardiac histology at later ages (PND70–90) during which only minor exposure-related changes in LV wall thickness and the collagen extracellular matrix were observed in the hearts of males. Clear changes in the expression of components of the ECM were evident in the gene expression analysis of LV cardiac tissues from males, with expression changes in numerous components of the ECM. The identified differentially expressed transcripts encode protein components of the ECM, regulatory proteases that regulate ECM composition, proteoglycan binding factors, mediators of matricellular signaling, and proteins involved in inflammatory responses. This pattern of concerted changes in expression support the findings that BPA exposures resulted in the observed histological alterations of the collagen ECM and pathological remodeling (44, 57). Overall, these finding indicate that dietary exposure to BPA and EE has minor effects on cardiac remodeling but do not appear to have major impacts on ventricular function in adult male or female CD1 mice.
Estrogens, especially estradiol and 17β-estradiol benzoate, have well-known effects on autonomic regulation of heart rate, baroreflex sensitivity, BP, and the responsiveness to β-adrenergic agonists (36–38). An estrogen-like effect of BPA exposure was most evident in the observed female-specific enhancement of the reflex bradycardia in response of phenylephrine. This effect was observed in females exposed to either BPA or EE and is reminiscent the normal estrogen-induced changes in baroreflex response of cycling females, effects that result from changes in central sympathetic regulation of the hindbrain baroreflex circuits (39, 41). Other exposure-related phenotypes, including the lengthening of the RR interval and decreased heart rate observed in BPA- or EE-exposed males only, are also consistent with BPA acting as a partial agonist of endogenous estrogen actions (eg, inhibitory effects at low endogenous ligand concentrations and either having no effect or enhancing effects at higher endogenous estrogen concentrations) and the reversal of sex-specific phenotypes related to cardioprotection. Because of the closely integrated nature of autonomic regulation of metabolism and cardiac and hemodynamic function, it is possible that many of the identified phenotypic changes resulting from exposures arose from alterations in autonomic functions that contribute to the observed modest levels of compensatory remodeling. Although the interpretation of the effects of exposure are complicated by estrogens having effects on both the heart and vasculature, further study is required to assess whether BPA's effects are centrally mediated, as suggested by the impacts on baroreflex response, or mediated by direct actions on the myocardium and/or vasculature. Based on the wide-ranging effects that the autonomic nervous system has on essentially all organ systems and its critical role in metabolic regulation and control of adipose tissue lipolysis (58), it seems reasonable to conclude that the observed effects of BPA exposure were the result of a dynamic interaction between centrally mediated and peripheral actions of BPA. It is possible that the dysregulation of autonomic functions may contribute to many of the diverse phenotypic effects that have been reported for BPA.
Acknowledgments
The authors are indebted to Dana Buesing, Susie Christie, and Charles Lo for their exceptional dedication and technical contributions to this study.
This work was supported by Research Grants R03ES023098 and RC2ES018765 from the National Institute of Environmental Health Sciences.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANCOVA
- analysis of covariance
- BP
- blood pressure
- BPA
- bisphenol A
- bw
- body weight
- CVD
- cardiovascular disease
- ECG
- electrocardiogram
- ECM
- extracellular matrix
- EE
- 17α-ethinyl estradiol
- ER
- estrogen receptor
- GO
- gene ontology
- Iso
- isoproterenol
- LV
- left ventricular
- MAP
- mean atrial pressure
- PND
- postnatal day
- RNAseq
- RNA sequence
- WBS
- Williams-Beuren syndrome.
References
- 1. Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ Res. 2011;111:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lang IA, Galloway TS, Scarlett A, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. [DOI] [PubMed] [Google Scholar]
- 4. Silver MK, O'Neill MS, Sowers MR, Park SK. Urinary bisphenol A and type-2 diabetes in US adults: data from NHANES 2003–2008. PLoS One. 2011;6:e26868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab. 2011;96:3822–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shankar A, Teppala S. Urinary bisphenol A and hypertension in a multiethnic sample of US adults. J Environ Public Health. 2012;2012:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khalil N, Ebert JR, Wang L, et al. Bisphenol A and cardiometabolic risk factors in obese children. Sci Total Environ. 2014; 470–471:726–732. [DOI] [PubMed] [Google Scholar]
- 8. Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5:e8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belcher SM, Chen Y, Yan S, Wang HS. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17β-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology. 2011;153:712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang HS. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One. 2011;6:e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Posnack NG, Jaimes R, 3rd, Asfour H, et al. Bisphenol A exposure and cardiac electrical conduction in excised rat hearts. Environ Health Perspect. 2014;122:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pant J, Ranjan P, Deshpande SB. Bisphenol A decreases atrial contractility involving NO-dependent G-cyclase signaling pathway. J Appl Toxicol. 2011;31:698–702. [DOI] [PubMed] [Google Scholar]
- 13. Babiker FA, De Windt LJ, van Eickels M, Grohe C, Meyer R, Doevendans PA. Estrogenic hormone action in the heart: regulatory network and function. Cardiovasc Res. 2002;53:709–719. [DOI] [PubMed] [Google Scholar]
- 14. Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. [DOI] [PubMed] [Google Scholar]
- 15. Wolbrette D, Naccarelli G, Curtis A, Lehmann M, Kadish A. Gender differences in arrhythmias. Clin Cardiol. 2002;25:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhupathy P, Haines CD, Leinwand LA. Influence of sex hormones and phytoestrogens on heart disease in men and women. Womens Health (Lond Engl). 2010;6:77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kendig EL, Buesing DR, Christie SM, et al. Estrogen-like disruptive effects of dietary exposure to bisphenol A or 17α-ethinyl estradiol in CD1 mice. Int J Toxicol. 2012;31:537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kendziorski JA, Kendig EL, Gear RB, Belcher SM. Strain specific induction of pyometra and differences in immune responsiveness in mice exposed to 17α-ethinyl estradiol or the endocrine disrupting chemical bisphenol A. Reprod Toxicol. 2012;34:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. FAO/WHO. Toxicological and health aspects of bisphenol A: report of joint FAO/WHO expert meeting and report of stakeholder meeting on bisphenol A. Geneva: World Health Organization; 2011. [Google Scholar]
- 20. European Food Safety Authority. http://www.efsa.europa.eu/en/topics/topic/bisphenol.htm Accessed September 2014.
- 21. Thigpen JE, Setchell KDR, Kissling GE, et al. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol a studies. J Am Assoc Lab Anim Sci. 2013;52:130–141. [PMC free article] [PubMed] [Google Scholar]
- 22. Chu V, Otero J, Lopez O, Morgan J, Amende I, Hampton T. Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol. 2001;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swoap SJ, Overton JM, Garber G. Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol. 2004;287:R391–R396. [DOI] [PubMed] [Google Scholar]
- 24. Feng M, DiPetrillo K. Non-invasive blood pressure measurement in mice. In: DiPetrillo K, ed. Cardiovascular Genomics. Vol 573 New York: Humana Press; 2009:45–55. [DOI] [PubMed] [Google Scholar]
- 25. Feng M, Whitesall S, Zhang Y, Beibel M, Alecy LD, DiPetrillo K. Validation of volume–pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21:1288–1291. [DOI] [PubMed] [Google Scholar]
- 26. Patisaul HB, Roberts SC, Mabrey N, et al. Accumulation and endocrine disrupting effects of the flame retardant mixture firemaster® 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morgan M, Anders S, Lawrence M, Aboyoun P, Pages H, Gentleman R. Short read: a bioconductor package for input, quality assessment and exploration of high-throughput sequence data. Bioinformatics. 2009;25:2607–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 29. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boyle EI, Weng S, Gollub J, et al. Go::Termfinder—open source software for accessing gene ontology information and finding significantly enriched gene ontology terms associated with a list of genes. Bioinformatics. 2004;20:3710–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saito R, Smoot ME, Ono K, et al. A travel guide to cytoscape plugins. Nat Meth. 2012;9:1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maere S, Heymans K, Kuiper M. Bingo: a cytoscape plug-in to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448–3449. [DOI] [PubMed] [Google Scholar]
- 33. Franceschini A, Szklarczyk D, Frankild S, et al. String v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haseman JK, Bailer AJ, Kodell RL, Morris R, Portier K. Statistical issues in the analysis of low-dose endocrine disruptor data. Toxicol Sci. 2001;61:201–210. [DOI] [PubMed] [Google Scholar]
- 35. Patel BB, Raad M, Sebag IA, Chalifour LE. Lifelong exposure to bisphenol A alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol Sci. 2013;133:174–185. [DOI] [PubMed] [Google Scholar]
- 36. Black DJ, Fregly MJ, Thrasher TN, Moreland AF. Reduced β adrenergic responsiveness in rats treated with estrogenic agents. J Pharmacol Exp Ther. 1976;197:362–370. [PubMed] [Google Scholar]
- 37. Fregly MJ, Thrasher TN. Response of heart rate to acute administration of isoproterenol in rats treated chronically with norethynodrel, ethinyl estradiol, and both combined. Endocrinology. 1977;100:148–154. [DOI] [PubMed] [Google Scholar]
- 38. Krause EG, Curtis KS, Markle JP, Contreras RJ. Oestrogen affects the cardiovascular and central responses to isoproterenol of female rats. J Physiol. 2007;582:435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mohamed MK, El-Mas MM, Abdel-Rahman AA. Estrogen enhancement of baroreflex sensitivity is centrally mediated. Am J Physiol. 1999;276:R1030–1037. [DOI] [PubMed] [Google Scholar]
- 40. Takezawa H, Hayashi H, Sano H, Saito H, Ebihara S. Circadian and estrous cycle-dependent variations in blood pressure and heart rate in female rats. Am J Physiol. 1994;267:R1250–R1256. [DOI] [PubMed] [Google Scholar]
- 41. Goldman RK, Azar AS, Mulvaney JM, Hinojosa-Laborde C, Haywood JR, Brooks VL. Baroreflex sensitivity varies during the rat estrous cycle: role of gonadal steroids. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1419–R1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanaka M, Sato M, Umehara S, Nishikawa T. Influence of menstrual cycle on baroreflex control of heart rate: comparison with male volunteers. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1091–R1097. [DOI] [PubMed] [Google Scholar]
- 43. Iacobellis G. Epicardial adipose tissue in endocrine and metabolic diseases. Endocrine. 2014;46:8–15. [DOI] [PubMed] [Google Scholar]
- 44. Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schubert C. The genomic basis of the Williams-Beuren syndrome. Cell Mol Life Sci. 2009;66:1178–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goergen CJ, Li HH, Francke U, Taylor CA. Induced chromosome deletion in a Williams-Beuren syndrome mouse model causes cardiovascular abnormalities. J Vasc Res. 2011;48:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362:239–252. [DOI] [PubMed] [Google Scholar]
- 48. Chen Y-R, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res. 2014;114:524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gastaldelli A, Morales MA, Marraccini P, Sicari R. The role of cardiac fat in insulin resistance. Curr Opin Clin Nutr Metab Care. 2012;15:523–528. [DOI] [PubMed] [Google Scholar]
- 50. Sironi AM, Petz R, De Marchi D, et al. Impact of increased visceral and cardiac fat on cardiometabolic risk and disease. Diabet Med. 2012;29:622–627. [DOI] [PubMed] [Google Scholar]
- 51. Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8:253–261. [DOI] [PubMed] [Google Scholar]
- 52. Street VA, Tempel BL. Physical mapping of potassium channel gene clusters on mouse chromosomes three and six. Genomics. 1997;44:110–117. [DOI] [PubMed] [Google Scholar]
- 53. Kelly BB, Narula J, Fuster V. Recognizing global burden of cardiovascular disease and related chronic diseases. Mt Sinai J Med. 2012;79:632–640. [DOI] [PubMed] [Google Scholar]
- 54. Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 2011;104:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rodrigues FL, de Oliveira M, Salgado HC, Fazan R. Effect of baroreceptor denervation on the autonomic control of arterial pressure in conscious mice. Exp Physiol. 2011;96:853–862. [DOI] [PubMed] [Google Scholar]
- 56. Trasande L. Further limiting bisphenol a in food uses could provide health and economic benefits. Health Aff (Millwood). 2014;33:316–323. [DOI] [PubMed] [Google Scholar]
- 57. Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2010;318:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]