Abstract
Diffusion- and perfusion-based imaging studies are regularly used in patients with ischemic stroke. Cerebral venous sinus thrombosis (CVST) is a rare cause of stroke and is primarily treated by systemic anticoagulation. Endovascular intervention can be considered in cases of failed medical therapy, yet the prognostic value of diffusion- and perfusion-based imaging for CVST has not been clearly established. We present a patient with CVST whose abnormal findings on MRI and CT perfusion images were largely reversed after endovascular treatment.
Keywords: Stroke, Vein, CT perfusion, MRI
Background
Cerebral venous sinus thrombosis (CVST) is a rare cause of stroke and affects mostly younger individuals, particularly women between the ages of 20 and 35 years who have risk factors such as use of oral contraceptives, pregnancy, and smoking.1 2 The clinical presentation of CVST most commonly involves headache, which may progress to neurological deficits, encephalopathy, and seizures.3 The prognosis of CVST has dramatically improved over the past few decades because of advances in treatment, a shift in associated risk factors, and enhancement of diagnostic measures.4
The primary treatment for CVST is systemic anticoagulation. Endovascular intervention, including local infusion of recombinant tissue plasminogen activator and mechanical thrombectomy, can be considered if anticoagulation fails. MR diffusion- and perfusion-based images have been studied to identify prognostic indicators for CVST. CT perfusion (CTP) imaging has been shown to be valuable in selecting patients for acute stroke intervention,5 6 but its application in CVST is unclear. Here we present a case of CVST in which the abnormal findings on MR and CTP images were essentially reversed after endovascular treatment.
Case presentation
A young patient aged in the late 20s presented with a 1-week history of progressively worsening headaches, nausea, and vomiting. On the day of presentation the patient was found to have decreased mental status and was unable to speak. On physical examination in the emergency room the patient was somnolent with sluggish pupils, dense right-sided hemiparesis, and significant expressive aphasia.
Investigations
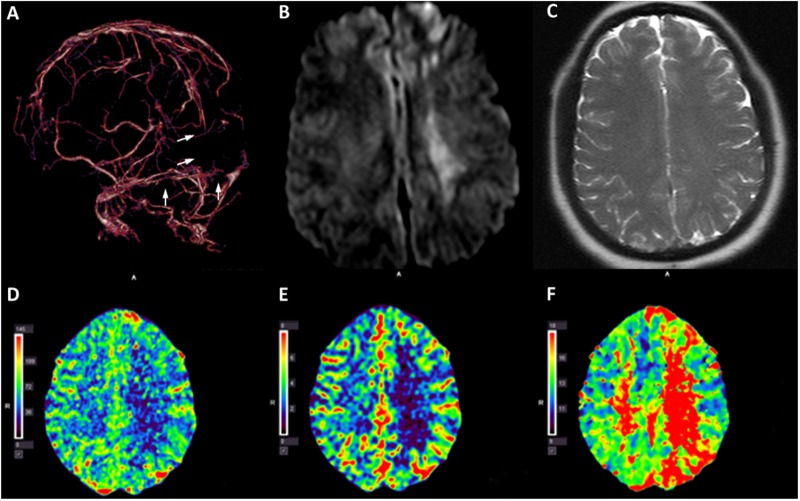
A non-contrast CT scan of the head demonstrated a hyperdense signal in the superior sagittal sinus (SSS) and bilateral transverse sinuses (images not shown). CT venography confirmed extensive CVST (figure 1A). Brain MRI showed hyperintensity at the left frontal and deep white matter regions on diffusion-weighted imaging (DWI) (figure 1B) without evidence of T2 shine-through (ie, high signal on DWI that is not due to restricted diffusion but rather to high T2 signal that ‘shines through’ to the diffusion-weighted image) (figure 1C). CTP imaging revealed decreased cerebral blood flow (CBF) (figure 1D) and cerebral blood volume (CBV) (figure 1E) and increased time-to-peak (TTP) (figure 1F) in the approximate areas of DWI abnormalities. The MR and CTP imaging findings were suggestive of a large area of parenchymal infarction with possible permanent brain tissue damage.
Figure 1.
Pre-procedural imaging studies. CT venogram of the brain shows occlusion of the superior sagittal sinus, torcula, and bilateral transverse sinuses (A, arrows). Brain MRI shows hyperintensity at the left frontal area and deep white matter region on diffusion-weighted imaging (B) without evidence of T2 shine-through (C). CT perfusion imaging study shows decreased cerebral blood flow (D) and cerebral blood volume (E) and increased mean time-to-peak (F) at the deep white matter region on the left side.
Treatment
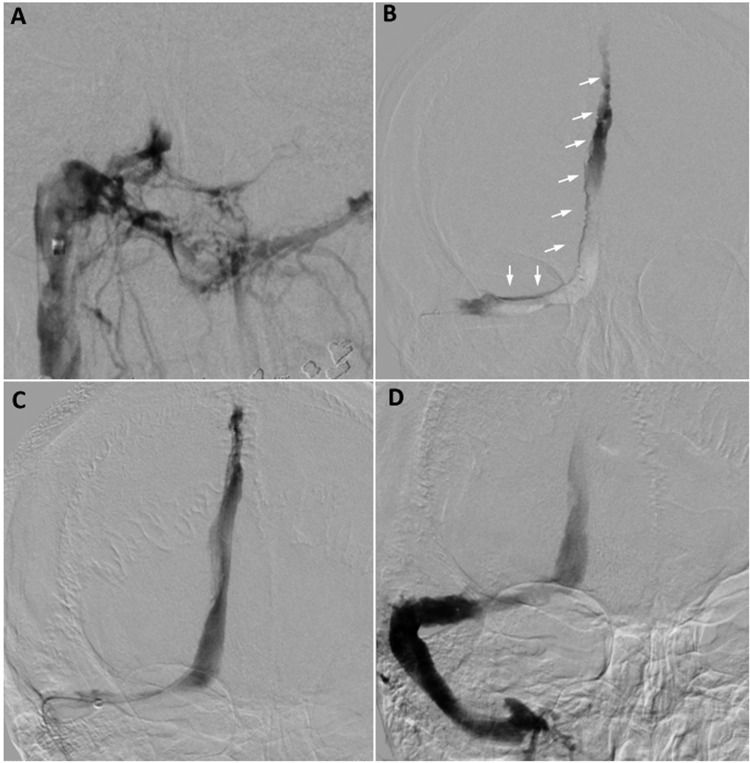
The patient was taken as an emergency for endovascular intervention because of the severity of the symptoms. A NeuronMax long sheath (Penumbra, Alameda, California, USA) was advanced from the right common femoral vein into the right internal jugular vein, and angiography demonstrated no filling of the right transverse sinus, torcula, or SSS (figure 2A). A triaxial system consisting of a 5MAX reperfusion catheter and a Velocity microcatheter (both from Penumbra) was used to access the SSS. Superselective angiography showed extensive thrombus within the SSS, torcula, and right transverse sinus (figure 2B). Thrombectomy was performed with a combination of a 6 mm×30 mm Solitaire FR device (Covidien, Irvine, California, USA) and aspiration via the reperfusion catheter. Post-intervention control angiography showed patency of the SSS, torcula, and right transverse sinus (figure 2C, D).
Figure 2.
Cerebral angiography and intervention. Angiogram from the right jugular vein shows no opacification of the right transverse sinus or the torcula (A). Microcatheter injection demonstrates extensive thrombus within the superior sagittal sinus (SSS), torcula, and right transverse sinus (B, arrows). After mechanical thrombectomy with the Solitaire FR, the control angiogram reveals patent SSS, torcula, and right transverse sinus (C, D).
Outcome and follow-up
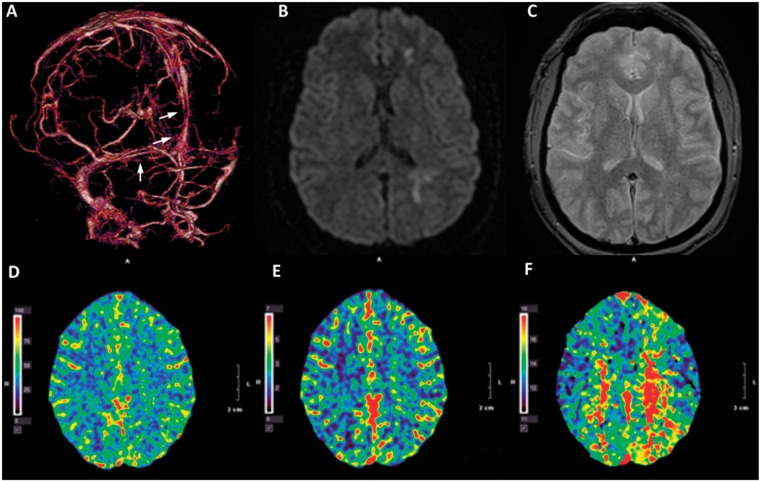
The patient's neurological status improved rapidly and the patient was able to carry on a conversation on post-intervention day 1. Repeat CT venography demonstrated patent SSS, torcula, and right transverse sinus, and partially thrombosed left transverse sinus (figure 3A, arrows). MRI showed that the DWI abnormalities were largely reversed (figure 3B) without fluid-attenuated inversion recovery (FLAIR) changes suggestive of permanent tissue damage (figure 3C). Post-intervention CTP demonstrated normalized CBF, CBV, and significantly smaller area of increased TTP (figure 3D–F). The patient was discharged home on post-intervention day 3 with minimal right hemiparesis and normal speech and was to be maintained on anticoagulation therapy with warfarin for 6 months. The patient was asymptomatic at clinical follow-up 3 months after the intervention.
Figure 3.
Post-procedural imaging studies. Post-thrombectomy CT venogram of the brain shows patent superior sagittal sinus, torcula, and right transverse sinus (A, arrows). Post-intervention brain MRI shows minimal abnormal signal at the left frontal area on (B) diffusion-weighted imaging and (C) fluid attenuated inversion recovery (FLAIR) sequences. CT perfusion study demonstrates normalized cerebral blood flow (D) and cerebral blood volume (E) and minimally elevated time-to-peak (F).
Discussion
The treatment course for patients with CVST is dependent on the neurological condition of the patient. For patients showing neurological improvement or stability, anticoagulation therapy (typically low molecular weight heparin) is used to deter progression of thrombosis, promote recanalization, and prevent further thrombotic events. Patients with neurological deterioration or coma not responding to medical treatment are considered for endovascular therapy or, if there is evidence of severe mass effect or intracranial hemorrhage, decompressive hemicraniectomy.7 Endovascular treatment includes thrombolysis and mechanical thrombectomy, which can be performed with multiple devices and approaches such as an AngioJet (MEDRAD, Warrendale, Pennsylvania, USA), balloon venoplasty, aspiration, or a Solitaire stent retriever. In our case, endovascular intervention was performed because the patient had rapidly deteriorating neurological symptoms and the venogram showed significant clot burden. The use of a large Solitaire FR stent retriever combined with an aspiration catheter for CVST is derived from experience with arterial thrombectomy in acute stroke cases, and this approach was successfully applied in a recently reported case of CVST.8
At our institution, CTP is part of the standard protocol for all patients who present with acute stroke symptoms. This technology offers rapid evaluation of cerebral infarction and ischemia to assess whether a patient with acute ischemic stroke would benefit from endovascular therapy.9 Whole-brain CTP is obtained from an Aquilion320 CT system (Toshiba Medical Systems, Nasu, Japan) and is performed by injecting a small bolus of a non-diffusible iodinated contrast agent followed by rapid sequential acquisitions. This creates a time–density curve (a signal-intensity vs time curve) in each tissue voxel that represents the measured concentration of contrast agent within each voxel at a certain time. Perfusion parameters of CBF, CBV, and TTP (also mean transit time, MTT) can subsequently be calculated from the time–density curve via a tracer delay invariant single-value decomposition plus (SVD+) deconvolution algorithm (Vital Images, Minnetonka, Minnesota, USA and Toshiba Medical Systems).10 11 In patients with large artery occlusion, a mismatch between core infarction (CBV <2 mL/100 g) and tissue under ischemic condition (TTP >6 s) indicates the presence of salvageable penumbra, whereas a large area of CBV abnormality is a contraindication for revascularization.5 6 However, it remains unclear how to interpret CTP findings in patients with CVST.
Doege et al12 studied perfusion- and diffusion-weighted MRIs in six patients with CVST who had a good recovery and found that, although MTT was increased in the affected brain region and was normalized after successful anticoagulation treatment, CBV and apparent diffusion coefficient in the same region remained unchanged before and after treatment. Gupta et al13 used CTP imaging to evaluate 20 patients with CVST and found a statistically significant correction between reduced CBF and CBV on initial CTP imaging and poor neurological outcome. However, our case showed that, unlike patients with ischemic stroke, pre-intervention DWI (without T2 shine-through) and CTP abnormalities did not correlate with structural damage and were largely reversed after recanalization of the dural sinuses and improvement of cerebral venous flow. These findings imply that DWI brightness and CBV reduction need not be considered an absolute contraindication to endovascular intervention in the setting of CVST.
The utility of CTP has largely been reported in the ischemic stroke literature, and the limitations of this technology need to be considered when CTP findings are interpreted in conjunction with CVST. The deconvolution algorithm varies among different software, potentially leading to differences in reported perfusion parameters. Artifacts and overestimation of core infarct size can be caused by other factors such as improper arterial input function selection, the presence of large peripheral blood vessels and perforating arteries, internal carotid artery stenosis, and/or vasospasm.14 DWI and CTP abnormalities could have different implications for patients with CVST than for those with ischemic stroke, as demonstrated in our case. DWI hyperintensity, even without T2 shine-through, and CBV loss observed on CTP may not suggest irreversible tissue damage and should not be considered a contraindication for intervention. Further investigation is necessary to determine prognostic indications of MR and CTP imaging for patients with CVST.
Learning points.
Treatment options for cerebral venous sinus thrombosis (CVST) include systemic anticoagulation and, if refractory to medical therapy or in the presence of significant neurological deficits, endovascular thrombolysis and thrombectomy.
CT perfusion (CTP) parameters (cerebral blood flow, cerebral blood volume (CBV), and mean transit time (MTT)) are calculated via a deconvolution algorithm of time–density curves obtained through sequential acquisition. A mismatched area of decreased CBV and increased MTT indicates the presence of salvageable penumbra, whereas a large area of CBV loss is a contraindication for endovascular intervention.
Diffusion-weighted imaging (DWI) and CTP findings that indicate core infarction in patients with arterial occlusion need to be interpreted differently for patients with CVST. Increased DWI, decreased CBV, and increased MTT can be reversed after successful revascularization of venous sinuses.
Footnotes
Contributors: Conception and design: NL and AHS. Data acquisition, analysis, and interpretation: all authors. Drafting the manuscript: AKW, NL. Critical revision and final approval of the manuscript: all authors.
Competing interests: MM has received a grant from Toshiba. AHS has received grants from National Institutes of Health (co-investigator: NINDS 1R01NS064592-01A1; NIBIB 5RO1EB002873-07) and University at Buffalo (Research Development Award), none related to the present study; financial interests: Hotspur, Intratech Medical, StimSox, Valor Medical, Blockade Medical, and Lazarus Effect; consultant: Codman & Shurtleff, Concentric Medical, ev3/Covidien Vascular Therapies, GuidePoint Global Consulting, Penumbra, Stryker Pulsar Vascular, MicroVention, Lazarus Effect, Blockade Medical; speakers’ bureau: Codman & Shurtleff; National Steering Committee member: Penumbra 3D Separator Trial, Covidien SWIFT PRIME trial, MicroVention FRED trial; advisory boards: Codman & Shurtleff, Covidien Neurovascular; honoraria: Abbott Vascular, Codman & Shurtleff, Penumbra, Snyder: consultant and speakers’ bureau: Toshiba; honoraria: Toshiba; speakers’ bureau and honoraria: Covidien, The Stroke Group.
Patient consent: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional data may be available on a per-request basis. Requests should be directed to the corresponding author.
References
- 1.Coutinho J, de Bruijn SF, Deveber G et al. . Anticoagulation for cerebral venous sinus thrombosis. Cochrane Database Syst Rev 2011;(8):CD002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renowden S. Cerebral venous sinus thrombosis. Eur Radiol 2004;14:215–26. 10.1007/s00330-003-2021-6 [DOI] [PubMed] [Google Scholar]
- 3.Ferro JM, Canhao P, Stam J et al. . Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke 2004;35:664–70. 10.1161/01.STR.0000117571.76197.26 [DOI] [PubMed] [Google Scholar]
- 4.Coutinho JM, Zuurbier SM, Stam J. Declining mortality in cerebral venous thrombosis: a systematic review. Stroke 2014;45:1338–41. 10.1161/STROKEAHA.113.004666 [DOI] [PubMed] [Google Scholar]
- 5.Prabhakaran S, Soltanolkotabi M, Honarmand AR et al. . Perfusion-based selection for endovascular reperfusion therapy in anterior circulation acute ischemic stroke. AJNR Am J Neuroradiol 2014;35:1303–8. 10.3174/ajnr.A3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rai AT, Raghuram K, Domico J et al. . Pre-intervention triage incorporating perfusion imaging improves outcomes in patients undergoing endovascular stroke therapy: a comparison with the device trials. J Neurointerv Surg 2013;5:121–7. 10.1136/neurintsurg-2011-010189 [DOI] [PubMed] [Google Scholar]
- 7.Bushnell C, Saposnik G. Evaluation and management of cerebral venous thrombosis. Continuum (Minneap Minn) 2014;20:335–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pukenas BA, Kumar M, Stiefel M et al. . Solitaire FR device for treatment of dural sinus thrombosis. J Neurointerv Surg 2014;6:e2 10.1136/neurintsurg-2012-010543.rep [DOI] [PubMed] [Google Scholar]
- 9.Murphy BD, Fox AJ, Lee DH et al. . Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke 2006;37:1771–7. 10.1161/01.STR.0000227243.96808.53 [DOI] [PubMed] [Google Scholar]
- 10.Lev MH, Gonzalez RG. CT angiography and CT perfusion imaging (chapter 17). In: Toga AW, Mazziotta J eds. Brain mapping: the methods. 2nd edn San Diego, CA: Academic Press, 2002:445–8. [Google Scholar]
- 11.Orrison WW Jr, Snyder KV, Hopkins LN et al. . Whole-brain dynamic CT angiography and perfusion imaging. Clin Radiol 2011;66:566–74. 10.1016/j.crad.2010.12.014 [DOI] [PubMed] [Google Scholar]
- 12.Doege CA, Tavakolian R, Kerskens CM et al. . Perfusion and diffusion magnetic resonance imaging in human cerebral venous thrombosis. J Neurol 2001;248:564–71. 10.1007/s004150170133 [DOI] [PubMed] [Google Scholar]
- 13.Gupta RK, Bapuraj JR, Khandelwal N et al. . Prognostic indices for cerebral venous thrombosis on CT perfusion: a prospective study. Eur J Radiol 2014;83:185–90. 10.1016/j.ejrad.2013.09.027 [DOI] [PubMed] [Google Scholar]
- 14.Mangla R, Ekhom S, Jahromi BS et al. . CT perfusion in acute stroke: know the mimics, potential pitfalls, artifacts, and technical errors. Emerg Radiol 2014;21:49–65. 10.1007/s10140-013-1125-9 [DOI] [PubMed] [Google Scholar]