Abstract
Because the estrogen-based hormone therapy (HT) in postmenopausal women typically contains a progestogen component, understanding the interactions between estrogens and progestogens is critical for optimizing the potential neural benefits of HT. An important issue in this regard is the use of continuous vs discontinuous hormone treatments. Although sex steroid hormone levels naturally exhibit cyclic fluctuation, many HT formulations include continuous delivery of hormones. Recent findings from our laboratory and others have shown that coadministration of progesterone (P4) can either attenuate or augment beneficial actions of 17β-estradiol (E2) in experimental models depending in part upon the delivery schedule of P4. In this study, we demonstrate that the P4 delivery schedule in combined E2 and P4 treatments alters degenerative and regenerative outcomes of unilateral entorhinal cortex lesion. We assessed how lesion-induced degeneration of layer II neurons in entorhinal cortex layer and deafferentation in dentate gyrus are affected by ovariectomy and treatments with E2 alone or in combination with either continuous or discontinuous P4. Our results demonstrate the combined efficacy of E2 and P4 is dependent on the administration regimen. Importantly, the discontinuous-combined E2+P4 regimen had the greatest neuroprotective efficacy for both end points. These data extend a growing literature that indicates qualitative differences in the neuroprotective effects of E2 as a function of cotreatment with continuous versus discontinuous P4, the understanding of which has important implications for HT in postmenopausal women.
Combined estrogen and progestogen hormone therapies (HTs) are widely used for the treatment of menopause-related climacteric symptoms. In addition to relief from menopausal symptoms, ovarian hormones also have well-established neuroprotective, neurotrophic, and cognitive benefits that may be useful in the prevention of Alzheimer's disease (AD). Despite a wealth of data supporting the independent neuroprotective and neurotrophic effects of estradiol (E2) and progesterone (P4), interactions between E2 and P4 in combined HT regimens on neural parameters are not well understood. Recent evidence suggests P4 may modulate the neural actions of E2 (1). Understanding E2 and P4 interactions on neuroprotective outcomes has important implications for combined HT use in postmenopausal women. An important issue in this regard is the effect of continuous vs discontinuous P4 delivery schedules, which may play an important role in determining the cognitive benefit of a combined HT.
Although sex steroid hormone levels naturally exhibit cyclic fluctuations, many HT formulations include continuous delivery of hormones (2). Prior experimental studies have found that P4 attenuates the effects of E2 in some models of neuroprotection (3–8) but increases E2 neuroprotection in other paradigms (9–11). These apparent discrepancies may reflect divergent effects of continuous vs discontinuous P4 administration regimens. Specifically, continuous P4 exposure for prolonged periods is often associated with attenuation of the beneficial E2 effects (3, 4, 12, 13), whereas discontinuous P4 administered in combination with E2 tends to yield improved outcomes (10, 11, 14, 15). Highlighting the importance of the E2/P4 delivery regimen, behavioral inhibition or facilitation by concurrent or sequential administration of E2/P4 is observed on lordosis (16), cocaine self-administration and sensitization (17), and wheel running (18).
In this study, we examine the effects of P4 delivery schedule in the presence and absence of E2 on both degenerative and compensatory changes after the unilateral transection of the perforant path [entorhinal cortex lesion (ECL)] in female rats (Figure 1A). The perforant path is a network of axons running from layer II of the lateral entorhinal cortex (EC) projecting to the molecular layer of the dentate gyrus (DG) of the hippocampal formation. This pathway plays an important role in memory consolidation and is severely affected in AD (19, 20). Selective degeneration of EC neurons and perforant path axons occurs early in AD pathogenesis, accompanied by compensatory axonal sprouting in response to deafferentation of the hippocampus (21). ECL is a model of entorhinal cortex degeneration in AD, with transection of the perforant path resulting in rapid loss of axotomized entorhinal cortex layer II neurons (22) and compensatory synaptic sprouting in the molecular layer of the dentate gyrus (23–25) (Figure 1). Compensatory sprouting is a potentially important regenerative mechanism as it can spare memory deficits associated with hippocampal deafferentation after ECL (25, 26). A significant advantage of the ECL knife lesion model is that it avoids seizures induced by electrolytic lesions or excitotoxic lesions, which would potentially confound interpretation of neuroprotective effects of P4 due to its anticonvulsant effects. To determine the effects of P4 delivery schedule on neuroprotection, we compared the effects of E2 alone or in combination with either continuous or discontinuous P4 on survival of layer II entorhinal cortex neurons and compensatory neuronal sprouting in dentate gyrus following ECL lesion.
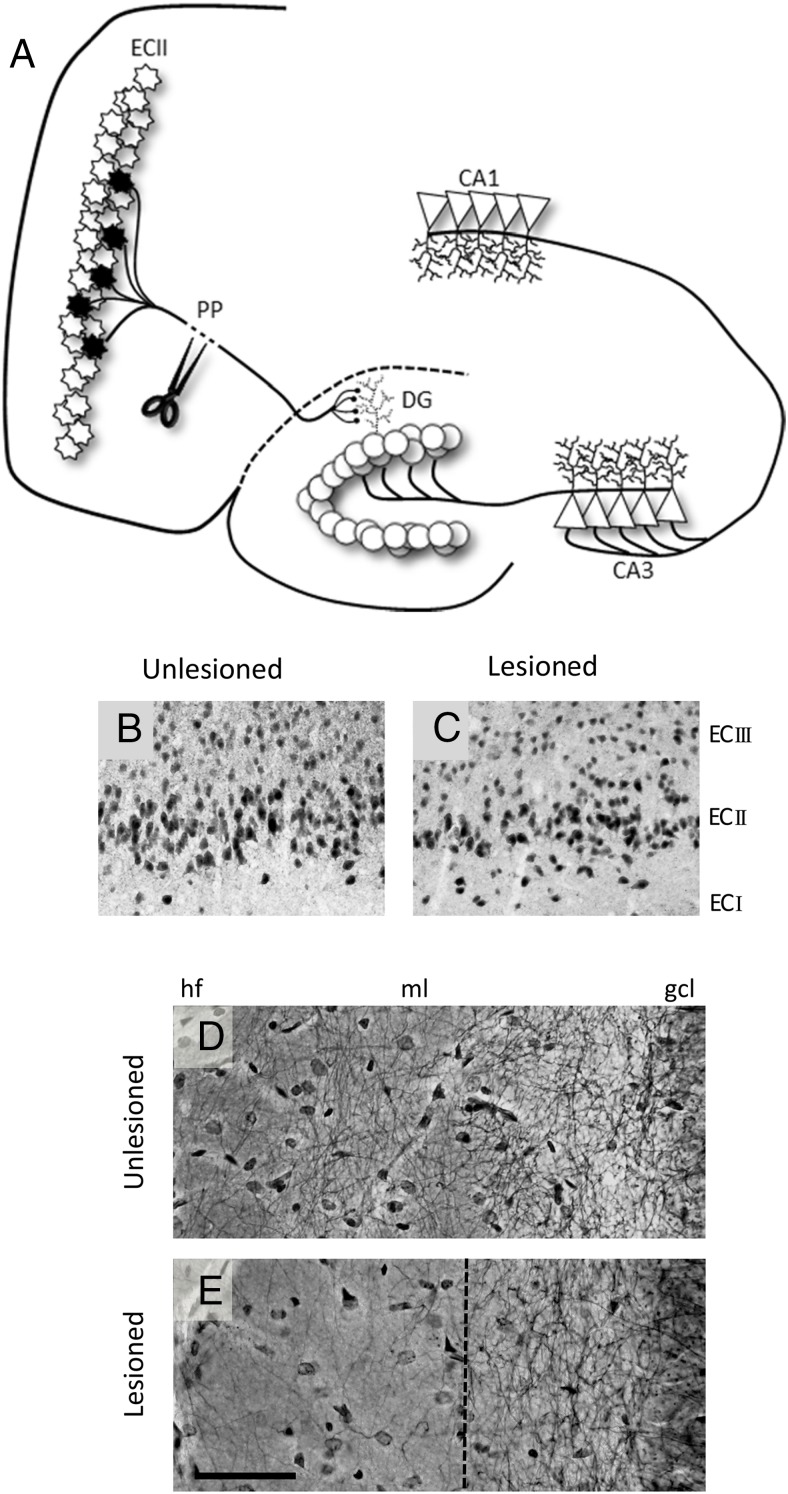
Figure 1.
Unilateral entorhinal cortex lesion model. A, Schematic representation of the entorhinal cortex/perforant path lesion model. Perforant path (PP) axons arise from the distinctly laminated layer II entorhinal cortex neurons (ECII) and terminate on the dendrites of the granule cells in the molecular layer of the DG. Unilateral lesion of the perforant path results in ipsilateral degeneration of axotomized ECII neurons, evidenced by reduced NeuN immunoreactive cells in the ECII region of the lesioned (ipsilateral, B) vs unlesioned hemisphere (contralateral, C). Neurite fiber density in the DG molecular layer, as assessed by Holmes staining, is reduced after the lesion (unlesioned, D; lesioned, E) but shows compensatory regrowth. Compensatory sprouting arises from reinnervation from multiple pathways including commissural/associational afferents, septohippocampal afferents, contralateral EC afferents, and local interneurons (71). Scale bar, 50 μm. hf, hippocampal fissure; ml, molecular layer; gcl, granule cell layer.
Materials and Methods
Animals and surgical procedures
Three-month-old Sprague Dawley rats were purchased ovariectomized (OVX) or sham-OVX from Harlan Laboratories. Seven days after the surgery, rats were administered two consecutive 30-day cycles of hormone treatment (Figure 2). Hormone delivery consisted of continuous E2, continuous P4, discontinuous P4 (10 d/mo), or a combinations of continuous E2 with either continuous P4 or discontinuous P4. These regimens of hormone exposure were chosen to model ongoing clinical trials of HT in postmenopausal women that involve continuous E2 with discontinuous delivery of P4 for 10–12 days per month (14, 15, 27–29). Control animals were treated with vehicle (cholesterol). Hormones were delivered to rats via sc implanted SILASTIC brand capsules (1.57 mm inner diameter, 3.18 mm outer diameter) containing vehicle (cholesterol, 1 × 1 cm), 1.2 mg E2 (15% E2 in cholesterol, 1 × 1 cm), and/or 110 mg crystalline P4 (3 × 3 cm) according to previously described methods (4, 30). Prior to implantation, capsules were primed overnight in sterile PBS to prevent an initial spike in hormone release. Cholesterol and hormones were purchased from Sigma-Aldrich.
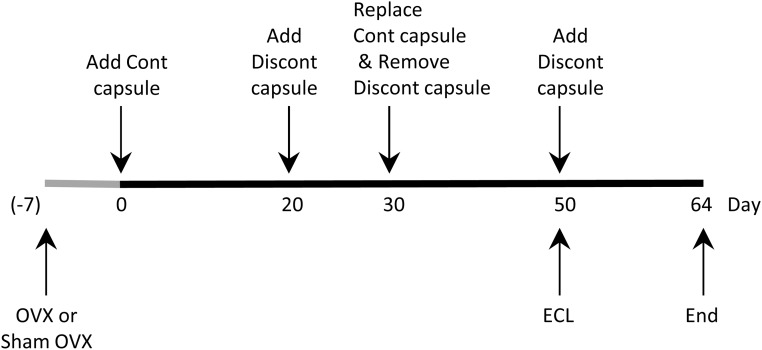
Figure 2.
Treatment schedule. Hormone treatment began at day 0, 7 days after Sham OVX or OVX surgery, with continuous (Cont) capsule insertion. Discontinuous P4 capsules (Discont) were inserted at day 20 and day 50 of the hormone treatment. The ECL lesion also occurred on day 50. On day 30, continuous capsules were replaced and discontinuous capsules were removed. Tissues were collected 64 days after the initiation of hormone treatment.
For the continuous hormone regimen groups, capsules were replaced on day 30. For discontinuous groups, P4 SILASTIC brand capsules were inserted on day 20, removed on day 30 (at the time of E2 insertion), and inserted again on day 50 (at the time of ECL surgery). SILASTIC brand capsules were inserted under general anesthesia (5% isoflurane for induction, 2%-3% isoflurane for maintenance). Mannino et al (30) previously characterized the pharmacokinetic profiles of SILASTIC brand capsule delivery of E2 and P4, reporting that the doses used in the current study resulted in plasma E2 levels of approximately 70 pg/mL, comparable with peak levels observed at proestrus (31–33), and plasma P4 levels of approximately 18 ng/mL, comparable with midrange levels observed during late proestrus (31–33). Previous studies have also demonstrated that the SILASTIC brand capsule method of delivery provides continuous delivery of a constant level of hormone for a period of months (30, 34, 35). Rats were divided into the following treatment groups: Sham + vehicle (Sham, n = 7); OVX + vehicle (OVX, n = 10); OVX + E2 (O+E, n = 7); OVX + continuous P4 (O+Pcont; n = 7); OVX + discontinuous P4 (O+P, n = 7); OVX + E2 + continuous P4 (O+E+Pcont, n = 7); or OVX +E2 + discontinuous P4 (O+E+P, n = 7).
Animals were maintained at the University of Southern California vivarium facilities with ad libitum access to standard rat chow (Lab Diet; PMI 5001) and water. All experimentation was approved by the University of Southern California Animal Resources and Institutional Care and Use Committee and carried out in accordance with National Institutes of Health guidelines.
Entorhinal cortex lesion
On day 50 of the hormone treatment period, animals were administered a unilateral stereotaxic knife-cut lesion of the perforant path (ECL), resulting in partial deafferentation of the hippocampal dentate gyrus and retrograde degeneration of axotomized layer II neurons in the entorhinal cortex (Figure 2). Because both E2 and P4 have established neuroprotective actions, the lesion was administered on day 50 of the hormone treatment to ensure all P4-treated groups would have P4 present at the time of lesion. Tissues were collected 14 days after lesion EC cell loss, and neurite sprouting in the dentate gyrus has been previous characterized at this time point (13, 22, 24). Rats were anesthetized (70 mg/kg ketamine per 5 mg/kg xylazine, ip) and then placed in a stereotaxic frame with their heads in a level position and the incisor bar at −3.3 mm. The retracted wire knife (Scouten wire knife; Kopf, Tujunga) was positioned perpendicular to the midline (270°) and then inserted 0.5 mm anterior to lambda and 5.5 mm lateral to the sagittal suture such that the lesion was always in the left hemisphere (36). The knife was lowered 1 mm below the dura and rotated 30° clockwise (to 300° position), and then the knife was extended and a vertical cut was made by lowering the knife 2 mm. The knife was then retracted and rotated counterclockwise 165° (to the 135° position) before extending the knife again and making a vertical cut by raising the knife 2 mm. Lesion efficacy was confirmed by histological examination and differences within each animal between outcome measures on the ipsilateral vs contralateral brain regions. After removal of the knife, forceps were used to push the inner edges of skin at the incision site together and three to five wound clips were used along the length of the incision to fully close the wound site. Betadine was applied to the wound site and buprenorphrine (0.02 mg/kg, im) was administered postoperatively for analgesia. Fourteen days after the lesion, rats were transcardially perfused with ice-cold PBS followed by 4% paraformaldehyde while under general anesthesia (70 mg/kg ketamine per 5 mg/kg xylazine). The uteri were dissected to 1 cm length and weighed. The brains were collected and postfixed for 24 hours in 4% paraformaldehyde and then stored in PBS with sodium azide at 4°C.
Assessment of neuron viability
The brain was sectioned in the horizontal plane at 50 μm using a vibratome. For the assessment of neuron viability, every sixth section was immunostained with the neuron-specific antibody NeuN (1:250; Chemicon) using the ABC Elite immunohistochemistry kit, as previously described (4, 37). Control experiments in which the primary antibody was omitted revealed the expected absence of specific staining.
The numbers of NeuN immunoreactive cells in layer II of the entorhinal cortex were estimated using an unbiased optical dissector method as previously described (37). Every sixth section was collected within a 2-mm dorsal-ventral aspect of the EC surrounding the lesion. An Olympus BX50 microscope with a microcator and motorized stage controlled by CAST-grid software (Olympusenmark) was used to obtain unbiased, randomly orientated counting frames (32 × 32 μm with X-Y steps of 75 μm × 75 μm) throughout the layer II EC region. Within each counting frame, immunolabeled cells that exhibited intact nuclear morphology and that came into focus within the dissector height (z = −10 to −30 μm) were counted by an experimenter blinded to treatment group. The anatomical boundaries for quantification were modified from a previously established protocol (22), with one boundary being the point where EC layer II neurons form a distinct laminar organization emerging from the loosely organized parasubiculum and the lateral boundary stretching from the splenium of the corpus callosum to the apex of the cortical curvature between posterior and lateral cortices. The dorsal border was defined from the last point at which the knife lesion could be seen in the tissue section. Total neuron estimates were calculated according to the following equation: N = (ζQ−) (t/h) (1/asf) (1/ssf); where Q− is the total number of neurons counted, t is section thickness (50 μm), h is dissector height (20 μm), asf is the area sampling fraction, and ssf is the section sampling fraction (1/15). NeuN-labeled cells in EC layer II were counted in both the ipsilateral (lesioned) and contralateral hemispheres (unlesioned), which allowed the calculation in each animal of the percentage of cell survival (ratio of cell numbers in lesioned relative to unlesioned hemisphere).
To qualitatively verify NeuN findings, a separate set of tissue sections from all animals were thionin, a general stain for DNA and Nissl substance. Sections were mounted onto gelatin-subbed slides and air dried overnight and then stained with 0.1% thionin, dehydrated with ethanol solutions, and coverslipped with Krystalon (EMD Chemicals).
Assessment of neurite sprouting
To assess compensatory neurite sprouting in the DG, three 50-μm sections were selected from each animal sample and stained with the Holmes fiber method using a previously described method (38). Briefly, sections were mounted on gelatin-subbed slides and air dried overnight. Rehydrated sections were bathed in 1% silver nitrate solution and incubated overnight at 37°C in impregnation solution (0.2 M boric acid buffer, 0.1 M borax buffer, 1% silver nitrate, and 10% pyridine). The following day, slides were bathed in reducing solution (0.09 M hydroquinone and 1.3 M sodium sulfite) followed by washes in 0.2% gold chloride, 2% oxalic acid solution, and 5% sodium thiosulfate. Finally, stained sections were dehydrated in a series of graded ethanol solutions, cleared with xylene, and coverslipped with permanent mounting medium.
The width of Holmes-positive neurite growth extending from the DG granule cell layer was measured. Slides were viewed using a Leica DMLB microscope (McBain Instruments) coupled to a MTI 3CCD camera (Dage-MTI), and images were captured using IPLabAlias software. The method of quantification was modified from a previously described protocol (13). In brief, Image J (National Institutes of Health, Bethesda, Maryland) was used to make three measurements from each image (five images per section, three sections per animal), representing the apparent longest, shortest and an average width of fiber outgrowth, as detected by Holmes fiber staining. These three measurements of neurite outgrowth width were averaged and used to calculate a percentage of total width. The total width was measured from the granule cell layer to the hippocampal fissure.
Statistical analyses
Data were analyzed using the Statistical Package for Social Sciences (version 11.5; SPSS Inc). All data are presented as mean ± SEM. One outlier from the O+P group was excluded from analyses. One-way ANOVA was performed to determine the effect of hormone status on percent neuronal loss and fiber staining. Homoscedasticity was assessed with Levene's test (neuronal survival, P = .487; neurite outgrowth, P < .001). Tukey-Kramer post hoc tests were used for between group comparisons of parametric data (neuronal survival), Games-Howell post hoc tests were used for between group comparisons of nonparametric data (neurite outgrowth). Significance was set at a threshold of P < .05.
Results
In this study, we examined the effects of OVX-induced ovarian hormone depletion and various E2 and P4 hormone treatment regimens on neuron survival in EC layer II and compensatory neuronal sprouting in the dentate gyrus after ECL. As a bioassay of estrogenic effects, body and uterine weights were assessed, with a significant uterotrophic effect of E2 but not P4 observed, confirming the efficacy of the E2 SILASTIC brand capsules (Supplemental Table 1).
Neuron viability
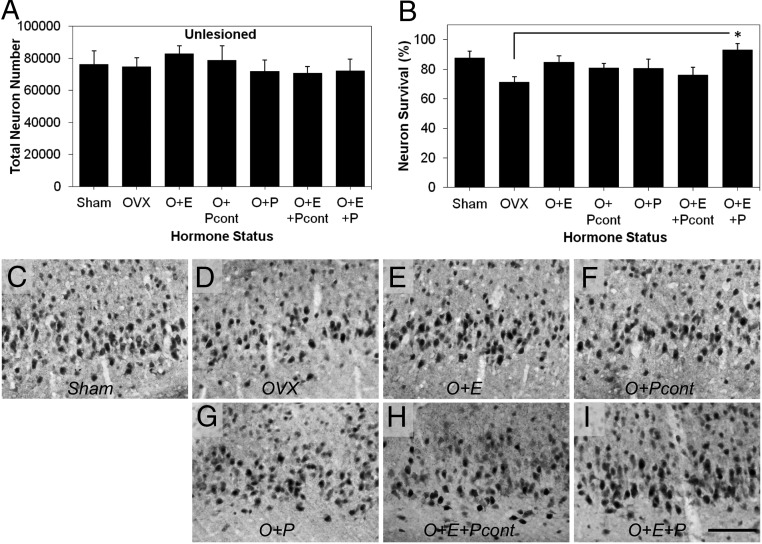
To investigate the effects of treatment conditions ECL-induced neuron loss in EC layer II, we compared stereological estimates of NeuN immunoreactive cells in lesioned (ipsilateral) relative to unlesioned (contralateral) hemisphere. In the absence of hormone manipulation (Sham), ECL resulted in a modest but significant reduction in the total number of EC layer II neurons in the lesioned (65 937 neurons ± 9290) compared with unlesioned hemisphere (76 172 neurons ± 9228; paired t test: t = 2.992, P = .024; Figure 1, B and C). Total neuron numbers in the unlesioned hemisphere were not affected by hormone manipulation (F = 0.34, P = .91; Figure 3A). Neuronal survival in the lesioned hemisphere was significantly affected by sex hormone status [F(6, 50) = 2.87, P < .02; Figure 3, B–I]. Mean neuronal survival in vehicle-treated OVX rats was not significantly different from Sham (P = .11), O+E (P = .28), O+Pcont (P = .67), O+P(P = .79), or O+E+Pcont groups (P = .98). E2 combined with discontinuous P4 (O+E+P) was the only hormone treatment to significantly increase the EC layer II neuronal survival in OVX rats (P < .02 vs OVX). The relative differences in neuron survival across the groups were confirmed with thionin staining (Supplemental Figure 1).
Figure 3.
E2 combined with discontinuous P4 promotes neuron viability after the ECL. A, Total neuron numbers in the EC layer II were unaffected by hormone manipulations in the contralateral hemisphere (unlesioned). B, Percentage neuron survival in the lesioned relative to the unlesioned hemisphere was significantly altered across treatment groups. E2 supplementation combination with discontinuous P4 (O+E+P) significantly reduced neuronal loss compared with OVX rats administered vehicle (OVX). C–I, Representative photomicrographs show NeuN immunoreactivity in the EC layer II after the ECL in Sham-OVX (C, Sham) and OVX rats administered vehicle (D, OVX), continuous E2 (E, O+E), discontinuous P4 (F, O+Pcont), discontinuous P4 (G, O+P), E2 combined with continuous P4 (H, O+E+Pcont), and E2 combined with discontinuous P4 (I, O+E+P). Data are presented as means ± SEM. *, P < .02 compared with OVX group. Scale bar, 100 μm.
Neurite outgrowth
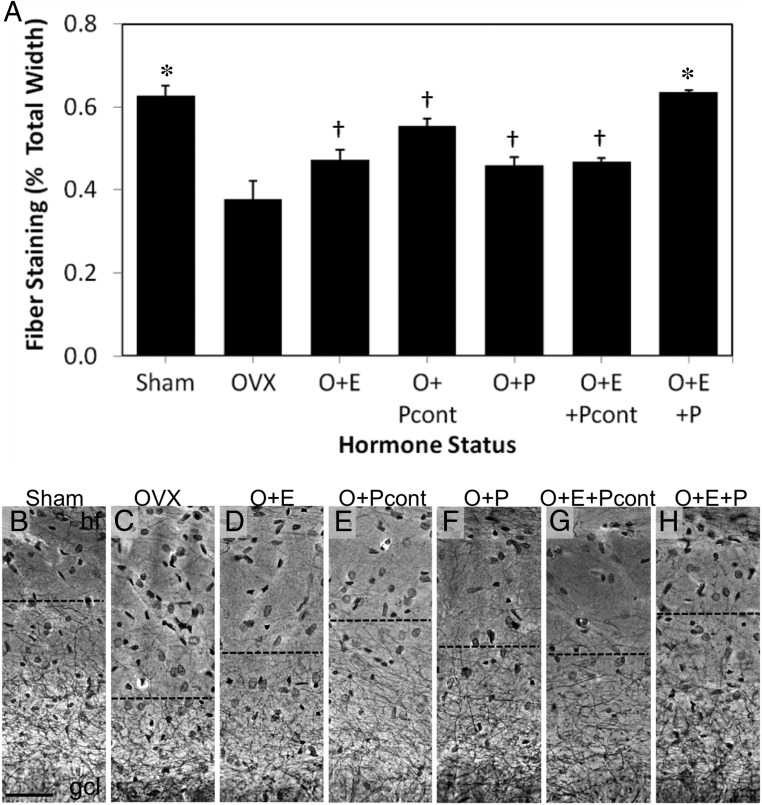
In the unlesioned hemisphere, Holmes fiber staining revealed neurite networks that extended from the DG granule cell layer throughout the entire molecular layer to the hippocampal fissure (Figure 1D). In the lesioned hemisphere, reduced fiber staining was observed in the molecular layer of the DG, with stained neurite networks terminating in the inner molecular layer (Figure 1E). To determine the effect of P4 delivery schedule on adaptive responses in the DG following ECL, we compared the effects of E2 alone or in combination with either continuous or discontinuous P4 on compensatory neuronal sprouting. Staining of neurite outgrowth was significantly affected by sex hormone status [F(6, 50) = 16.511, P < .001; Figure 4]. Compared with the Sham group, OVX reduced fiber staining by more than 40% (P < .05). E2 combined with discontinuous P4 was the only hormone treatment to significantly increase neurite sprouting in OVX rats (O+E+P, P < .05), rescuing neurite sprouting to sham levels (P = 1 vs Sham). Fiber outgrowth in rats treated with E2 combined with discontinuous P4 (O+E+P) was significantly increased compared with all other hormone treatments (P < .05).
Figure 4.
E2 and P4 promote neurite sprouting after the ECL. A, Quantification of neurite fiber staining in the DG of the hippocampus after the ECL shows significant differences across treatment groups. B–H, Representative photomicrographs of Holmes fiber staining in the DG after the ECL in gonadally intact (B, Sham) and OVX rats administered vehicle (C, OVX), E2 alone (D, O+E), continuous P4 alone (E, O+Pcont), cyclic P4 alone (F, O+P), E2 combined with continuous P4 (G, O+E+Pcont), and E2 combined with cyclic P4 (H, O+E+P). Data are presented as means ± SEM. gcl, granule cell layer; hf, hippocampal fissure. *, P < .05 relative to OVX group; †, P < .05 relative to O+E+P group. Scale bar, 50 μm.
Discussion
In this study, we demonstrate that varying P4 delivery schedule in combined E2 and P4 treatments alters degenerative and regenerative outcomes in female rats after unilateral ECL, a model of AD-like EC layer II cell loss and DG deafferentation. Our findings suggest that that the combined efficacy of E2 and P4 on neuroprotective and regenerative outcomes is dependent on the administration regimen. Importantly, the discontinuous-combined E2+P4 regimen was the only treatment condition to improve neuronal survival and neurite outgrowth in the OVX rats. Prior work demonstrated that short-term interactions between E2 and P4 are important in the regulation of a variety of neural sex steroid actions including sexual receptivity (39), gonadotropin secretion (40), and synaptic plasticity (41). The current findings extend a growing literature that indicates neural effects of long-term E2 and P4 exposure also depend on the treatment regimen, the understanding of which has important implications for HT in postmenopausal women.
Diverse protective actions of both E2 and P4 have been described in several models of neural injury (42–49). In the current study, neither E2 nor P4 significantly promoted EC layer II neuronal survival or neurite outgrowth when administered independently. Although the absence of E2 neuroprotection is unexpected, it may be more reflective of a modest lesion than lack of hormone benefit. Note that OVX-induced hormone depletion showed only a nonsignificant trend of reduced neuron survival, which showed a nonsignificant trend of reversal by treatment with E2 alone. Notably, E2 combined with discontinuous (but not continuous) P4 significantly increased both neuronal survival and neurite outgrowth. One potential limitation of these data is that neuronal viability was quantitatively assessed using the postmitotic neuronal specific nuclear protein NeuN, which can be down-regulated in surviving neurons under stress (50, 51) and thus may underestimate neuronal survival after injury. To address this possibility, NeuN findings were qualitatively confirmed with thionin, a general cell stain that labels DNA and Nissl bodies.
In general agreement with the current findings, we have previously observed that continuous P4 attenuated E2-mediated neuroprotection after kainate lesion (3, 4). The mechanism by which continuous P4 inhibits E2/P4-mediated neuroprotection remains unclear, although previous studies have demonstrated that P4 attenuates E2-induced elevations in key neurotrophins including brain-derived neurotrophic factor (BDNF) (5, 6). For example, in cultured hippocampal slices, E2 protected against N-methyl-D-aspartate toxicity by the up-regulation of BDNF expression and activation of the BDNF receptor tropomyosin receptor kinase B, whereas P4 antagonized these protective actions of E2 (5). BDNF has been shown to reduce EC layer II cell loss after ECL (52), suggesting that interactions between E2 and P4 on BDNF expression may contribute to the observed effects on neuron survival.
Ovariectomy significantly reduced compensatory sprouting after ECL-induced deafferentation in the DG, consistent with previous findings (13, 53, 54). Prior work suggests that the ovarian hormones, E2 and P4 may promote neurite outgrowth through several mechanisms, including E2 mediated effects on apolipoprotein E (55, 56), P4 mediated promotion of myelination (57–59), and P4 induced proliferation of neural progenitor cells derived from the DG (60, 61). Newborn neurons have increased plasticity compared with mature neurons after ECL, innervating the inner molecular layer at a higher density after ECL (62). However, in the current study, the effect of OVX was not reversed by E2 or P4 administered alone or combined in a continuous treatment regimen. These findings differ from observations by Wong et al (13), who found increased synaptic sprouting in the DG of continuous E2- but not P4-treated OVX rats after ECL lesion, with E2-mediated neurite sprouting attenuated by combination with continuous P4. These discrepancies may be the consequence of key differences in hormone doses and delivery regimens. First, Wong et al administered E2 and P4 using a pellet delivery system that has been reported to release a hormone surge in the first week of administration that is 5- to 15-fold higher than more physiological levels observed subsequent weeks (63). Second, the use of two consecutive cycles of hormone treatment in the current study prior to the lesion surgery may have altered the efficacy of the neurite-promoting actions of E2.
E2 combined with discontinuous P4 was the most beneficial regimen for both EC layer II neuron survival and DG neurite sprouting. However, because discontinuous P4 groups underwent one additional capsule insertion under isoflurane anesthesia (d 20, 1 mo prior to lesion), we cannot exclude that the increased efficacy of the E2 combined with discontinuous P4 treatment could potentially be attributed to indirect effects of the additional exposure to anesthesia. For example, isoflurane anesthesia has been shown to stimulate adrenal corticosteroid production and can modulate expression of BDNF (64, 65), although this effect may be minimal with brief exposure, as is required for capsule insertion (66). Furthermore, isoflurane has been demonstrated to elicit diverse effects on the hippocampus and cortex, ranging from increased susceptibility to neuronal degeneration (67, 68) to increased resistance to neurotoxic insult (69, 70). Despite this limitation, the findings are consistent with our previous findings of qualitative differences in the neural effects of discontinuous vs continuous P4 regimens. For example, E2 combined with discontinuous but not continuous P4 reduces β-amyloid accumulation in 3xTg-AD mice (12, 14) and in wild-type rats (27). Others have reported that E2 combined with discontinuous but not continuous P4 improves cholinergic function in OVX female rats (72). Recent evidence suggests that observed qualitative differences in functional outcomes across P4 regimens are related to underlying changes in gene expression (15).
Although the mechanisms underlying interactions between P4 and E2 remain incompletely defined, in vitro and ex vivo studies suggest P4 may modulate estrogen receptor (ER) expression. In cultured hippocampal slices, E2 treatment up-regulated ERβ expression, whereas priming with the P4-blocked E2 induced the up-regulation of ERβ and inhibited E2-mediated neuroprotection (73). In primary hippocampal neuron cultures, P4 decreased the expression of both ERα and ERβ as well as ER-dependent transcriptional activity (74). Likewise, the progesterone receptor membrane component-1 (Pgrmc1) and the classical P4 receptor (Pgr) can be regulated by both E2 and P4 (15, 75). For example, Bali et al (75) reported that mRNA and protein levels of Pgrmc1 were increased in the DG by independent and combined treatments of E2 and P4. Zhao et al (15) demonstrated that the effects of E2 and P4 on Pgr and Pgrmc1 mRNA expression are dependent upon the P4 treatment regimen, such that continuous but not discontinuous P4 (alone or in combination with E2), down-regulated Pgr expression.
Our results indicate that differences in the P4 administration regimen can markedly alter the neuroprotective and regenerative outcomes in female rats, findings that may be predictive of the neural efficacy of HT regimens in postmenopausal women. Consistent with this possibility, Tierney et al (76) reported E2 combined with intermittent progestin (norethindrone) prevented declines in delayed verbal recall in women with normal memory function. The recent clinical trials Kronos Early Estrogen Prevention Study (29) and Early vs Late Intervention Trial with Estradiol (77) should provide further insight into the potential cognitive benefits of HT with discontinuous P4. Continued preclinical and clinical investigation of this issue is needed to optimize the therapeutic potential of HT against age-related cognitive decline and neurodegenerative disorders including AD.
Acknowledgments
We thank Dr Liqin Zhao for preparing the SILASTIC brand capsules and Dr Amy Christensen, Dr Sharon Lin, Natalie Kintz, and Dr Namrata Bali for technical assistance.
This work was supported by National Institutes of Health Grant P01AG026572 (R. Brinton/Project 3 CJP). A.M.B. is supported by the American Australian Neurological Fellowship and the Japanese Society for the Promotion of Science.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AD
- Alzheimer's disease
- BDNF
- brain-derived neurotrophic factor
- DG
- dentate gyrus
- E2
- 17β-estradiol
- EC
- entorhinal cortex
- ECL
- entorhinal cortex lesion
- ER
- estrogen receptor
- HT
- hormone therapy
- OVX
- ovariectomized
- P4
- progesterone
- Pgr
- P4 receptor
- Pgrmc1
- Pgr membrane component-1.
References
- 1. Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer's disease. Front Biosci (Elite Ed). 2012;4:976–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawrence M, Jones L, Lancaster T, Daly E, Banks E. Hormone replacement therapy: patterns of use studied through British general practice computerized records. Fam Pract. 1999;16(4):335–342. [DOI] [PubMed] [Google Scholar]
- 3. Carroll JC, Rosario ER, Pike CJ. Progesterone blocks estrogen neuroprotection from kainate in middle-aged female rats. Neurosci Lett. 2008;445(3):229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099(1):206–210. [DOI] [PubMed] [Google Scholar]
- 5. Aguirre CC, Baudry M. Progesterone reverses 17β-estradiol-mediated neuroprotection and bdnf induction in cultured hippocampal slices. Eur J Neurosci. 2009;29(3):447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neurorep. 2004;15(17):2659–2663. [DOI] [PubMed] [Google Scholar]
- 7. Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinol. 2008;149(6):3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia-Segura LM, Cardona-Gomez P, Naftolin F, Chowen JA. Estradiol upregulates bcl-2 expression in adult brain neurons. Neuroreport. 1998;9(4):593–597. [DOI] [PubMed] [Google Scholar]
- 9. Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: Synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinol. 2002;143(1):205–212. [DOI] [PubMed] [Google Scholar]
- 10. Azcoitia I, Fernandez-Galaz MC, Sierra A, Garcia-Segura LM. Gonadal hormones affect neuronal vulnerability to excitotoxin-induced degeneration. J Neurocytol. 1999;28:699–710. [DOI] [PubMed] [Google Scholar]
- 11. Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24(10):1160–1166. [DOI] [PubMed] [Google Scholar]
- 12. Carroll JC, Rosario ER, Chang L, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xtg-ad mice. J Neurosci. 2007;27(48):13357–13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong AM, Rozovsky I, Arimoto JM, et al. Progesterone influence on neurite outgrowth involves microglia. Endocrinology. 2009;150(1):324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carroll JC, Rosario ER, Villamagna A, Pike CJ. Continuous and cyclic progesterone differentially interact with estradiol in the regulation of Alzheimer-like pathology in female 3×transgenic-Alzheimer's disease mice. Endocrinology. 2010;151(6):2713–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao L, Morgan TE, Mao Z, et al. Continuous versus cyclic progesterone exposure differentially regulates hippocampal gene expression and functional profiles. PLoS One. 2012;7(2):e31267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marrone BL, Rodriguez-Sierra JF, Feder HH. Lordosis: inhibiting effects progesterone in the female rat. Horm Behav. 1977;8(3):391–402. [DOI] [PubMed] [Google Scholar]
- 17. Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behav Brain Res. 2007;184(2):174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodier WI., 3rd Progesterone-estrogen interactions in the control of activity-wheel running in the female rat. J Comp Physiol Psychol. 1971;74(3):365–373. [DOI] [PubMed] [Google Scholar]
- 19. Gómez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer ii entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16(14):4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hyman B, Van Hoesen G, Damasio A, Barnes C. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225(4667):1168–1170. [DOI] [PubMed] [Google Scholar]
- 21. Geddes JW, Monaghan DT, Cotman CW, Lott IT, Kim RC, Chui HC. Plasticity of hippocampal circuitry in Alzheimer's disease. Science. 1985;230(4730):1179–1181. [DOI] [PubMed] [Google Scholar]
- 22. Peterson D, Lucidi-Phillipi C, Eagle K, Gage F. Perforant path damage results in progressive neuronal death and somal atrophy in layer ii of entorhinal cortex and functional impairment with increasing postdamage age. J Neurosci. 1994;14(11):6872–6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cabalka LM, Hyman BT, Goodlett CR, Ritchie TC, Van Hoesen GW. Alteration in the pattern of nerve terminal protein immunoreactivity in the perforant pathway in Alzheimer's disease and in rats after entorhinal lesions. Neurobiol Aging. 1992;13(2):283–291. [DOI] [PubMed] [Google Scholar]
- 24. Cummings BJ, Yee GJ, Cotman CW. Bfgf promotes the survival of entorhinal layer ii neurons after perforant path axotomy. Brain Res. 1992;591(2):271–276. [DOI] [PubMed] [Google Scholar]
- 25. Ramirez JJ, McQuilkin M, Carrigan T, MacDonald K, Kelley MS. Progressive entorhinal cortex lesions accelerate hippocampal sprouting and spare spatial memory in rats. Proc Natl Acad Sci USA. 1996;93(26):15512–15517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramirez JJ, Bulsara K, Moore SC, Ruch K, Abrams W. Progressive unilateral damage of the entorhinal cortex enhances synaptic efficacy of the crossed entorhinal afferent to dentate granule cells. J Neurosci. 1999;19(22):RC42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jayaraman A, Carroll JC, Morgan TE, et al. 17β-estradiol and progesterone regulate expression of β-amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology. 2012;153(11):5467–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harman SM, Brinton EA, Cedars M, et al. KEEPS: the Kronos Early Estrogen Prevention Study. Climacteric. 2005;8(1):3–12. [DOI] [PubMed] [Google Scholar]
- 29. Miller VM, Black DM, Brinton EA, et al. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). J Cardiovasc Transl Res. 2009;2(3):228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mannino CA, South SM, Inturrisi CE, Quinones-Jenab V. Pharmacokinetics and effects of 17β-estradiol and progesterone implants in ovariectomized rats. J Pain. 2005;6(12):809–816. [DOI] [PubMed] [Google Scholar]
- 31. Edwards HE, Burnham WM, Mendonca A, Bowlby DA, MacLusky NJ. Steroid hormones affect limbic afterdischarge thresholds and kindling rates in adult female rats. Brain Res. 1999;838(1–2):136–150. [DOI] [PubMed] [Google Scholar]
- 32. Haim S, Shakhar G, Rossene E, Taylor AN, Ben-Eliyahu S. Serum levels of sex hormones and corticosterone throughout 4- and 5-day estrous cycles in Fischer 344 rats and their simulation in ovariectomized females. J Endocrinol Invest. 2003;26(10):1013–1022. [DOI] [PubMed] [Google Scholar]
- 33. Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96(1):219–226. [DOI] [PubMed] [Google Scholar]
- 34. Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136(5):2320–2324. [DOI] [PubMed] [Google Scholar]
- 35. Strom J, Theodorsson E, Holm L, Theodorsson A. Different methods for administering 17β-estradiol to ovariectomized rats result in opposite effects on ischemic brain damage. BMC Neurosci. 2010;11(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed San Diego: Academic Press; 2007. [Google Scholar]
- 37. Ramsden M, Berchtold NC, Patrick Kesslak J, Cotman CW, Pike CJ. Exercise increases the vulnerability of rat hippocampal neurons to kainate lesion. Brain Res. 2003;971(2):239–244. [DOI] [PubMed] [Google Scholar]
- 38. Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. Columbus, OH: Battelle Press; 1980. [Google Scholar]
- 39. Feder HH. Hormones and sexual behavior. Annu Rev Psychol. 1984;35:165–200. [DOI] [PubMed] [Google Scholar]
- 40. Mahesh VB, Muldoon TG. Integration of the effects of estradiol and progesterone in the modulation of gonadotropin secretion. J Steroid Biochem. 1987;27(4–6):665–675. [DOI] [PubMed] [Google Scholar]
- 41. Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34(2):140–148. [DOI] [PubMed] [Google Scholar]
- 42. Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129(1):64–69. [DOI] [PubMed] [Google Scholar]
- 43. Stein DG, Hoffman SW. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr Rehabil. 2003;6(1):13–22. [DOI] [PubMed] [Google Scholar]
- 44. Labombarda F, Gonzalez SL, Deniselle MC, et al. Effects of injury and progesterone treatment on progesterone receptor and progesterone binding protein 25-dx expression in the rat spinal cord. J Neurochem. 2003;87(4):902–913. [DOI] [PubMed] [Google Scholar]
- 45. Cervantes M, Gonzalez-Vidal MD, Ruelas R, Escobar A, Morali G. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res. 2002;33(1):6–14. [DOI] [PubMed] [Google Scholar]
- 46. Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp Neurol. 2005;193(2):522–530. [DOI] [PubMed] [Google Scholar]
- 47. Garay L, Deniselle MC, Meyer M, et al. Protective effects of progesterone administration on axonal pathology in mice with experimental autoimmune encephalomyelitis. Brain Res. 2009;1283:177–185. [DOI] [PubMed] [Google Scholar]
- 48. Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66(9):916–928. [DOI] [PubMed] [Google Scholar]
- 49. Rhodes ME, Frye CA. Progestins in the hippocampus of female rats have antiseizure effects in a pentylenetetrazole seizure model. Epilepsia. 2004;45(12):1531–1538. [DOI] [PubMed] [Google Scholar]
- 50. McPhail LT, McBride CB, McGraw J, Steeves JD, Tetzlaff W. Axotomy abolishes neun expression in facial but not rubrospinal neurons. Exp Neurol. 2004;185(1):182–190. [DOI] [PubMed] [Google Scholar]
- 51. Unal-Cevik I, Kilinc M, Gursoy-Ozdemir Y, Gurer G, Dalkara T. Loss of neun immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res. 2004;1015(1–2):169–174. [DOI] [PubMed] [Google Scholar]
- 52. Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15(3):331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kadish I, Van Groen T. Low levels of estrogen significantly diminish axonal sprouting after entorhinal cortex lesions in the mouse. J Neurosci. 2002;22(10):4095–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morse JK, DeKosky ST, Scheff SW. Neurotrophic effects of steroids on lesion-induced growth in the hippocampus: Ii. Hormone replacement. Exp Neurol. 1992;118(1):47–52. [DOI] [PubMed] [Google Scholar]
- 55. Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: Implications for Alzheimer's disease. J Neurosci. 1998;18(9):3180–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Hajian H, Finch CE. Astrocytes and microglia respond to estrogen with increased ApoE mRNA in vivo and in vitro. Exp Neurol. 1997;143(2):313–318. [DOI] [PubMed] [Google Scholar]
- 57. Magnaghi V, Veiga S, Ballabio M, Gonzalez LC, Garcia-Segura LM, Melcangi RC. Sex-dimorphic effects of progesterone and its reduced metabolites on gene expression of myelin proteins by rat Schwann cells. J Peripher Nerv Syst. 2006;11(2):111–118. [DOI] [PubMed] [Google Scholar]
- 58. Schumacher M, Guennoun R, Ghoumari A, et al. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev. 2007;28(4):387–439. [DOI] [PubMed] [Google Scholar]
- 59. Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Brain Res Rev. 2005;48(2):273–286. [DOI] [PubMed] [Google Scholar]
- 60. Liu L, Wang J, Zhao L, et al. Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology. 2009;150(7):3186–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu L, Zhao L, She H, et al. Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology. 2010;151(12):5782–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Perederiy JV, Luikart BW, Washburn EK, Schnell E, Westbrook GL. Neural injury alters proliferation and integration of adult-generated neurons in the dentate gyrus. J Neurosci. 2013;33(11):4754–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Singh M, Sumien N, Kyser C, Simpkins JW. Estrogens and progesterone as neuroprotectants: what animal models teach us. Front Biosci. 2008;13:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Altholtz LY, Fowler KA, Badura LL, Kovacs MS. Comparison of the stress response in rats to repeated isoflurane or co2 anesthesia used for restraint during serial blood collection via the jugular vein. J Am Assoc Lab Anim Sci. 2006;45(3):17–22. [PubMed] [Google Scholar]
- 65. Zhang F, Zhu ZQ, Liu DX, Zhang C, Gong QH, Zhu YH. Emulsified isoflurane anesthesia decreases brain-derived neurotrophic factor expression and induces cognitive dysfunction in adult rats. Exp Ther Med. 2014;8(2):471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zardooz H, Rostamkhani F, Zaringhalam J, Faraji Shahrivar F. Plasma corticosterone, insulin and glucose changes induced by brief exposure to isoflurane, diethyl ether and co2 in male rats. Physiol Res. 2010;59(6):973–978. [DOI] [PubMed] [Google Scholar]
- 67. Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61(8):1354–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wise-Faberowski L, Zhang H, Ing R, Pearlstein RD, Warner DS. Isoflurane-induced neuronal degeneration: an evaluation in organotypic hippocampal slice cultures. Anesth Analg. 2005;101(3):651–657. [DOI] [PubMed] [Google Scholar]
- 69. Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA. Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular ca2+ and mitogen-activated protein kinases. Anesthesiology. 2005;102(3):606–615. [DOI] [PubMed] [Google Scholar]
- 70. Bickler PE, Zhan X, Fahlman CS. Isoflurane preconditions hippocampal neurons against oxygen–glucose deprivation: role of intracellular Ca2+ and mitogen-activated protein kinase signaling. Anesthesiology. 2005;103(3):532–539. [DOI] [PubMed] [Google Scholar]
- 71. Steward O, Loesche J. Quantitative autoradiographic analysis of the time course of proliferation of contralateral entorhinal efferents in the dentate gyrus denervated by ipsilateral entorhinal lesions. Brain Res. 1977;125(1):11–21. [DOI] [PubMed] [Google Scholar]
- 72. Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neurosci. 2000;101(4):931–938. [DOI] [PubMed] [Google Scholar]
- 73. Aguirre C, Jayaraman A, Pike C, Baudry M. Progesterone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-β. J Neurochem. 2010;115(5):1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jayaraman A, Pike CJ. Progesterone attenuates oestrogen neuroprotection via downregulation of oestrogen receptor expression in cultured neurones. J Neuroendocrinol. 2009;21(1):77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bali N, Arimoto JM, Iwata N, et al. Differential responses of progesterone receptor membrane component-1 (pgrmc1) and the classical progesterone receptor (pgr) to 17β-estradiol and progesterone in hippocampal subregions that support synaptic remodeling and neurogenesis. Endocrinology. 2012;153(2):759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tierney MC, OH P, Moineddin R, et al. A randomized double-blind trial of the effects of hormone therapy on delayed verbal recall in older women. Psychoneuroendocrinology. 2009;34(7):1065–1074. [DOI] [PubMed] [Google Scholar]
- 77. Henderson VW. Estrogens, episodic memory, and Alzheimer's disease: a critical update. Semin Reprod Med. 2009;27(3):283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]