Abstract
We investigated how the dual inhibition of the molecular mechanism of the mammalian target of the rapamycin (mTOR) downstreams, P70S6 kinase (P70S6K) and eukaryotic initiation factor 4E (eIF4E), can lead to a suppression of the proliferation and progression of urothelial carcinoma (UC) in an orthotopic mouse non-muscle invasive bladder tumor (NMIBT) model. A KU-7-luc cell intravesically instilled orthotopic mouse NMIBC model was monitored using bioluminescence imaging (BLI) in vivo by interfering with different molecular components using rapamycin and siRNA technology. We then analyzed the effects on molecular activation status, cell growth, proliferation, and progression. A high concentration of rapamycin (10 µM) blocked both P70S6K and elF4E phosphorylation and inhibited cell proliferation in the KU-7-luc cells. It also reduced cell viability and proliferation more than the transfection of siRNA against p70S6K or elF4E. The groups with dual p70S6K and elF4E siRNA, and rapamycin reduced tumor volume and lamina propria invasion more than the groups with p70S6K or elF4E siRNA instillation, although all groups reduced photon density compared to the control. These findings suggest that both the mTOR pathway downstream of eIF4E and p70S6K can be successfully inhibited by high dose rapamycin only, and p70S6K and Elf4E dual inhibition is essential to control bladder tumor growth and progression.
Graphical Abstract
Keywords: Urinary Bladder Neoplasms, Mouse Orthotopic Model, mTOR
INTRODUCTION
Urothelial carcinoma (UC) is one of the most common malignant tumors. UC is divided into non-invasive and invasive subtypes, with non-invasive carcinoma further subdivided into low- and high-grade lesions according to pathology and clinical features (1, 2, 3). More than 75% of all bladder cancer cases are non-muscle-invasive bladder cancer (NMIBC) that can be treated by transurethral resection (TUR) (4). The intravesical administration of Bacillus Calmette-Guerin (BCG) is known as the most effective therapy for patients with high grade superficial bladder cancer with carcinoma in situ, as well as for preventing intravesical recurrence (5, 6). Unfortunately, of the patients who undergo complete TUR for non-muscle-invasive bladder, 31%-78% were reported to experience relapse or progression to muscle-invasive bladder cancer within 5 yr of follow-up (7, 8). Thus, constant efforts have been made to prevent disease progression and recurrences through research on the aberrant activation of cell signaling pathways that are involved in NMIBC, in order to identify novel molecular targets.
The mammalian target of rapamycin (mTOR), a ubiquitous serine-threonine kinase and a downstream component of the phosphatidylinositol 3'-kinase (PI3K)/AKT/phosphatase and the tensin homologue (PTEN)-signaling pathway, has been shown to play a critical role in the regulation of protein synthesis, cell growth, proliferation, apoptosis, survival, and angiogenesis (9). Furthermore, mTOR has been demonstrated to act as a transitional activator of hypoxia-inducible factor (HIF) through its activated downstream molecules, namely phosphorylated p70S6 kinase protein (p-p70S6K) and phosphorylated eukaryotic translation initiation factor 4E-binding protein-1 (p-4E-BP1) (10, 11). Phosphorylation by mTOR 4E-BP1 disrupts binding to eukaryotic initiation factor 4E (eIF4E), a protein that binds the 5'-cap structure of mRNA. The released eIF4E allows the formation of a functional translation initiation complex containing elF4G, elF4A, and elF3, thereby allowing translation (12). Also, several studies have revealed that activating the mTOR pathway, as assessed by p-4E-BP1, is related to bladder cancer tumorigenesis and that p-P70S6K is associated with a high level of disease recurrence and progression as well as poor cancer-specific survival (13) .
In a previous study, we demonstrated that the mTOR downstream, p70S6K, and eIF4E, are involved in regulating cell proliferation to a similar extent and inhibiting cells by high dose of rapamycin alone, effectively preventing cellular growth in vitro (14). On the basis of these findings, we also planned an in vivo study for a preclinical test. Non-muscle invasive bladder tumor (NMIBT) animal models are essential in preclinical research to evaluate the effect of novel molecular targets and understand the progression of NMIBC. A recent study showed that the orthotopic bladder transplantable tumor in mice can be a practical model because it is analogous to the clinical pathological process in humans (15). Furthermore, by using bioluminescence imaging (BLI) in an orthotopic NMIBT model, the evaluation of various intravesical therapy methods can be performed more easily and more rapidly (16).
In this study, we investigated how the dual inhibition of the molecular mechanism of mTOR downstream by high dose of rapamycin can lead to the suppression of the proliferation and progression of UC in an orthotopic mouse NMIBT model.
MATERIALS AND METHODS
Experimental animal
Twenty-seven-week old female nude (nu/nu) mice were provided by Orient Bio Co. (Seongnam, Korea). The animals were accommodated for one week as an adaptation period under routine laboratory conditions before the experiments were started. All animals were housed in cages containing five animals and kept on a daily 12-hr cycle of light and dark. The mice were fed a standard balanced diet and water ad libitum.
Cell culture and reagents
The high grade human bladder cancer cell line KU-7 engineered to stably express firefly luciferase and green fluorescent protein (KU-7-luc) was provided by Dr. H.K. Seo (National Cancer Center, Goyang, Korea) and purchased from Caliper Life Sciences (Hopkinto, MA, USA). KU-7-luc was cultured in a Roswell Park Memorial Institute medium (RPMI 1640; Gibco, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS). The media contained 50 µg/mL gentamycin sulfate. The KU-7-luc cell line was maintained at 37℃ in 5% CO2. Rapamycin was purchased from Sigma (St. Louis, MO, USA) and antibodies were purchased from different manufacturers: mTOR, phophrylated mTOR (p-mTOR, Ser 2448), p70S6 kinase (p70S6K), p-p70S6K (Ser371), 4E-BP1, p-4E-BP1, eIF4E, and p-eIF4E (Ser209) were obtained from Cell Signaling (Beverly, MA, USA), while β-actin was obtained from Sigma.
Western blot analysis
The cells were washed with ice-cold phosphate buffered saline (PBS) and trypsinized, a lysis buffer (Intron, Seoul, Korea) was pipetted onto the cells, and the lysates were stored at -20℃ until analysis. The amounts of protein were quantified by a Bradford assay (Biorad). Equal amounts of protein were loaded onto Readygels (4%-20% Tris-HCL; Biorad, Hercules, CA, USA), and electrophoresis was performed according to the manufacturer's instructions. Proteins were blotted onto poly-vinyl difluoride membranes (Invitrogen, USA), and then incubated in 5% skim milk blocking for 1 hr at room temperature. Blots were incubated with primary antibodies overnight at 4℃ and HRP-conjugated secondary antibodies for 1 hr at room temperature. The membranes were then developed into films using ECL.
siRNA constructs and transfection
siRNA oligonucleotides against p70S6 kinase (p70S6K I/II) and eIF4E were designed by and purchased from Cell signaling. Transient transfections were performed using X-treme gene transfection or lipofectamin reagent (Roche Diagnostics, Pleasanton, CA, USA) according to the manufacturer's instruction, using 2 µg/siRNC/6-well. The cells were harvested at 48 hr.
Wound-healing migration assay
This assay was performed using the Cytoselect Wound Healing kit (Cell Biolabs, San Diego, CA, USA) according to the manufacturer's instructions. Briefly, 2.5×104 mock, siRNA transfected, or rapamycin-treated cells were added to each well and incubated overnight to form a monolayer. The inserted wells were then removed to create a wound field of 0.9 mm diameter. After washing, the cells were incubated at 37℃ for 24 hr in 5% FBS growth medium. The extent of wound closure was determined and photographed with a Zeiss 8 Axiovert 200M live cell microscope.
Invasion assay
The cell invasion assay was performed with a CytoSelect 24-well Cell Invasion Assay kit (Cell Biolabs) according to manufacturer's protocol. Briefly, basement membranes of Boyden chambers were rehydrated with 300 µL serum free RPMI, and 2.5×104 mock siRNA transfected, or rapamycin-treated cells were then seeded into the upper area of the chamber in serum-free RPMI. The bottom wells were filled with RPMI supplemented with 10% FBS. After a 48 hr incubation (37℃, 5% CO2), non-invasive cells were removed from the upper chamber, and cell invasion was assessed by light microscopy after staining of invaded cells with Crystal Violet Cell Stain Solution (Cell Biolabs). The inserts were then placed in extraction buffer (200 µL, 10 min), and absorbance at 560 nm was determined using a VersaMax microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA) after transferring the medium to a 96 well plate (100 µL per well).
Cell viability assay
Cell viability was determined using an EZ-Cytoz viability assay kit (Dail lab, Seoul, Korea) according to the manufacturer's instructions. The KU-7-luc cells were seeded in 96 well plates at a density of 2×104 cells/well. After 24 hr in culture, the cells were treated with rapamycin, p70S6K siRNA, eIF4E siRNA, and dual p70S6K siRNA and eIF4E siRNA at given concentrations for 48 hr. The cell proliferation assay was performed 1, 2, and 3 days after treatment. The medium was replaced with fresh medium (200 µL), and 10 µL EZ-cytox reagent was added to each well. The cell culture was continued for 1 hr and the culture medium was then removed and placed in each well of a new plate. The optical density (OD) was quantified at a wavelength of 450 nm.
Intravesical orthotopic model of bladder cancer
For intravesical implantation of KU-7-luc, female nude (nu/nu) mice at 7 weeks of age (Orient Bio Co., Seongnam, Korea) were anaesthetized with isoflurane. Chemical lesions to the bladder urothelium were carried out by injecting 100 µL of Poly-L-lysine (PLL) (molecular weight 70,000-150,000, Sigma) into the bladder of each animal through a 24-gauge catheter (B/BrAUN, Melsungen, Germany). After instilling 0.1 mL of 0.1 mg/mL PLL for 20 min, the bladders were washed with PBS and subsequently instilled with KU-7-luc cells (2.0×106) suspended in 50 µL PBS via a catheter. The cells were retained in the bladder for 2 hr by tying off the orifice to the urethra.
In vivo bioluminescence imaging of KU-7-luc cells
The mice were administrated the KU-7-luc cell on day 0. On the 7th day, each mouse was observed using an IVIS 200 (Xenogen Corp., Alameda, CA, USA), and in vivo BLI of KU-7-luc cells was evaluated 10 min after intraperitoneal administration of 150 mg/kg D-luciferin (Invitrogen, Carlsbad, CA, USA). A bioluminescence signal (BLS) was acquired and analyzed using Living Image software version 2.50 (Xenogen, Alameda, CA, USA). Regions of interest (ROI) were defined manually to encompass the bladder and quantify signal intensity. In vivo bioluminescence imaging (BLI) confirmed Ku-7-luc cells intravesically instilled in the mice were randomized into control, p70S6K siRNA, eIF4E siRNA, dual p70S6K and eIF4E, and rapamycin groups. Each groups contained 5 mice and at the beginning of the 7th day, each mouse was administered p70S6K siRNA, eIF4E siRNA, dual p70S6K and eIF4E siRNA, and rapamycin through a catheter into the bladder lumen, twice a week. Dimethyl sulfoxide (DMSO) was administered to the control group. Rapamycin was delivered at doses of 10 µM in DMSO, and p70S6K siRNA, and eIF4E siRNA was delivered at doses of 100 nM in liposomes. Intravesical delivery was carried out with a dwelling time of 1 hr. We monitored bladder cancer progression by serial BLI (Fig. 1). The in vivo BLI of KU-7-luc cells was evaluated 10 min after intraperitoneal administration of 150 mg/kg D-luciferin (Invitrogen) on days 7, 10, 14, 17, and 21 after tumor cell implantation. Bioluminescence imaging analysis have been described and discussed in Fig. 2 of our previous study (17).
Fig. 1.
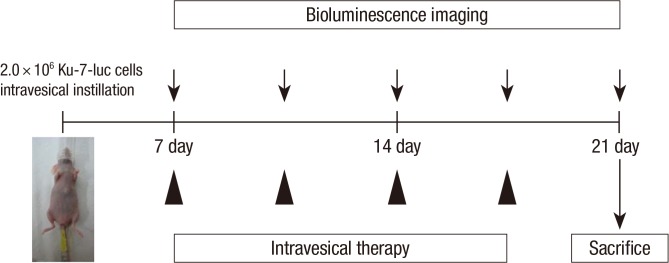
Experimental strategy of mTOR signaling in bladder cancer progression in vivo. Bioluminescence imaging confirmed Ku-7-luc cells intravesical instilled mice which were confirmed by bioluminescence imaging were randomised into control, pS6K siRNA, elF4E siRNA, dual pS6K and elF4E, and rapamycin groups. Beginning at 7th days, mice were delivered pS6K siRNA, elF4E siRNA, dual pS6K and elF4E, and rapamycin through a catheter into the bladder lumen, respectively twice a week, and we monitored bladder cancer progression by serial bioluminescence imaging. At 21th days mice were sacrificed for analysis of bladder histophatology.
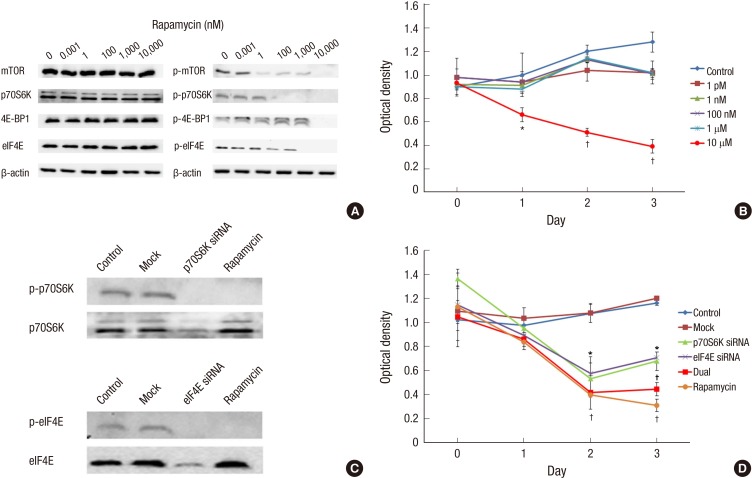
Fig. 2.
Experimental strategy for inhibition of mTOR signaling in bladder cancer progression at in vitro study. (A) Western blot analysis for mTOR pathway downstream protein expression in Ku-7-luc cell line treated with rapamycin. The expression of p-mTOR, and p-p70S6K, the activated form of proteins, is decreased dose-dependent manner by rapamycin concentration, but the expression of p-4E-BP1 and p-elF4E is blocked at high concentration (10 µM) of rapamycin in Ku-7-luc cell line. (B) Cell viability assay of Ku-7-luc cell line treated with rapamycin. Cell viability is inhibited at high concentration (10 µM) of rapamycin on 1, 2, and 3 days compared to control, but other concentration of rapamycin is not inhibit cell viability. (C) The transfection of siRNA against pS6K or elF4E, and rapamycin in Ku-7-luc cell line. We confirmed that the transfection of siRNA against pS6K or elF4E reduced protein expression respectively, and the dual pS6K and elF4E phosphorylation inhibited by rapamycin, simultaneously. (D) Cell viability assay of Ku-7-luc cell line treated with siRNA oligonucleotide directed against pS6K or elF4E, or high concentration of rapamycin. Cells silenced for pS6K or elF4E expression exhibit significantly reduced cell viability compared to control at 2 and 3 days, but the dual pS6K and elF4E phosphorylation inhibition by rapamycin reduced cell viability more than the transfection of siRNA against pS6K or elF4E at 3 days. *P < 0.05; †P < 0.01.
Histopathologic examination
We sacrificed all mice at day 21. The bladders were harvested and opened in the sagittal plane. After gross examination, the bladders were fixed in 4% paraformaldehyde, routinely processed and paraffin included, and stained with hematoxylin and eosin (H&E). We evaluated the tumor stage by observing histological staining through the microscope. For tumor volume measurement (largest width2×largest length×0.5), bladder slices were sectioned into 5 µm sections using a microtome and stained with H&E.
Statistical analysis
The SPSS software package, version 14.0 (Statistical Package for Social Sciences™, Chicago, IL, USA) was used for all statistical analyses. One-way analysis of variance (ANOVA) was used to detect significant differences among the groups and a Student t-test was used to compare the means in different groups, and P<0.05 was considered significant.
Ethics statement
The animal study was carried out according to a protocol approved by the institutional animal care and use committee (NCC-12-178) of the National Cancer center.
RESULTS
High concentration of rapamycin and dual blocking of both mTOR downstream targets
In order to characterize the functional relevance of the mTOR pathway in UC, we used rapamycin. Ku-7-luc cells were treated with rapamycin (1 pM, 1 nM, 100 nM, 1 µM, and 10 µM) for 48 hr and western blot analysis was performed for the mTOR pathway downstream targets. As shown in Fig. 2A, the expression of p-mTOR and p-p70S6K, the activated form of proteins, was decreased in a dose-dependent manner by rapamycin concentration, but the expression of p-4E-BP1 and p-elf4E was blocked at high concentration (10 µM) of rapamycin. Therefore, we showed that high concentration of rapamycin can block both of the mTOR downstream targets effectively.
Anti-proliferative effect of rapamycin shown by dual block of both mTOR downstream targets
Next, we examined the effect of rapamycin, a known mTOR inhibitor, on UC cell viability in the Ku-7-luc cell line. Ku-7-luc cells were treated with various concentrations of rapamycin (1 pM, 1 nM, 100 nM, 1 µM, and 10 µM) for 48 hr and the cell viability assay was performed 1, 2, and 3 days after treatment. Each of the concentrations above was regarded as one treatment group, while rapamycin was not administered in the control group. As shown in Fig. 2B, the cell viability of the Ku-7-luc cells were inhibited at high concentration (10 µM) of rapamycin on 1 (P<0.05), 2 (P<0.01), and 3 days (P<0.01) compared to the control, but other concentrations of rapamycin did not inhibit cell viability.
Combined activity of p70S6K and elf4E on cell proliferation in Ku-7-luc cells
Next, we addressed the question of whether inhibiting both p70S6K and eIF4E phosphorylation by rapamycin would affect cell viability more effectively than inhibiting p70S6K or eIF4E individually. We demonstrated that a high concentration of rapamycin influenced both p70S6K and eIF4E phosphorylation. Thus, we used siRNA oligonucleotide directed against p70S6K or eIF4E individually or simultaneously, and a high concentration of rapamycin to mimic dual inhibition of p70S6K and eIF4E phosphorylation. Reduced protein expression was observed at 24 hr after siRNA transfection against p70S6K or eIF4E, and we showed that rapamycin inhibited both P70S6K and eIF4E phosphorylation simultaneously (Fig. 2C). The Ku-7-luc cells silenced for p70S6K or eIF4E expression exhibited significantly reduced viability compared to those in the control at 1, 2, and 3 days, but dual p70S6K and eIF4E inhibition by siRNA reduced cell viability more than transfection of siRNA against p70S6K or eIF4E individually, similar to the high-dose rapamycin in the Ku-7-luc cells at 3 days (Fig. 2D).
In summary, both the mTOR downstream targets of p70S6K and eIF4E were involved in regulating UC cell proliferation, and inhibiting both p70S6K and eIF4E phosphorylation by rapamycin showed a more potent anti-proliferative effect than inhibiting p70S6K or 4E-BP1 phosphorylation alone.
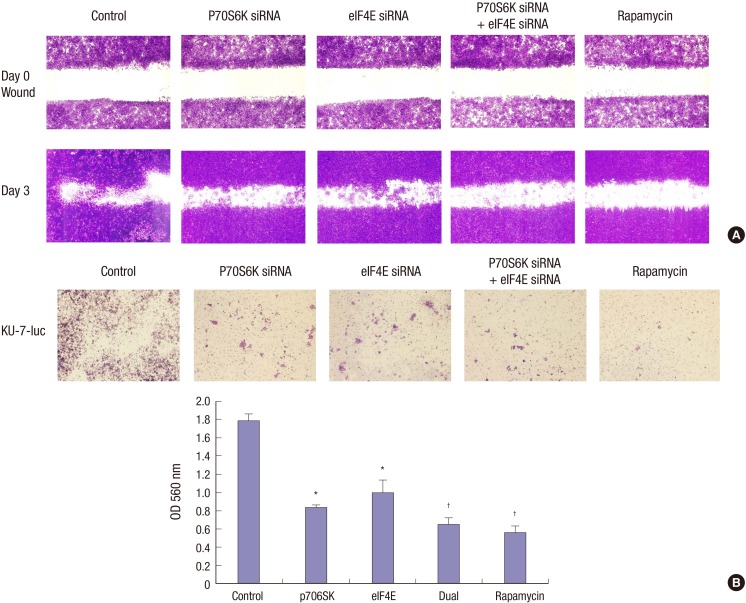
The combined activity of pS6K and elf4E on cell migration and invasion in KU-7-luc cells
We further evaluated whether inhibiting S6K1 and eIF4E phosphorylation by rapamycin affected cell migration more efficaciously than S6K1 or eIF4E inhibition alone using an in vitro wound healing motility assay. Control, S6K1, eIF4E siRNA transfected, or rapamycin-treated KU-7-luc bladder cancer cells were plated at high density in serum containing medium overnight. Cells were wounded and then incubated for 3 days. In Fig. 3A, we determined that inhibiting both S6K1 and eIF4E phosphorylation by rapamycin reduced cell migration more than transfection of siRNA against S6K1 or eIF4E in KU-7-luc cells. Next, an invasion assay was performed to evaluate the effect of rapamycin, S6K1, and eIF4E siRNA on bladder cancer cell invasion (Fig. 3B). Silencing of S6K1 or eIF4E expression resulted in significantly reduced cell invasion in KU-7-luc cells compared to that of the control but inhibiting S6K1 and eIF4E phosphorylation with rapamycin reduced cell invasion more than transfection of siRNA against S6K1 or eIF4E in KU-7-luc cells (Fig. 3B). In summary, both mTOR downstream targets S6K1 or eIF4E were similarly involved in regulating UC cell migration and invasion, and inhibiting phosphorylation of both S6K1 and eIF4E by rapamycin showed more potent anti-migration and anti-invasive effects than inhibiting S6K1 or eIF4E phosphorylation.
Fig. 3.
Wound healing assay and invasion assay of the UC cell lines treated with siRNA oligonucleotides directed against pS6K or elF4E or a high concentration of rapamycin. (A) Inhibiting phosphorylation of both pS6K and elF4E by rapamycin reduce cell migration more than transfection of siRNA against pS6K or elF4E in KU-7 cells. (B) An invasion assay was performed to evaluate the effect of rapamycin, pS6K, and elF4E siRNA on bladder cancer cells invasion. Cells silenced for pS6K or elF4E expression exhibit significantly reduced cell invasion compared to that of the control (P < 0.05) but inhibiting phosphorylation of both pS6K and elF4E by rapamycin reduced cell invasion more than the transfection of siRNA against pS6K or elF4E in KU-7 cells. *P < 0.05; †P < 0.01.
Effects of P70S6K and Elf4E dual inhibition on control bladder cancer progression in orthotopic mouse non-muscle invasive bladder cancer model
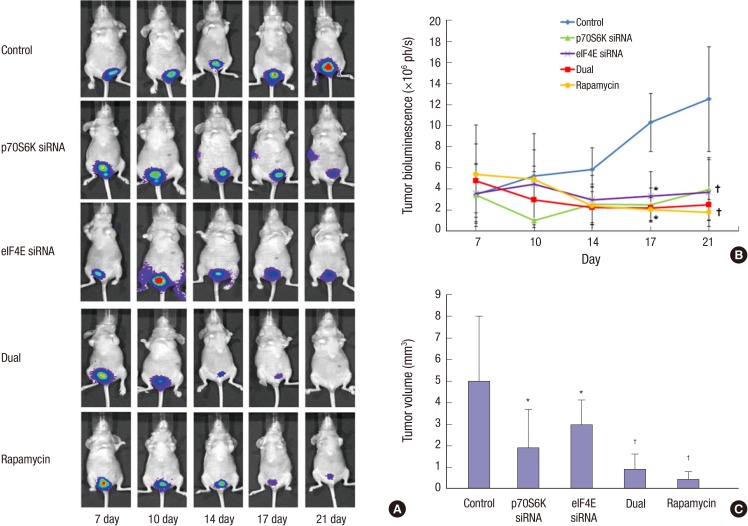
After intravesical instillation of 2×106 Ku-7-Luc cells on day zero, the mice were imaged on days 7, 10, 14, 17, and 21 after tumor cell implantation in the 5 groups of control, p70S6K siRNA, eIF4E siRNA, dual p70S6K and eIF4E siRNA, and rapamycin groups (Fig. 4A), respectively. The mice were delivered p70S6K siRNA, eIF4E siRNA, dual p70S6K and eIF4E siRNA, and rapamycin through a catheter into the bladder lumen. Fig. 4B shows the BLI photon densities according to the groups, and the photon densities (mean±SD, ×106 ph/s) of the control, p70S6K siRNA, eIF4E siRNA, dual p70S6K and eIF4E siRNA, and rapamycin groups were 10.31±2.76, 2.5±1.61, 3.34±2.31, 2.22±0.01, and 2.07±1.12×106 ph/s, respectively, after 17 days and 12.53 ±4.96, 3.96±2.85, 3.73±3.26, 2.51±0.51, and 1.81±0.79×106 ph/s, respectively, after 21 days. The photon densities of the control were higher than those of the other groups after 17 and 21 days, although no difference was observed between all groups, except the control.
Fig. 4.
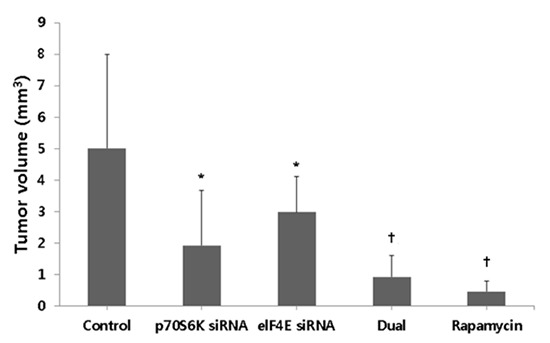
The effects of S6K1 and Elf4E dual inhibition to control bladder cancer progression at orthotopic mouse non-muscle invasive bladder cancer model. (A) In vivo imaging of tumor growth over time according to groups. After intravesical instillation of 2×106 Ku-7-Luc cells on day zero, mice are imaged at 4, 7, 14, and 21 days. (B) Comparison of bioluminescence between groups. The photon densities of control showed higher than other groups at 17 and 21 days. (C) Comparison of tumor volumes between groups. The groups with pS6K or elF4E siRNA instillation show the decreased tumor volumes compared to control, but the groups with dual pS6K and elF4E siRNA and rapamycin instillation reduce tumor volumes more than the groups with pS6K or elF4E siRNA instillation. *P < 0.05; †P < 0.01.
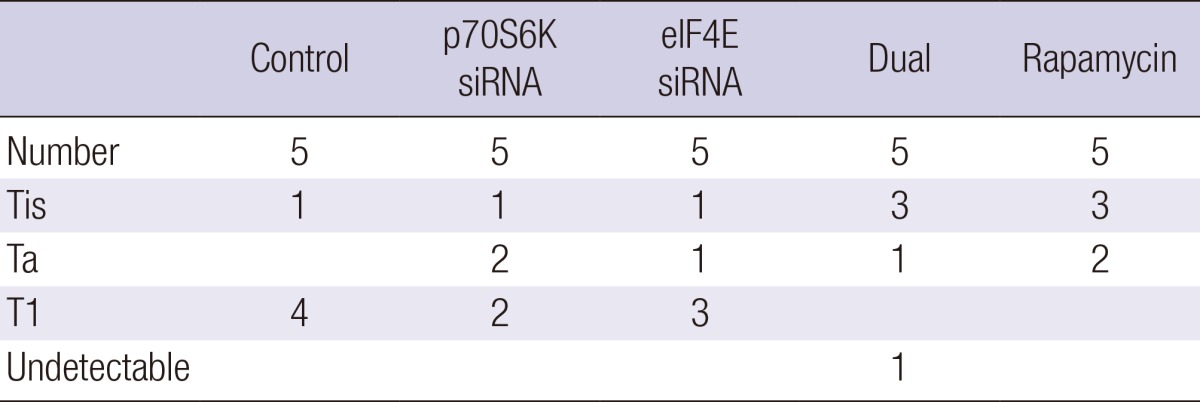
Table 1 shows the histopathological stage of bladder tumors among the groups. All dual p70S6K and eIF4E siRNA, and rapamycin groups showed Tis or Ta, although the p70S6K siRNA and eIF4E siRNA groups showed lamina propria invasion (T1) in 2 and 3 mice, respectively. In the control groups, 4 of the 5 mice showed T1. We then compared the tumor volumes between groups (Fig. 4C). The tumor volume (mean±SD, mm3) of the control, p70S6K siRNA, eIF4E siRNA, dual p70S6K and eIF4E siRNA, and rapamycin groups was 5.02±2.98, 1.92±1.76, 2.98±1.13, 0.92±0.67, and 0.45±0.36 mm3, respectively. The groups with p70S6K siRNA or eIF4E siRNA instillation showed decreased tumor volumes compared to the control (P<0.05), but the groups with dual p70S6K and eIF4E siRNA, and rapamycin instillation reduced tumor volumes more than the groups with p70S6K or eIF4E siRNA instillation (P<0.05).
Table 1.
Histological stage of five mice with bladder tumors among the groups
Thin sections were prepared from each bladder sample followed by hematoxylin and eosin staining.
DISCUSSION
To enable preclinical testing of intravesical therapies based on the mTOR signaling pathway against NMIBC, we validated an orthotopic mouse bladder tumor model, and augmented the model by serial BLI for in vivo tumor assessments. Our overall tumor establishment with 2.0×106 KU-7-Luc cells was >80% in mice at the scheduled time of 4-21 days. The tumor formed initially on day 4 and remained NMIBT at up to 21 days. Moreover, we confirmed that the established orthotopic NMIBT model showed similar mTOR signaling pathway expression to that in the urothelial cancer cell lines. The KU-7-luc is believed to be the only cell line to date in which reproducibly provides reliable tumor take rates as orthotopic xenografts without the use of secondary agents such as trypsin or electrocautery that traumatize the urothelium to promote tumor cell adhesion. KU-7-luc xenografts represent an epithelial intravesical carcinoma model that clinically resembles human bladder cancer and the key characteristic of this model is the lack of deeper invasion at early time points, which makes these tumors amenable to intravesical therapy (18). Thus, we believe that an orthotopic mouse NMIBT model using KU-7-luc cells may be suitable for our experiment.
We revealed that a high dose of rapamycin showed dual blocking of both the mTOR downstream targets. The natural product rapamycin has potent immunosuppressant and antiproliferative properties stemming from its ability to modulate signal transduction pathways linking growth stimuli to the synthesis of specific proteins required for cell cycle progression from the G1 to the S phase. The first downstream regulator modulated by the phosphorylation status of mTOR, 4E-BP1, is a low molecular-weight protein that inhibits the initiation of translation through its association with eIF-4E, the mRNA cap-binding subunit of the eukaryotic initiation factor-4 (eIF-4F) complex. Treatment with rapamycin results in the dephosphorylation of 4E-BP1, an increase in eIF-4E binding, and a concomitant decrease in the translation of mRNAs for cell cycle progression from the G1 to the S phase (19). The second downstream target modulated by mTOR is the kinase p70S6K. Upon activation by proliferative stimuli mediated by the PI3K/Akt signal transduction pathway, mTOR phosphorylates/activates p70S6K, which in turn phosphorylates the 40S ribosomal protein S6 (20). Rapamycin treatment results in a rapid and profound dephosphorylation of p70S6K, suppressing its activity (21, 22).
We demonstrated that both the mTOR downstream targets of p70S6K and eIF4E were involved in regulating KU-7-luc cell proliferation, and that inhibiting both p70S6K and eIF4E phosphorylation by rapamycin showed a more potent anti-proliferative effect than inhibiting p70S6K or 4E-BP1 phosphorylation alone. Moreover, the experiment was conducted with the orthotopic animal model that we had developed. In addition, from a BLI comparison of tumor volume, the dual p70S6K and eIF4E siRNA, and rapamycin installation groups reduced tumor volumes more than the groups with p70S6K or eIF4E siRNA instillation. This means that high dose rapamycin is able to prevent the progression of NMIBC. These results may explain why previous studies with mTOR inhibitors showed a low efficacy (23). Our assumption is that the low concentration of rapamycin is not able to block both mTOR downstream targets effectively, as shown in Fig. 2B. Injecting the high dose of rapamycin into a human vein is dangerous due to toxicity, but the bladder is a closed organ so the instillation of a high dose of rapamycin can be performed. This is strongly supported by our experiment, which demonstrated that all mice survived after a high dose of rapamycin instillation. Thus, a high dose of rapamycin instillation might be applicable for humans. If following research guarantees the safety of rapamycin instillation, the anticancer effect of rapamycin instillation which blocks both of mTOR pathways, would be more potent than that of non-specific immune reaction related BCG instillation. Moreover, unlike BCG therapy, it has a few systemic side effects and it is supposed to be widely used even in immunosuppressed patients.
Our study has some limitations. First, a report has been presented on the propriety of KU-7 as a human bladder cancer cell line. The report indicated that the cross contamination of KU-7 with HeLa occurred before 1984 at the source institution (24). However, this has not yet been definitely proved. Rather, many researches support the appropriateness of KU-7 as a human bladder cancer cell line (16, 25, 26). Second, in our study, the group with dual p70S6K and eIF4E siRNA, and the rapamycin instillation groups showed lower tumor volume and lower invasiveness than groups with p70S6K or eIF4E siRNA, although no difference was observed between groups in photon densities. Our hypothesis suggests that although the BLI photon density is increased according to tumor volume increase, it has a tendency to be exaggerated (27); this may be correlated with the factors that influence the intensity of bioluminescent emissions including the time courses of luciferase-luciferin reactions, and effective plasmid DNA and D-luciferin doses and combinations. Although BLI needs to be improved in terms of cost-effectiveness and non-invasiveness, it has many advantages. Finally, the toxicities of high dose of rapamycin must be considered since it is an immunosuppressant. A reasonable concern regarding the suppression of the immune response by rapamycin might promote rather than inhibit tumor growth. However, available evidence supports the contrary in clinical and preclinical studies, that rapamycin inhibits the growth of the tumor with no reports of promoting tumor growth (28). Even though rapamycin suppresses the immune system, it can also inhibit tumor cell proliferation and angiogenesis, and can thus lead to apoptosis of tumor cell with a potent cytostatic nature and growth inhibitory properties triggered by a nutrient deprivation-like response. Despite these limitations, our results are reasonably accurate because the orthotopic mice model using a human cancer cell line is practically analogous to the clinical pathological process in humans.
In conclusion, the experiment with the NMIBT animal model has established the effect of high dose rapamycin on NMIBC and it seems very optimistic in the near future to be used as an intravesical instillation therapy if more clinical trials humans are taken.
Footnotes
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2012R1A1A1002559).
The authors have no potential conflicts of interest to disclose.
Conception and coordination of the study: Chang IH. Design of ethical issues: Kwon JK. Acquisition of data: Seo HK, Seo HH, Lee SJ. Data review: Kim SJ. Statistical analysis: Chi BH. Chang IH. Manuscript preparation: Chi BH. Chang IH. Manuscript approval: All authors.
References
- 1.Sharir S. Update on clinical and radiological staging and surveillance of bladder cancer. Can J Urol. 2006;13:71–76. [PubMed] [Google Scholar]
- 2.Bulbul MA, Husseini N, Houjaij A. Superficial bladder cancer epidemiology, diagnosis and management. J Med Liban. 2005;53:107–113. [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Murta-Nascimento C, Schmitz-Dräger BJ, Zeegers MP, Steineck G, Kogevinas M, Real FX, Malats N. Epidemiology of urinary bladder cancer: from tumor development to patient's death. World J Urol. 2007;25:285–295. doi: 10.1007/s00345-007-0168-5. [DOI] [PubMed] [Google Scholar]
- 5.Catalona WJ, Ratliff TL. Bacillus Calmette-Guerin and superficial bladder cancer. Clinical experience and mechanism of action. Surg Annu. 1990;22:363–378. [PubMed] [Google Scholar]
- 6.Holmäng S, Hedelin H, Anderström C, Holmberg E, Busch C, Johansson SL. Recurrence and progression in low grade papillary urothelial tumors. J Urol. 1999;162:702–707. doi: 10.1097/00005392-199909010-00019. [DOI] [PubMed] [Google Scholar]
- 7.Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. 1997;158:62–67. doi: 10.1097/00005392-199707000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Kavoussi LR, Torrence RJ, Gillen DP, Hudson MA, Haaff EO, Dresner SM, Ratliff TL, Catalona WJ. Results of 6 weekly intravesical bacillus Calmette-Guerin instillations on the treatment of superficial bladder tumors. J Urol. 1988;139:935–940. doi: 10.1016/s0022-5347(17)42722-4. [DOI] [PubMed] [Google Scholar]
- 9.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 10.Ching CB, Hansel DE. Expanding therapeutic targets in bladder cancer: the PI3K/Akt/mTOR pathway. Lab Invest. 2010;90:1406–1414. doi: 10.1038/labinvest.2010.133. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau D, Gingras AC, Pause A, Sonenberg N. The eIF4E-binding proteins 1 and 2 are negative regulators of cell growth. Oncogene. 1996;13:2415–2420. [PubMed] [Google Scholar]
- 12.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5'TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SJ, Lee TJ, Chang IH. Role of the mTOR pathway in the progression and recurrence of bladder cancer: an immunohistochemical tissue microarray study. Korean J Urol. 2011;52:466–473. doi: 10.4111/kju.2011.52.7.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon JK, Kim SJ, Kim JH, Lee KM, Chang IH. Dual inhibition by S6K1 and Elf4E is essential for controlling cellular growth and invasion in bladder cancer. Urol Oncol. 2014;32:51.e27–51.e35. doi: 10.1016/j.urolonc.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Yang XH, Ren LS, Wang GP, Zhao LL, Zhang H, Mi ZG, Bai X. A new method of establishing orthotopic bladder transplantable tumor in mice. Cancer Biol Med. 2012;9:261–265. doi: 10.7497/j.issn.2095-3941.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadaschik BA, Black PC, Sea JC, Metwalli AR, Fazli L, Dinney CP, Gleave ME, So AI. A validated mouse model for orthotopic bladder cancer using transurethral tumour inoculation and bioluminescence imaging. BJU Int. 2007;100:1377–1384. doi: 10.1111/j.1464-410X.2007.07165.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim SJ, Seo HK, Seo HH, Lee SJ, Kwon JK, Lee TJ, Chi BH, Chang IH. Establishment of an orthotopic mouse non-muscle invasive bladder cancer model expressing the mammalian target of rapamycin signaling pathway. J Korean Med Sci. 2014;29:343–350. doi: 10.3346/jkms.2014.29.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadaschik BA, Zhang K, So AI, Fazli L, Jia W, Bell JC, Gleave ME, Rennie PS. Oncolytic vesicular stomatitis viruses are potent agents for intravesical treatment of high-risk bladder cancer. Cancer Res. 2008;68:4506–4510. doi: 10.1158/0008-5472.CAN-08-0238. [DOI] [PubMed] [Google Scholar]
- 19.Brunn GJ, Fadden P, Haystead TA, Lawrence JC., Jr The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J Biol Chem. 1997;272:32547–32550. doi: 10.1074/jbc.272.51.32547. [DOI] [PubMed] [Google Scholar]
- 20.Tang S, Hu RG, Liu WY, Ruan KC. Non-specific depurination activity of saporin-S6, a ribosome-inactivating protein, under acidic conditions. Biol Chem. 2000;381:769–772. doi: 10.1515/BC.2000.098. [DOI] [PubMed] [Google Scholar]
- 21.Seufferlein T, Rozengurt E. Rapamycin inhibits constitutive p70s6k phosphorylation, cell proliferation, and colony formation in small cell lung cancer cells. Cancer Res. 1996;56:3895–3897. [PubMed] [Google Scholar]
- 22.Grewe M, Gansauge F, Schmid RM, Adler G, Seufferlein T. Regulation of cell growth and cyclin D1 expression by the constitutively active FRAP-p70s6K pathway in human pancreatic cancer cells. Cancer Res. 1999;59:3581–3587. [PubMed] [Google Scholar]
- 23.Mansure JJ, Nassim R, Chevalier S, Rocha J, Scarlata E, Kassouf W. Inhibition of mammalian target of rapamycin as a therapeutic strategy in the management of bladder cancer. Cancer Biol Ther. 2009;8:2339–2347. doi: 10.4161/cbt.8.24.9987. [DOI] [PubMed] [Google Scholar]
- 24.Jäger W, Horiguchi Y, Shah J, Hayashi T, Awrey S, Gust KM, Hadaschik BA, Matsui Y, Anderson S, Bell RH, et al. Hiding in plain view: genetic profiling reveals decades old cross contamination of bladder cancer cell line KU7 with HeLa. J Urol. 2013;190:1404–1409. doi: 10.1016/j.juro.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang MR, Yang G, Charisse K, Epstein-Barash H, Manoharan M, Li LC. An orthotopic bladder tumor model and the evaluation of intravesical saRNA treatment. J Vis Exp. 2012 doi: 10.3791/4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe T, Shinohara N, Sazawa A, Harabayashi T, Ogiso Y, Koyanagi T, Takiguchi M, Hashimoto A, Kuzumaki N, Yamashita M, et al. An improved intravesical model using human bladder cancer cell lines to optimize gene and other therapies. Cancer Gene Ther. 2000;7:1575–1580. doi: 10.1038/sj.cgt.7700261. [DOI] [PubMed] [Google Scholar]
- 27.Jung SY, Willard ST. Quantitative bioluminescence imaging of transgene expression in intact porcine antral follicles in vitro. Reprod Biol Endocrinol. 2014;12:11. doi: 10.1186/1477-7827-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luan FL, Hojo M, Maluccio M, Yamaji K, Suthanthiran M. Rapamycin blocks tumor progression: unlinking immunosuppression from antitumor efficacy. Transplantation. 2002;73:1565–1572. doi: 10.1097/00007890-200205270-00008. [DOI] [PubMed] [Google Scholar]