Abstract
Immunosuppressive checkpoints mediated by IDO, CTLA4, and PD1/PDL1 play a critical role in glioma progression and the efficacy of immunotherapies. Combined blockade of these immunosuppressive checkpoints in a glioma model elicited long-term survival. This combined blockade adds to the armamentarium of anti-glioma therapies, which could be implemented in clinical trials.
In this issue of Clinical Cancer Research, Wainwright and colleagues (1) report that by using a combinatorial approach aimed at blocking three immunosuppressive checkpoints in malignant glioma, they could elicit tumor regression and long-term survival in a syngeneic intracranial mouse glioma model. The model used by the authors is very challenging and difficult to treat, and because the tumor is located within the brain in immune-competent, syngeneic mice, the data have imminent, strong translational relevance. The model harbors many characteristics of human glioma, i.e., the tumor is highly immunosuppressive; the authors treated established, large tumors, and due to the fact that the tumors were implanted within the brain parenchyma lacking proper afferent antigen presenting cells, without immune-stimulatory treatments, antitumor immune responses are poor. Thus, the model used is highly relevant to test immune-stimulatory strategies (2).
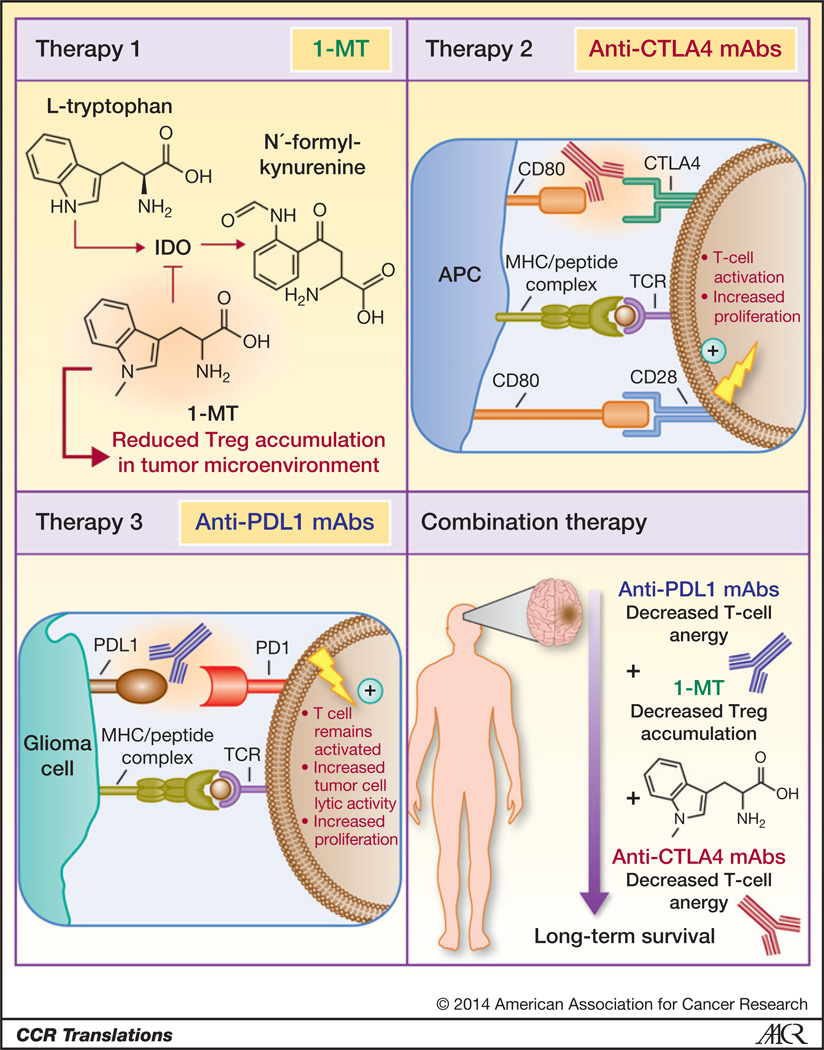
The authors blocked individually and simultaneously three immunosuppressive checkpoints, which play an important role in cancer immune suppression: (i) indoleamine 2,3-dioxygenase 1 (IDO; refs. 3, 4), (ii) cytotoxic T-lymphocyte antigen 4 (CTLA4; refs. 5, 6), and (iii) programmed death 1 receptor ligand, PDL1 (refs. 7, 8; Fig. 1). IDO is a cytosolic enzyme produced by tumor cells, macrophages, and dendritic cells within draining lymph nodes and the tumor microenvironment (9, 10).IDO catalyzes the limiting reaction in the degradation of tryptophan (Trp); a decrease in the levels of Trp, together with an increase in the production of active Trp metabolites (kynurenine), inhibits effector T cells and induces immunosuppressive regulatory T cells (Tregs; ref. 10). IDO is not normally expressed within the brain, but its expression is found in a high percentage of gliomas, thus making it an attractive immune-therapeutic target (10). Work by the authors and other groups had previously shown that immune suppression in glioma is associated with the recruitment of myeloid-derived suppressor cells, increased levels of interleukin-10, transforming growth factor-β, and the accumulation of Tregs: CD4+CD25+FoxP3+; ref. 11). In glioma, Tregs and T cells express high levels of CTLA4, a powerful immunosuppressive receptor. CTLA4 exerts its immunosuppressive activity by binding with higher affinity to CD80 and CD86, thereby reducing their binding to the immune-stimulatory receptor CD28; thus, CTLA4 blockade inhibits negative signals that prevent T-cell activation and expansion (5, 6). As opposed to CTLA4 signaling which occurs early, during T-cell activation in lymphatic organs, PD1 signaling takes place during the effector phase of T cells’ functions (7, 8). PD1 interacts with its two ligands, i.e., PDL1 (B7-H1) and PDL2 (B7-DC) in the tumor microenvironment, leading to T-cell apoptosis and inhibition of effector functions (7, 8).
Figure 1.
Molecular targets and associated therapies to block three immunosuppressive checkpoints in a malignant brain tumor (glioma) model. Glioma cells overexpress IDO to convert l-tryptophan to N′-formyl-kynurenine, the first step in the kynurenine metabolic pathway, which induces the accumulation of immunosuppressive Tregs in the tumor microenvironment. Therapy 1, the tryptophan analog, 1-MT, used as an IDO-specific competitive inhibitor. CD80 molecules on the surface of antigen-presenting cells (i.e., APC) interact with CTLA4 on the T-cell surface in the context of antigen-presenting MHC class I, inhibiting T-cell activation and decreasing proliferation, resulting in suppressed antitumor effector function. Therapy 2, anti-CTLA4 monoclonal antibodies bind to CTLA4, inhibiting its immunosuppressive signal, while promoting the interaction of CD80 with CD28, a T cell–activating receptor. Glioma cells express PDL1, which interacts with its cognate receptor PD1 on the T-cell surface to downregulate tumor lytic capacity and promote T-cell anergy in the context of tumor antigen-presenting MHC class I. Therapy 3, anti-PDL1 monoclonal antibodies bind to PDL1 on the surface of tumor cells to enable T cells to remain in an activated state characterized by high tumor lytic capacity and increased proliferation.
In this study, the authors targeted the inhibition of IDO in combination with therapies aimed at inhibiting CTLA4 and PD1/PDL1 function to develop an effective immune therapeutic strategy for the treatment of glioma, a strategy that could be potentially translated to human clinical trials (Fig. 1). To inhibit IDO, the authors used the tryptophan analog, 1-methyltryptophan (1-MT), an IDO-specific competitive inhibitor that blocks l-tryptophan conversion to N′-formyl-kynurenine and thus prevents Treg accumulation in the tumor microenvironment. The authors inhibited CTLA4 using anti-CTLA4 monoclonal antibodies, which bind to CTLA4, inhibiting its immunosuppressive activity, while freeing up CD80 and CD86 to bind to CD28, a T cell–activating receptor. The third immune checkpoint targeted by the authors is the PD1–PDL1 axis. Malignant glioma cells express PDL1, which interacts with its cognate receptor programmed death 1 (PD1) on the T-cell surface to downregulate tumor lytic capacity and promote T-cell anergy in the context of tumor antigen–presenting MHC class I molecules. Anti-PDL1 monoclonal antibodies bind to PDL1 on the surface of tumor cells, thus enabling T cells to remain activated, with high tumor lytic capacity and T-cell proliferation. The survival data presented by the authors in the glioma model indicate that the combined triple immunosuppressive checkpoint blockade provides maximum efficacy when compared with monotherapies using 1-MT, anti-CTLA4, or anti-PD1 alone or when compared with blockade of two immunosuppressive checkpoints (1). The data shown indicate that blockade of the inhibitory CTLA4 and PD1/PDL1 costimulatory pathways in combination with IDO inhibition, to decrease levels of Tregs in the tumor microenvironment, enables anti–tumor-specific effector T cells to continue to expand and display potent cytotoxic effector functions. Interestingly, when testing the triple combined blockade in a model of aggressive intracranial melanoma, the overall survival benefit was days, as opposed to months as observed in the glioma model. The authors hypothesize that the triple combination blockade would be more effective in tumors that exhibit highly prevalent immunosuppressive characteristics, i.e., glioma as opposed to tumors that evade antitumor immune responses using alternative mechanisms (1). These results also highlight the role that different tumor types exert to subvert antitumor immunity and reinforce the importance of testing novel therapies in the most relevant in vivo models before implementation in the clinic.
To date, several immune-stimulatory approaches have been proposed for treating glioma, and many of these have been tested in the clinic with some indication of biologic activity. These include vaccination strategies using dendritic cells, specific peptide tumor antigens, and engineered T cells (12). Nevertheless, these immune-stimulatory activities have not yet been translated into increased median survival in the glioma patient population. The report by Wainwright and colleagues is the first to test the combined blockade of three critical immunosuppressive checkpoints, IDO, CLTA4 and PD1/PDL1; the data reported indicate that the approach is highly effective, yielding a robust decrease in tumor-infiltrating Tregs concurrent with tumor regression, long-term survival (measured in months!), and immunologic memory in a relevant glioma model. Interestingly, another multipronged immunologic approach for glioma, pioneered by our group (2) also achieves survival that can be measured in months in a comparable model, and is currently being tested in a phase I clinical trial for glioma. We anticipate that the approach proposed by Wainwright and colleagues could be translated into phase I clinical trials for glioma in the near future, as these approaches have already been used individually in patients suffering from systemic tumors and clinical grade reagents are readily available. In addition, the data reported highlight that for immunotherapies to succeed, multiple pronged strategies are key.
Acknowledgments
Grant Support
This work was supported by NIH/National Institute of Neurological Disorders and Stroke (NINDS) grants U01-NS052465, U01-NS052465-S1, R01-NS074387, and R01-NS057711 to M.G. Castro; NIH/NINDS grants R01-NS054193, R01-NS061107, R01-NS082311, and R21-NS084275 to P.R. Lowenstein; and the Department of Neurosurgery at the University of Michigan School of Medicine.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: M.G. Castro, G.J. Baker, P.R. Lowenstein
Development of methodology: G.J. Baker, P.R. Lowenstein
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): M.G. Castro
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): P.R. Lowenstein
Writing, review, and or revision of the manuscript: M.G. Castro, G.J. Baker, P.R. Lowenstein
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): M.G. Castro
Study supervision: M.G. Castro, P.R. Lowenstein
References
- 1.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20:5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, et al. Hmgb1 mediates endogenous Tlr2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 7.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase Shp2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 11.Zou JP, Morford LA, Chougnet C, Dix AR, Brooks AG, Torres N, et al. Human glioma-induced immunosuppression involves soluble factoRs) that alters monocyte cytokine profile and surface markers. J Immunol. 1999;162:4882–4892. [PubMed] [Google Scholar]
- 12.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]