Summary
Ipilimumab, a novel therapy for metastatic melanoma, inhibits cytotoxic T-lymphocyte apoptosis, causing both antitumor activity and significant autoimmunity, including autoimmune thyroiditis. Steroids are frequently used in treatment of immune-related adverse events; however, a concern regarding the property of steroids to reduce therapeutic antitumor response exists. This study describes the first reported case of ipilimumab-associated thyroid storm and implicates iopanoic acid as an alternative therapy for immune-mediated adverse effects. An 88-year-old woman with metastatic melanoma presented with fatigue, anorexia, decreased functional status, and intermittent diarrhea for several months, shortly after initiation of ipilimumab – a recombinant human monoclonal antibody to the cytotoxic T-lymphocyte-associated antigen 4 (CTLA4). On arrival, she was febrile, tachycardic, and hypertensive with a wide pulse pressure, yet non-toxic appearing. She had diffuse, non-tender thyromegaly. An electrocardiogram (EKG) revealed supraventricular tachycardia. Blood, urine, and stool cultures were collected, and empiric antibiotics were started. A computed tomography (CT) angiogram of the chest was negative for pulmonary embolism or pneumonia, but confirmed a diffusely enlarged thyroid gland, which prompted thyroid function testing. TSH was decreased at 0.16 μIU/ml (normal 0.3–4.7); free tri-iodothyronine (T3) was markedly elevated at 1031 pg/dl (normal 249–405), as was free thyroxine (T4) at 5.6 ng/dl (normal 0.8–1.6). With iopanoic acid and methimazole therapy, she markedly improved within 48 h, which could be attributed to lowering of serum T3 with iopanoic acid rather than to any effect of the methimazole. Ipilimumab is a cause of overt thyrotoxicosis and its immune-mediated adverse effects can be treated with iopanoic acid, a potent inhibitor of T4-to-T3 conversion.
Learning points
While ipilimumab more commonly causes autoimmune thyroiditis, it can also cause thyroid storm and clinicians should include thyroid storm in their differential diagnosis for patients who present with systemic inflammatory response syndrome.
Immune-related adverse reactions usually occur after 1–3 months of ipilimumab and baseline thyroid function testing should be completed before initiation with ipilimumab.
Conflicting data exist on the use of prednisone for treatment of CTLA4 adverse effects and its attenuation of ipilimumab's antitumor effect. Iopanoic acid may be considered as an alternative therapy in this setting.
Background
Developments in cancer therapeutics have shifted toward novel target-specific immunotherapies that act along signaling pathways. Ipilimumab, an antineoplastic agent used in unresectable metastatic melanoma, is a recombinant human MAB that acts against surface receptor cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) to modulate immune response (1). Through its inhibition of CTLA4, ipilimumab disinhibits the proliferation of effector T-lymphocytes, leading to antitumor activity. It has been shown to prolong the overall survival in patients with metastatic melanoma, although the greatest impact appears to be in those with previously treated metastatic disease (2, 3). However, due to its enhancement of immunity, ipilimumab, similar to other agents including interferon (4, 5), has the potential for significant immune-mediated toxicity, including inflammatory colitis, dermatitis, and endocrinopathies (2, 6, 7). Ipilimumab has been associated with autoimmune hypophysitis with an incidence of 5–17% (4) and a wide phenotypic spectrum of thyroid function abnormalities, most commonly, thyroiditis and/or hypothyroidism, in roughly 1–2% of patients (2), and less commonly orbitopathy and subclinical hyperthyroidism (2, 3, 4, 5, 8, 9). We describe herein a case of ipilimumab-induced thyroid storm in a patient with metastatic melanoma. To our knowledge, severe hyperthyroidism has not yet been reported in the setting of treatment with ipilimumab.
Case presentation
An 88-year-old Caucasian female with a history of desmoplastic melanoma presented to the hospital in March 2012 with 3 months of fatigue, poor appetite, and failure to thrive. She had previously been treated with wide excision in November 2009, but was subsequently found to have diffusely metastatic melanoma of the liver, lungs, and bone on CT imaging in October 2011. She received rounds of ipilimumab on January 30 and February 21, 2012, with the second dose 3 weeks before admission. After starting immunotherapy, the patient developed progressive weakness, anorexia, and fatigue, and was unable to complete her activities of daily living. She also endorsed a 5-lb weight loss, anxiety, abdominal pain, and non-bloody diarrhea.
On presentation to the Emergency Department, her initial vital signs were significant for a temperature of 38.1 °C, heart rate of 105 b.p.m., and blood pressure of 144/64 mmHg. Two hours later, she developed a fever to 38.6 °C, worsened hypertension with a wide pulse pressure to 173/75 mmHg, and tachycardia to the 150 b.p.m. Physical examination was notable for an elderly female with moist skin, tachycardia, and non-tender thyromegaly.
Investigation
Initial laboratory studies were significant for a white blood cell count of 6.6×103/μl with a normal differential and an elevated ionized calcium level at 1.35 mmol/l (1.09–1.29). An EKG demonstrated sinus tachycardia with frequent premature atrial contractions. Blood, urine, and stool cultures were collected, and empiric antibiotics with i.v. fluids were started. She was admitted to the general medicine service for the systemic inflammatory response syndrome with suspected occult infection in the setting of recent chemotherapy.
The first night of hospitalization, she developed intermittent chest pain and was found to have paroxysmal supraventricular tachycardia with a heart rate elevated to the 160 b.p.m. Her heart rhythm then converted to atrial fibrillation with rapid ventricular rate, requiring i.v. metoprolol and digitalis loading. Serial cardiac enzymes were negative. In the setting of her active malignancy, recent immobility, and unexplained tachycardia (modified Wells score >4), a CT angiogram of the chest was obtained and demonstrated metastatic melanoma, hydrostatic pulmonary edema, and diffuse thyroid gland enlargement. This latter finding prompted subsequent thyroid function testings. Her thyroid-stimulating hormone (TSH) in February 2012, before her second round of ipilimumab, was normal at 1.2 μIU/ml. However, TSH on admission was low at 0.16 μIU/ml (0.3–4.7), free tri-iodothyronine (FT3) elevated at 1031 pg/dl (249–405), and free thyroxine (FT4) elevated at 5.6 ng/dl (0.8–1.6) (Fig. 1). Pan-cultures returned negative and an 0800 h cortisol was normal at 30 μg/dl (8–25). Ipilimumab-induced thyroiditis was suspected. Given the recent iodine contrast load from the CT angiogram, radioactive iodine uptake scan was not performed. A number of serologies including TPO antibody, thyroglobulin antibody, thyroid-stimulating Ig, TSH-binding inhibitor, and TSH receptor antibody returned negative. Ultrasound of her thyroid demonstrated numerous small (<3 mm) cysts, but none amenable for fine-needle aspiration; there were no solid thyroid nodules.
Figure 1.
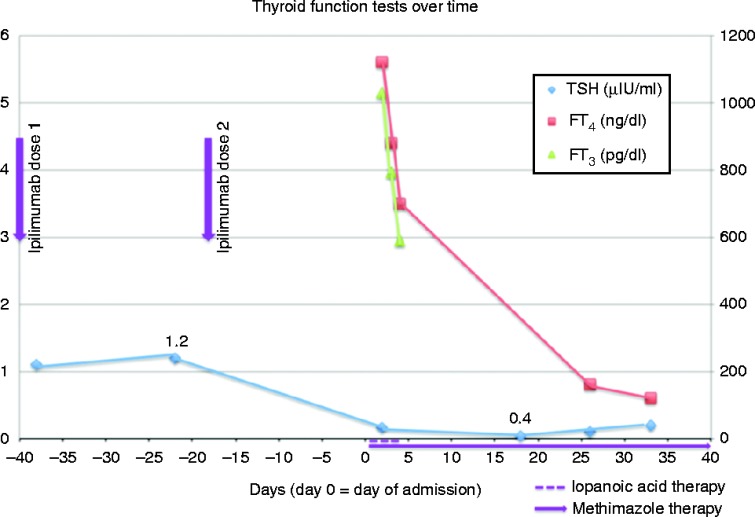
Thyroid function tests throughout the patient's course indicating a dramatic decline in free T3 and free T4 immediately after therapy with iopanoic acid and methimazole. Please note day 0 represents the day of admission.
Treatment
Given her overt thyrotoxicosis, she was started on iopanoic acid 500 mg three times daily for 3 days and methimazole 10 mg daily. Steroids were considered, but after consulting with the patient's oncologist, this option was deferred given the patient's acute illness and the possible abrogation of ipilimumab-induced antitumor activity. After 48 h of treatment with iopanoic acid and methimazole, her FT3 and FT4 had dropped to 590 pg/dl and 3.5 ng/dl respectively. She defervesced, had no further tachyarrhythmias, and her hypercalcemia resolved.
Outcome and follow-up
Three weeks after discharge, her TSH was still low at 0.11 μIU/ml but with a normal FT4 level of 0.8 ng/dl. She was clinically euthyroid, and her methimazole was discontinued. Her digoxin and metoprolol were weaned off given improvement of her symptoms after thyroid-suppressive therapy. She was eventually placed on hospice care after staging scans demonstrated progression of the melanoma despite ipilimumab.
Discussion
This case, to our knowledge, highlights the first report of ipilimumab-induced thyroid storm. While improvement of the patient's thyroid function tests may have been the natural history of the adverse reaction, the brisk decline in FT3 when compared with FT4 is consistent with rapid inhibition of T4-to-T3 conversion observed with iopanoic acid therapy. In comparison, resolution of hyperthyroidism of Graves' disease with antithyroid medications such as methimazole would have taken much longer (weeks to months) before a substantial (clinical or laboratory) improvement.
Interestingly, the patient's initial presentation masqueraded as possible sepsis in the setting of recent immunotherapy, with two of the four systemic inflammatory reaction syndrome criteria including temperature >38.3 °C and heart rate >90 b.p.m., but not respiratory rate >20 breaths/min or white blood cell count >12×103/μl. The parallel presentation raises the question of diagnostic criteria for thyroid storm that may help to differentiate sepsis from frank thyrotoxicosis. While Burch & Wartofsky (10) first proposed a scoring system using clinical criteria including thermoregulatory, cardiovascular, and CNS dysfunction, currently no standardized, universally accepted, or validated clinical tools for diagnosis exist. According to this system, scores 45 or more, highly suggestive of thyroid storm, could also easily describe sepsis, as in our patient who had obtained a score of 70 (points for temperature of 38.6 °C, diarrhea, pulse >140 b.p.m., atrial fibrillation, and precipitant history). Hence, there is a need for a more specific scoring system. More recently, Akamizu et al. (11) have developed diagnostic criteria based on retrospective data from the largest epidemiological and outcomes data to date, in which thyroid storm was defined as thyrotoxicosis with a combination of fever, tachycardia, congestive heart failure, and/or gastrointestinal/hepatic manifestations. Thyroid storm, based on these criteria, has an annual incidence of 0.2 patients/100 000 population and a mortality rate of 9.5–11%. Limitations include the retrospective nature of the study and the strictly Japanese study population.
Akamizu et al. (11) identified irregular use or cessation of antithyroid drug therapy and infections as the most common triggers for thyroid storm. Our patient, however, had neither of these; rather, we believe she was exposed to two other possible triggers, the most likely of which is ipilimumab. The second trigger or exacerbating factor may have been the large iodine load from the CT angiogram, inducing a Jod Basedow effect in the setting of ipilimumab-induced autoimmunity. This phenomenon, also known as iodine-induced hyperthyroidism, occurs when excess iodine leads to a sustained increase in hormone synthesis and secretion by autonomous thyroid nodule, causing elevated thyroid levels (12). However, our patient showed no solid thyroid nodules, rendering the Jod Basedow phenomenon rather unlikely.
Given that our patient was acutely ill, iodine contrast agents and thionamides were selected for therapy. The use of prednisone was considered for treatment of CTLA4 adverse effects, but deferred out of the oncology team's concern that steroids may attenuate the antitumor effect of ipilimumab. There are conflicting data on the validity of this interaction (13, 14, 15). Across three phase II studies for ipilimumab, 26 out of 117 patients achieved complete or partial response, or stable disease without disease progression (13). Of these 26 people, 11 of 14 people maintained a response after steroids when compared with nine of 12 who did not receive any steroids. Hence, the authors suggested that systemic steroids did not affect the development or maintenance of ipilimumab activity. Conversely, Weber et al. (16) found different results when they conducted a randomized, double-blinded, placebo-controlled phase II study on 115 patients comparing ipilimumab with and without prophylactic budesonide. The authors found that those treated with prophylactic budesonide had a decreased response rate of 12.1% and the median overall survival of 17.7 months compared with 15.8% and 19.3 months in the placebo group. While there is no definitive conclusion regarding the potential dampening effects of steroids on immune-mediated antitumor activity, this remained a concern and we chose an alternative therapy for hyperthyroidism in our patient.
Based on a compilation of case report data, immune-related adverse reactions usually occur after 1–3 months (2) or two to four sessions (16) of ipilimumab. Adverse events from treatment-related toxic effects may be confused with thyroid dysfunction, causing delay in dose adjustments or even cessation of therapy. Hence, while there are no official screening guidelines for thyroid function in patients undergoing ipilimumab therapy (4), thyroid function tests should be obtained before initiating treatment and monitored frequently, every 3 months, or sooner if the patient develops symptoms. Presently, treatment guidelines for immune-related adverse reactions per ipilimumab package insert and trial protocol revolve primarily around cessation of ipilimumab and the initiation of prednisone, dosed at 1–2 mg/kg per day. While there is little evidence to support the efficacy of this therapeutic regimen, the hypothesis that heightened autoimmunity leads to the endocrinopathies makes the aforementioned treatments logical choices. Data are limited in describing the successful treatment of asymptomatic hypo- and hyperthyroidism with steroids (16, 17, 18). Our patient's prompt and successful response highlights the usefulness of iopanoic acid as an alternative therapy for hyperthyroidism. This treatment is not known to specifically target the immune system. Additionally, it is still unclear whether steroids abrogate ipilimumab-induced immune antitumor activity. Data comparing steroids and alternative treatment are needed to clarify management of ipilimumab-induced hyperthyroidism, including thyroid storm. Additionally, more prospective data are necessary to evaluate whether ipilimumab can be continued safely in patients who develop severe hyperthyroidism that responds to antithyroid medications.
Patient consent
The patient is deceased.
Author contribution statement
C Yu, MD contributed to clinical care of the patient and wrote the manuscript. I J Chopra, MD edited the manuscript and was a content expert. E Ha, MD contributed to clinical care of the patient and edited the manuscript.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Dillard T, Yedinak CG, Alumkal J & Fleseriu M. 2010Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 1329–38 10.1007/s11102-009-0193-z [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JCet al. 2010Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine 363711–723 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain J-F, Testori A, Grob J-Jet al. 2011Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New England Journal of Medicine 3642517–2526 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 4.Hamnvik OP, Larsen PR & Marqusee E. 2011Thyroid dysfunction from antineoplastic agents. Journal of the National Cancer Institute 1031572–1587 10.1093/jnci/djr373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs EL, Clare-Salzler MJ, Chopra IJ & Figlin RA. 1991Thyroid function abnormalities associated with the chronic outpatient administration of recombinant interleukin-2 and recombinant interferon-α. Journal of Immunotherapy 10448–455 10.1097/00002371-199112000-00009 [DOI] [PubMed] [Google Scholar]
- 6.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T Jret al. 2010Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomized, double-blind, multicentre, phase 2, dose-ranging study. Lancet. Oncology 11155–164 10.1016/S1470-2045(09)70334-1 [DOI] [PubMed] [Google Scholar]
- 7.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner Det al. 2006Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. Journal of Clinical Oncology 242283–2289 10.1200/JCO.2005.04.5716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min L, Vaidya A & Becker C. 2011Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. European Journal of Endocrinology 164303–307 10.1530/EJE-10-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronstein Y, Ng CS, Hwu P & Hwu WJ. 2011Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR. American Journal of Roentgenology 197W992–W1000 10.2214/AJR.10.6198 [DOI] [PubMed] [Google Scholar]
- 10.Burch HB & Wartofsky L. 1993Life-threatening thyrotoxicosis. Thyroid storm. Endocrinology and Metabolism Clinics of North America 22263–277. [PubMed] [Google Scholar]
- 11.Akamizu T, Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, Tsuboi K, Monden T, Kouki T, Otani Het al. 2012Diagnostic criteria and clinico-epidemiological features of thyroid storm based on a nationwide survey. Thyroid 22661–679 10.1089/thy.2011.0334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wartofsky L. 2012Clinical criteria for the diagnosis of thyroid storm. Thyroid 22659–660 10.1089/thy.2012.2207.ed1 [DOI] [PubMed] [Google Scholar]
- 13.Amin A, DePril V, Hamid O, Wolchock J, Maio M, Neyns B, Chin K, Ibrahim R, Hoos A & O'Day S. 2009Evaluation of the effect of systemic corticosteroids for the treatment of immune-related adverse events (irAEs) on the development or maintenance of ipilimumab clinical activity. Journal of Clinical Oncology 2715s.(suppl; abstr 9037) 10.1200/JCO.2008.20.6235 [DOI] [Google Scholar]
- 14.El-Shirbiny AM, Stavrou SS, Dnistrian A, Sonenberg M, Larson SM & Divgi CR. 1997Jod-Basedow syndrome following oral iodine and radioiodinated-antibody administration. Journal of Nuclear Medicine 381816–1817. [PubMed] [Google Scholar]
- 15.Harmankaya K, Erasim C, Koelblinger C, Ibrahim R, Hoos A, Pehamberger H & Binder M. 2011Continuous systemic corticosteroids do not affect the ongoing regression of metastatic melanoma for more than two years following ipilimumab therapy. Medical Oncology 281140–1144 10.1007/s12032-010-9606-0 [DOI] [PubMed] [Google Scholar]
- 16.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman Det al. 2009A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clinical Cancer Research 155591–5598 10.1158/1078-0432.CCR-09-1024 [DOI] [PubMed] [Google Scholar]
- 17.Borodic G, Hinkle DM & Cia Y. 2011Drug-induced graves disease from CTLA-4 receptor suppression. Ophthalmic Plastic and Reconstructive Surgery 27e87–e88 10.1097/IOP.0b013e3181ef72a1 [DOI] [PubMed] [Google Scholar]
- 18.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis Set al. 2007Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. Journal of Immunotherapy 30825–830 10.1097/CJI.0b013e318156e47e [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a