Abstract
An essential aspect of goal-directed action selection is differentiating between behaviors that are more, or less, likely to be reinforced. Habits, by contrast, are stimulus-elicited behaviors insensitive to action–outcome contingencies and are considered an etiological factor in several neuropsychiatric disorders. Thus, isolating the neuroanatomy and neurobiology of goal-directed action selection on the one hand, and habit formation on the other, is critical. Using in vivo viral-mediated gene silencing, we knocked down Gabra1 in the orbitofrontal prefrontal cortex (oPFC) in mice, decreasing oPFC GABAAα1 expression, as well as expression of the synaptic marker PSD-95. Mice expressing Green Fluorescent Protein or Gabra1 knockdown in the adjacent M2 motor cortex served as comparison groups. Using instrumental response training followed by action–outcome contingency degradation, we then found that oPFC GABAAα1 deficiency impaired animals' ability to differentiate between actions that were more or less likely to be reinforced, though sensitivity to outcome devaluation and extinction were intact. Meanwhile, M2 GABAAα1 deficiency enhanced sensitivity to action–outcome relationships. Behavioral abnormalities following oPFC GABAAα1 knockdown were rescued by testing mice in a distinct context relative to that in which they had been initially trained. Together, our findings corroborate evidence that chronic GABAAα1 deficiency remodels cortical synapses and suggest that neuroplasticity within the healthy oPFC gates the influence of reward-related contextual stimuli. These stimuli might otherwise promote maladaptive habit-based behavioral response strategies that contribute to—or exacerbate—neuropsychiatric illness.
INTRODUCTION
Abundant evidence indicates that both humans and rodents can select actions based on the likelihood that they will be reinforced with a desired outcome (Balleine and O'Doherty, 2010). With repeated performance or exposure to stressors or drugs of abuse, these ‘goal-directed' actions can take on the characteristics of stimulus–response habits—motor behaviors elicited by conditioned stimuli rather than their association with specific outcomes. Stimulus-elicited habits may be etiological factors in several psychopathologies, including obsessive-compulsive disorder and addiction, and in animals, a potential model of ruminative thought and behavior in depression. Thus, isolating the neuroanatomy and neurobiology of action selection on the one hand, and habit formation on the other, is a critical research imperative.
Within the frontal cortex, a neural substrate closely associated with goal-directed action selection is the prelimbic prefrontal cortex. For example, lesions in this region result in insensitivity to the predictive relationship between a behavior and its outcome, as well as insensitivity to reinforcer devaluation (Balleine and Dickinson, 1998; Corbit and Balleine, 2003; Killcross and Coutoureau, 2003). The orbitofrontal prefrontal cortex (oPFC) is ventral and lateral to the prelimbic cortex, yet its contributions to action selection based on action–outcome contingencies remain somewhat unclear. On the one hand, rats and mice with oPFC lesions are behaviorally sensitive to reinforcer devaluation, providing evidence of knowledge of action–outcome relationships (Ostlund and Balline, 2007; Gourley et al, 2013a). On the other hand, surgical disconnection of the oPFC from the dorsal striatum results in insensitivity to modifications in the predictive relationship between a response and the associated outcome (Gourley et al, 2013a). Moreover, the oPFC mediates the pleasurable properties of rewards, which likely has a role in goal-directed reward seeking (Grabenhorst and Rolls, 2011).
The current study aimed to further clarify the role of the oPFC in goal-directed action selection. We selectively knocked down the α1 subunit of the GABAA receptor, the predominant inhibitory ligand-gated ion channel in the brain. The α1 subunit confers fast inhibitory properties, and chronic knockdown increases the expression of immature filopodial-like dendritic spine protrusions that are unlikely to contain synapses, and also decreases the density of mature, mushroom-shaped spines in the cerebral cortex (Heinen et al, 2003). Diminished GABAAα1 expression and function are implicated in depression and other chronic stressor-related psychopathologies, and in utero cocaine exposure also downregulates GABAAα1 in rodent prefrontal cortex (Lu et al, 2009; Skilbeck et al, 2010; Hines et al, 2012), suggesting that GABAAα1 expression is a factor in disease risk or etiology.
Using site-selective knockdown of Gabra1, which encodes GABAAα1, we discovered divergent roles for GABAAα1 in the oPFC and the adjacent motor region, M2, in action selection. Specifically, chronic oPFC GABAAα1 silencing impairs the performance of goal-directed response strategies, while M2 GABAAα1 inhibition enhances sensitivity to action–outcome relationships. The effects of oPFC GABAAα1 knockdown are context selective, consistent with evidence that spatial information is represented within the ventrolateral oPFC during appetitive choice tasks (Feierstein et al, 2006), and that the oPFC regulates behavioral sensitivity to reward-associated contextual cues (Lasseter et al, 2009, 2014).
SUBJECTS AND METHODS
Subjects
Transgenic Gabra1-tm1Geh mice back-crossed onto a C57BL/6 background were used (Vicini et al, 2001). Mice possess loxP sites on both sides of the α1 exon encoding an essential transmembrane domain. In the presence of Cre recombinase (Cre), the transmembrane domain is deleted, and local expression of Gabra1, which encodes GABAAα1, is reduced (Heldt and Ressler, 2010). Initial oPFC experiments used both males and females. No sex differences were observed, and experiments proceeded using males. Mice were >8 weeks of age, maintained on a 12-h light cycle (0700 hours on), and were provided food and water ad libitum except during instrumental conditioning when body weights were reduced to 90–93% of baseline to motivate responding. Procedures were approved by the Emory University IACUC.
Surgery
Lentiviruses were generated as described (Heldt and Ressler, 2010). Mice were anaesthetized with ketamine/xylazine and placed in a digitized stereotaxic frame (Stoelting). The scalp was incised, skin retracted, bregma and lambda identified, the head leveled, and coordinates located. Viral vectors expressing Cre or Green Fluorescent Protein (GFP) under the CMV promoter were infused over 2.5 min in a volume of 0.25 μl at AP+2.6, ML±1.2, DV-2.8 for the oPFC, and 0.5 mm dorsally for M2 (Gourley et al, 2010). Needles were left in place for an additional 5-min period prior to withdrawal, suturing, and recovery.
Immunoblotting
We aimed to quantify the degree to which Cre-mediated knockdown decreased regional expression of GABAAα1 and related targets. For this purpose, ∼50% of mice with oPFC-targeted lenti-Cre or lenti-GFP in our final behavioral experiment were killed by rapid decapitation 11 weeks following surgery for immunoblotting experiments. An additional group of behaviorally-naïve mice expressing lenti-Cre or lenti-GFP in M2 were killed by rapid decapitation 2 weeks after surgery. Brains were frozen at −80 °C, and then sectioned into 1 mm sections. The oPFC or M2, hippocampus, and dorsal striatum were dissected by a single experimenter using a 1-mm tissue core. Tissue was homogenized by sonication in lysis buffer (160 μl: 137 mM NaCl, 20 mM tris-Hcl (pH=8), 1% igepal, 10% glycerol, 1 : 100 Phosphatase Inhibitor Cocktails 2 and 3 (Sigma), 1 : 1000 Protease Inhibitor Cocktail (Sigma)). Protein concentrations were determined by Bradford colorimetric assay (Pierce), and 15 μg/sample was separated by SDS-PAGE on a 12% gradient tris-glycine gel (Bio-rad). Following transfer to PVDF membrane, membranes were blocked with 5% nonfat milk.
Anti-GABAAα1 (Millipore; Rb; 1 : 500); anti-GABAAα2 (Abcam; Rb; 1 : 250); anti-GABAAα3 (Abcam; Rb; 1 : 250); anti-PSD-95 (Cell Signaling; Rb; 1 : 5000); anti-synaptophysin (AbCam; Rb; 1 : 5000); and anti-HSP-70 (Santa Cruz; Ms; 1 : 5000) were used. Membranes were incubated at 4 °C overnight and then incubated in horseradish peroxidase goat anti-rabbit (Vector; 1 : 8000) and goat anti-mouse (Jackson Immunoresearch; 1 : 8000) secondary antibodies. Immunoreactivity was assessed using a chemiluminescence substrate (Pierce) and measured using a ChemiDoc MP Imaging System (Bio-rad). Densitometry values were normalized to the corresponding loading control (HSP-70) and then normalized to the control sample mean from the same membrane to control for variance between gels.
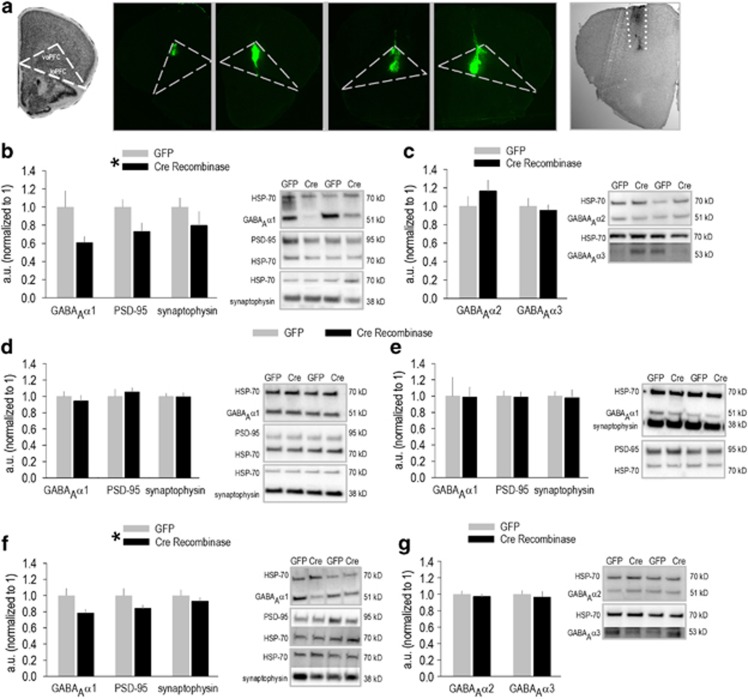
Figure 1.
GABAAα1 silencing regulates synaptic marker expression. (a) A coronal section from the Mouse Brain Library (Rosen et al, 2000) is shown adjacent to images of Green Fluorescent Protein (GFP) expression within the orbitofrontal prefrontal cortex (oPFC) of multiple mice. ‘voPFC' refers to the ventral oPFC, and ‘loPFC' refers to the lateral oPFC. Expression patterns contrast those following M2-targeted infusions. For example, Cre recombinase (Cre) immunoreactivity in M2 (between the dotted white lines) is shown at far right. (b) Regional GABAAα1 protein expression was decreased in homogenized oPFC tissue punches collected 11 weeks following viral vector infusion; PSD-95 and synaptophysin were also reduced. (c) By contrast, expression of the GABAAα2 and GABAAα3 subunits was unaffected. (d) No effects were identified off-target, in the hippocampus, or (e) dorsal striatum (n=5/group). (f) GABAAα1 and synaptic marker expression were also reduced in M2 following Cre delivery, and effects were detectable 2 weeks after infusion. (g) As in the oPFC, GABAAα2 and GABAAα3 were unaffected (n=10–11/group). Representative blots are adjacent throughout. Mean+SEM, *p<0.05, main effect of group.
Instrumental Response Training and Experimental Design for Behavioral Experiments
Three weeks following viral vector delivery, mice were trained to nose poke for food reinforcers using illuminated Med-Associates conditioning chambers with two nose poke apertures and a separate magazine. Training was initiated using a continuous reinforcement schedule (ie, fixed ratio 1). Thirty reinforcers were available for responding on each aperture, resulting in 60 reinforcers/session. 5–7 daily training sessions were conducted, during which all mice acquired the responses; the final 5 of these sessions for each mouse are shown, presented as sessions 1–5 in Figures 2b and 3a. Next, mice were shifted to a random interval (RI) 30-second schedule of reinforcement for 2 sessions, presented as sessions 6–7.
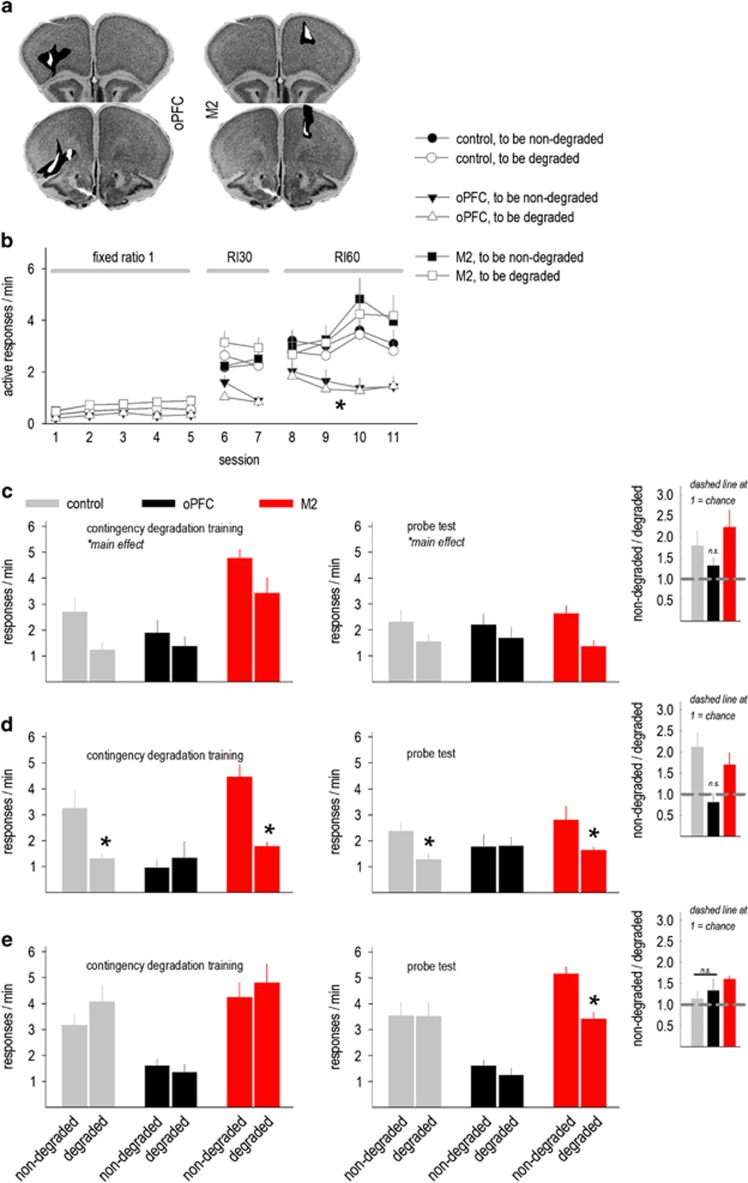
Figure 2.
Dissociable effects of orbitofrontal prefrontal cortex (oPFC)- and M2-targeted GABAAα1 silencing on action selection strategies. (a) Histological representations of viral vector infection are represented on images from the Mouse Brain Library (Rosen et al, 2000). Black represents the largest infusion spread, and white the smallest. Infusions were bilateral. (b) Response rates during instrumental response training are shown, with the schedules of reinforcement indicated and breaks in the response acquisition curves signaling tests for sensitivity action–outcome contingency degradation. oPFC GABAAα1 deficiency decreased response rates. (c) Initially, a main effect of response type indicated that mice inhibited responding when responding was less likely to be reinforced, both during contingency degradation training and in a subsequent probe test. Inset: response rates during the probe test are also represented as a preference for the ‘non-degraded' over ‘degraded' response. Here, only control and M2 mice preferred the non-degraded response, a preference ratio significantly >1. Meanwhile, oPFC knockdown mice, as a group, did not significantly differ from 1. (d) With additional training, mice with oPFC GABAAα1 deficiency developed marked insensitivity to instrumental contingency degradation, detectable both during the contingency degradation training period and during the subsequent probe test. Meanwhile, other mice inhibited responding when responding was unlikely to be reinforced. Inset: a comparison of preference scores again indicated that mice oPFC knockdown responded at chance levels. (e) With further training, control mice also developed habit-like insensitivity to modifications in action–outcome relationships as expected. Mice with M2 GABAAα1 deficiency retained sensitivity, although this effect was detected only during the probe test. Inset: a comparison of preference scores indicated that only mice with M2-targeted knockdown generated a score significantly >1. Mean+SEM, *p<0.05. Combined control group n=15 including 4 intact control mice that did not differ from Green Fluorescent Protein (GFP)-expressing control mice; M2 n=6; oPFC n=7.
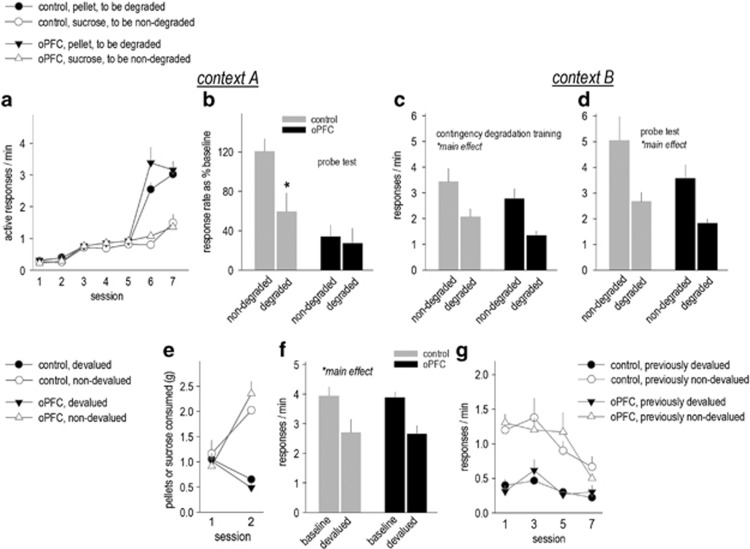
Figure 3.
Orbitofrontal prefrontal cortex (oPFC) GABAAα1 deficiency impairs action selection in a context-specific manner. (a) Mice were trained in context A to respond for two distinct outcomes, food pellets and sucrose. Using this two-outcome approach, we detected no group differences in response acquisition, however, response rates associated with pellets were higher during the final two sessions. (b) Nonetheless, when the action–outcome contingency associated with the pellet was degraded, control mice inhibited responding. Mice with oPFC-selective GABAAα1 deficiency failed to selectively modify response strategies. (c) Sensitivity to instrumental contingency degradation was retested in a separate context, ‘context B.' Here, both groups differentiated between the degraded and non-degraded instrumental contingencies during training and (d) the probe test, inhibiting the response less likely to be reinforced. (e) Next, the pellet was paired with LiCl. Ad libitum consumption decreased, while the ingestion of a sucrose solution paired with NaCl remained robust. (f) Response rates associated with the now-devalued pellet were also reduced. (g) In addition, mice extinguished responding when the reinforcer was withheld. Mean+SEM, *p<0.05. Control n=6; oPFC n=7.
First (Figure 2), we characterized the development of habitual response strategies using a within-subject design, eg, according to the study by Dias-Ferriera et al (2009) and others. Here, we tested sensitivity to instrumental contingency degradation three times, first after continuous reinforcement training, next after RI30-second training, and a final time after four additional training sessions using an RI60-second schedule. Both responses were reinforced with 20 mg grain-based Bioserv precision pellets.
In a separate group of mice (Figure 3), we next assessed whether habit-like behavior was context specific, by testing sensitivity to contingency degradation twice following RI30-second training as described under ‘Context shift.' Here we also employed a two-outcome approach such that each response was reinforced with a unique outcome—either one pellet or a liquid sucrose solution (10% w/v; 100 μl). The rationale for this decision is discussed in the Results section.
Instrumental Contingency Degradation
To assess whether mice selected actions based on their consequences, or based on stimulus–response habit-based strategies, we used a modified contingency degradation task (Gourley et al, 2012, 2013a, 2013b; Swanson et al, 2013). Here, one nose poke aperture is occluded, and reinforcers are delivered for 25 min independent of animals' interactions with the remaining aperture, thus ‘degrading' the action–outcome contingency associated with that nose poke response. The reinforcer delivery rate is yoked to each animal's individual reinforcement rate from the previous session. In another session, only the opposite aperture is available, and responding is reinforced using a variable ratio 2 schedule of reinforcement for 25 min. Thus, one response becomes significantly more predictive of reinforcer delivery than the other (Hinton et al, 2014). The order of these sessions and the location of the ‘degraded' aperture within the chambers are counter balanced.
The following day, both apertures are available for 5 min, and responding during this probe test is nonreinforced. Preferential engagement of the response that was more likely to be reinforced is considered ‘goal-directed,' while non-discriminate responding is considered ‘habitual' (Balleine and O'Doherty, 2010). When sensitivity to contingency degradation was tested multiple times, the location of the ‘degraded' aperture was opposite of that in the previous test.
Context Shift
Sensitivity to instrumental contingency degradation was tested twice, once in the original testing chambers, and then again using the same 3-day protocol, but in unique chambers located in a separate room in the laboratory. The chambers were contextually distinct and configured differently, with two nose poke apertures located on opposite sides of the chambers relative to the original chambers, and relative to each other. In addition, mice were tested at a different time of the day.
Outcome Devaluation, Response Extinction
In the same mice tested in the ‘Context shift,' responding on the pellet-associated aperture was then reinstated during three 70-min training sessions using a continuous reinforcement schedule. Then, mice were placed individually in a novel cage with ad libitum access to the reinforcer pellets for 1 h. Immediately following, mice were injected with 0.15 M LiCl (40 ml/kg; Quinn et al, 2007) to induce conditioned taste aversion. Alternately, mice had access to a sucrose solution (10% w/v) for 1 h, followed by an injection of NaCl. Pellet and sucrose intake were compared by two-factor ANOVA (group × outcome) to confirm specificity of conditioned taste aversion. The following day, mice were returned to the conditioning chambers, and responding in extinction was monitored for 10 min. Response rates were compared to a 10-min extinction test conducted prior to conditioned taste aversion.
Finally, responding was extinguished by placing mice in the conditioning chambers for 15 min per day for 7 days. Reinforcement was withheld, and response rates were quantified. This protocol is sufficiently sensitive to detect deficiencies in extinction conditioning (Gourley et al, 2009).
Histology
Infusion sites in fixed brains were verified by immunostaining for Cre as described (DePoy et al, 2013) with amplification using DAB (SIGMAFAST 3,3′-Diaminobenzidine tablets, Sigma) in accordance with the manufacturer's instructions. Alternatively, GFP was imaged. Mice lacking viral vector infection selective to the oPFC or M2 were excluded (n=1 oPFC-targeted GFP, n=3 oPFC-targeted Cre and n=1 M2-targeted Cre).
Statistical Analyses
Data were analyzed by SPSS. Densitometry values from immunoblotting experiments were compared by two-factor (protein target × knockdown) ANOVA within each brain region. Response rates from behavioral experiments were compared by two-factor (response × knockdown) ANOVA with repeated measures when appropriate. In the case of significant interaction effects, post-hoc comparisons were made using Tukey's t-tests and are indicated graphically.
RESULTS
Using viral vector strategies, we selectively expressed GFP or Cre in the oPFC or M2 of ‘floxed' Gabra1 mice to knock down GABAAα1 expression (Figure 1a). Eleven weeks following viral vector delivery, GABAAα1 expression was reduced by ∼40% in homogenized oPFC tissue, and in concert, PSD-95 and synaptophysin expression were reduced (main effect of knockdown F(1,8)=7.1, p=0.01; Figure 1b). Meanwhile, expression of GABAAα2 and GABAAα3 subunits were unchanged (F's<1; Figure 1c). Moreover, GABAAα1, PSD-95, and synaptophysin levels were unchanged off-target in the hippocampus and dorsal striatum (F's<1; Figure 1d and e).
In another experiment, we quantified protein expression 2 weeks following viral vector delivery to M2. Again, regional GABAAα1 was significantly reduced, in concert with reduced PSD-95 and a modest decrement in synaptophysin (main effect of knockdown F(1,19)=8.5, p=0.005; Figure 1f). The GABAAα2 and GABAAα3 subunits were again unaffected (F's<1; Figure 1g). Notably, comparable protein expression levels at 2 and 11 weeks (compare Figure 1b and f) suggest that knockdown reaches near-maximal levels by 2 weeks.
Divergent Roles for oPFC and M2 GABAAα1 in Complex Decision-Making
The oPFC can be sub-divided into multiple compartments (Ongur and Price, 2000). Our histological analyses indicated that viruses largely infected the ventrolateral compartments and also confirmed that M2-targeted infusions were contained within M2 (Figures 1a and 2a). When mice were trained to nose poke for food reinforcement, mice with oPFC GABAAα1 knockdown generated lower response rates overall (main effect F(2,50)=23.7, p<0.001) (Figure 2b). Importantly, however, there were no differences between the response rates on the ‘to be degraded' vs ‘to be non-degraded' response apertures (F's<1; Figure 2b).
Breaks in the response acquisition curves annotate tests for sensitivity to instrumental contingency degradation (Figure 2b). Initially, main effects of response selection (‘non-degraded' vs ‘degraded') indicated that overall, mice responded more when responding was reinforced, relative to when pellets were delivered non-contingently (F(1,25)=7.8, p=0.01; Figure 2c). During a probe test when both nose poke apertures were available simultaneously, a main effect again indicated that mice preferentially generated the response most predictive of reinforcement (F(1,25)=14.2, p<0.001; interaction p=0.2; Figure 2c). In other words, mice were initially able to use information about action–outcome associative contingencies to guide action selection strategies.
Notably, when the number of ‘non-degraded' responses was divided by the number of ‘degraded' responses to generate a preference score (Hinton et al, 2014), control mice and mice with M2-targeted knockdown significantly preferred the non-degraded response, a ratio significantly >1 (one-sample t-test compared to 1, both p<0.03; Figure 2c inset). Meanwhile, the oPFC knockdown mice, as a group, responded at chance levels not differing from 1 (t6=1.8, p=0.13), suggesting that mice with oPFC-targeted knockdown may be biased toward habit-based response strategies.
In a second test after RI training, a response pattern parsimonious with this interpretation emerged: Control mice and mice with M2-targeted knockdown generated higher response rates when pellets were delivered contingently, providing evidence of sensitivity to action–outcome associations. By contrast, mice with oPFC-targeted knockdown generated identical response rates regardless of whether responding was explicitly reinforced or not (interaction F(2,25)=7.3, p=0.003; Figure 2d). In a probe test when both nose poke apertures were presented simultaneously, mice with oPFC GABAAα1 knockdown again failed to differentiate between the ‘non-degraded' and ‘degraded' contingencies (interaction F(2,25)=4.6, p<0.05; Figure 2d). By contrast, other mice preferentially generated the response that had been most predictive of reinforcement. And again, an analysis of preferences scores indicated that mice with oPFC-selective knockdown responded at chance levels (t6=−0.3, p=0.8 compared to 1; Figure 2d inset).
Next, we further trained mice using an RI60-second schedule of reinforcement that can cause stimulus–response habits. Consequently, all mice failed to modify response rates during action–outcome contingency degradation (main effect F(1,25)=1.9, p=0.2; interaction p=0.2; Figure 2e). Surprisingly, however, when both nose poke apertures were simultaneously available in a probe test, mice with M2 knockdown preferentially generated the response that had been more predictive of reinforcement, evidence of prolonged sensitivity to action–outcome relationships (interaction F(2,25)=3.5, p<0.05; Figure 2e). Accordingly, an analysis of preference scores indicated that the M2 group responded above chance levels (t5=7.2, p=0.001 compared to 1; other groups p>0.3; Figure 2e inset).
Habit-Like Behavioral Inflexibility Following oPFC GABAAα1 Knockdown is Context Dependent
To summarize, mice with oPFC-selective GABAAα1 deficiency generate low response rates for food reinforcement, and they develop habit-like response strategies more rapidly than control mice. Next, we trained a new group of mice with oPFC-targeted knockdown to respond for two unique outcomes—food pellets or a liquid sucrose solution. This approach—relative to reinforcing two responses with identical outcomes, as above—enhances response acquisition in intact rats (Trapold, 1970), and it rescued response rates in mice with oPFC-targeted GABAAα1 knockdown here, such that response rates during training in control and knockdown mice did not differ (effect of knockdown F(1,11)=1.2, p=0.3; Figure 3a).
When the schedule of reinforcement escalated from a continuous reinforcement schedule to an RI30-second schedule, response rates diverged, with both groups responding at higher rates for the pellets (interaction F(1,11)=68.5, p<0.001; Figure 3a). Thus, we degraded the action–outcome contingency associated with the preferred pellet in all mice (rather than using a counter-balanced approach). Response rates are expressed as a percent of the final day of training to normalize response rates associated with the two outcomes. Control mice decreased responding on the pellet-associated nose poke aperture as expected, and as in our initial experiments, mice with oPFC-targeted knockdown failed to differentiate between the two responses (interaction F(1,11)=5.3, p<0.05; Figure 3b). These findings indicate that low response rates during training in Figure 2 cannot obviously account for insensitivity to degradation of the instrumental contingency (see also Corbit and Balleine, 2003).
Insensitivity to instrumental contingency degradation can result from interference by reward-associated Pavlovian stimuli (Colwill and Rescorla, 1986), so we hypothesized that the context may have served as a conditioned stimulus that interfered with goal-directed action selection. We thus re-tested mice in chambers that were contextually distinct and configured differently relative to those in which they had been originally trained to nose poke. In this case, both groups responded at higher rates when reinforcer delivery was contingent upon responding, evidence of sensitivity to instrumental contingency degradation (main effect F(1,11)=9.8, p=0.01; Figure 3c). When both apertures were simultaneously available during a subsequent probe test, both groups again preferentially engaged the non-degraded response (main effect F(1,11)=10, p=0.009; Figure 3d). In other words, sensitivity to action–outcome relationships was intact in a unique context, despite oPFC-selective GABAAα1 knockdown. We do not believe this rescue could be attributable to additional experience with action–outcome contingency degradation because response differentiation was instead impaired in a second test in the training-associated environment in Figure 2.
Sensitivity to Reinforcer Devaluation and Extinction are Intact
We next paired the preferred reinforcer, food pellets, with repeated LiCl injections. All but two mice developed rapid conditioned taste aversion (interaction F(1,9)=30, p<0.001; Figure 3e); these mice were excluded (n=1/group), and no group differences in food consumption were detected (F<1). When returned to the conditioning chambers, all mice inhibited responding on the nose poke aperture associated with the now-devalued food (main effect F(1,9)=18, p=0.002; Figure 3f).
In addition, in the absence of any reinforcer, responding decreased across sessions with no differences between groups, demonstrating intact extinction conditioning despite oPFC GABAAα1 knockdown (F's<1; Figure 3g).
DISCUSSION
The GABAA receptor is the predominant inhibitory ligand-gated ion channel in the brain. The α1 subunit confers fast inhibitory properties, and as such, reducing prefrontal cortical GABAAα1 function can dampen sensitivity to local GABA release and augment long-term potentiation (eg, Lu et al, 2010). Prolonged GABAAα1 deficiency can cause the proliferation of immature filopodial-like dendritic spine protrusions, and a loss of mature dendritic spines, the primary sites of excitatory synapses in the brain (Heinen et al, 2003). Prior studies concerning the effects of cortical GABAAα1 deficiency have largely utilized mice with unconditional heterozygous deletion of Gabra1 or forebrain-selective knockdown with early-life onset (eg, Kralic et al, (2002); Sonner et al, (2005); Zhou et al, (2013)). Instead, we knocked down GABAAα1 selectively in the cortex of mature mice. Synaptic marker expression was reduced as early as 2 weeks following viral vector delivery, and in the absence of compensatory upregulation of GABAAα2 or GABAAα3. By contrast, GABAAα3 is upregulated following heterozygous deletion (Zhou et al, 2013). Mice with oPFC-targeted GABAAα1 knockdown were unable to select actions based on their consequences, developing instead habit-like behavioral inflexibility, recapitulating the effects of early-life cocaine exposure (Hinton et al, 2014) and chronic stressor exposure (Dias-Ferreira et al, 2009), among other manipulations modeling chronic psychiatric illness in rodents (reviewed by Schwabe et al, (2011); Schwabe and Wolf, (2013)). Our findings thus provide a neuroanatomical substrate by which GABAAα1 dysfunction may contribute to addiction, depression, and other stressor-related psychopathologies.
Regulation of Action Selection by the oPFC
In classical instrumental contingency degradation experiments, rats generated a single response reinforced with a single food reinforcer. Then, the likelihood of reinforcement was decreased by the experimenter, and response rates decreased in concert, providing evidence of knowledge of the response–reinforcement relationship (Hammond, 1980; Dickinson, 1980). Similarly, if rats were trained to perform two discrete responses for two discrete reinforcers and the likelihood of reinforcement associated with one response was decreased, typical rats preferentially performed the remaining response (eg, Colwill and Rescorla, (1986); Balleine and Dickinson, (1998)). Rodents can also differentiate between two actions when both actions result in identical outcomes, as here (Trapold, 1970; Gourley et al, 2012, 2013a, 2013b; Swanson et al, 2013; Hinton et al, 2014), providing further evidence that like humans, rodents can select specific behaviors based on their likely outcomes, even in the absence of other factors such as conditioned reactions associated with each specific outcome (Overmier and Linwick, 2001).
Here, mice initially differentiated between responses that were more, or less, likely to be reinforced following instrumental contingency degradation. Nonetheless, oPFC-selective GABAAα1 knockdown accelerated the adoption of habitual response strategies, such that knockdown mice developed habit-like insensitivity to action–outcome contingency degradation with less response training than control mice or mice with M2-selective knockdown. The presentation of reward-associated Pavlovian stimuli can impinge upon goal-directed action selection, resulting in habit-like behavioral inflexibility, as here (Colwill and Rescorla, 1986). Further, cocaine self-administration studies indicate that prolonged oPFC inactivation enhances context-elicited reinstatement of drug seeking, sparing drug-seeking behaviors induced by other conditioned stimuli (Fuchs et al, 2004; Lasseter et al, 2009). Thus, we hypothesized that mice with oPFC-selective GABAAα1 deficiency failed to engage outcome-based decision-making strategies due to exaggerated sensitivity to reward-related contextual cues. This hypothesis predicts that action selection in a novel environment should be intact, and indeed, behavioral deficiencies were undetectable in contextually distinct chambers, relative to those in which mice had originally been trained. These findings provide new evidence that the healthy oPFC gates the influence of contextual cues associated with appetitive outcomes to promote the expression of goal-directed response strategies.
Given that the oPFC knockdown mice additionally responded less overall, an alternative perspective is that oPFC GABAAα1 silencing results in over-generalization of action–outcome contingency degradation, resulting in reduced responding regardless of the likelihood of reinforcement. While our current findings do not dissociate these possibilities, the ‘context shift' experiment suggests that in either case, the oPFC is essential for guiding instrumental response strategies in a context-dependent manner.
How might the oPFC be acting? Current models suggest that local dopamine D1 receptor stimulation signals the salience of contextual stimuli by interacting with the glutamatergic input to oPFC (Lasseter et al, 2014). In addition, glutamatergic projections from the cortex and dopamine input from the ventral tegmental area converge on dendritic spines within the rodent nucleus accumbens (Bouyer et al, 1984); the targeted medium spiny neurons may then coordinate information being shuttled to the basal ganglia to regulate the expression of instrumental decision-making strategies or habits, depending on an animal's drug, stress, or training history. In addition, oPFC neurons, particularly within the ventrolateral region affected here, encode information regarding space, context, and motor requirements as they pertain to acquiring a reinforcer (Feierstein et al, 2006). All of these functions could be degraded by chronic oPFC GABAAα1 silencing and associated molecular sequelae.
Lesions of the entorhinal cortex, a primary source of input to the hippocampus, also impair sensitivity to action–outcome contingency degradation (Corbit et al, 2002; Lex and Hauber, 2010). Entorhinal cortex lesions do not, however, impact sensitivity to outcome devaluation, presumably because response regulation is not influenced by the discord between the reinforcement-associated context and the validity of responding following noncontingent pellet delivery (discussed by Corbit et al, (2002); and Lex and Hauber, (2010)). Consistent with this model, mice with oPFC GABAAα1 deficiency were also sensitive to reinforcer devaluation here, reducing responding in the original training context following conditioned taste aversion.
M2 Regulates Action Selection
Lesion studies in rodents indicate that M2 is involved in action differentiation—specifically, mice with M2 lesions can acquire food-reinforced instrumental responses, but are unable to perform a series of responses in the correct sequence (Yin, 2009) or select actions based on the value of an outcome (Gremel and Costa, 2013). Here, M2 GABAAα1 knockdown enhanced sensitivity to modifications in action–outcome contingencies, even after extensive response training that produced habit-like insensitivity in control mice. This finding was unexpected but provocative because there are many reported manipulations that can cause habits, but few that prevent them.
M2 is largely analogous to the supplementary motor cortex in nonhuman primates and has been closely associated with timing and serial motor learning (eg, Chen et al, (1995)). Neurons in the supplementary motor area in primates fire prior to the production of a specific, trained series of actions (Tanji et al, 1996). Moreover, supplementary motor cortex lesions impair primates' ability to initiate goal-directed motor actions; notably, responding is rescued by the presentation of reward-associated conditioned stimuli (Chen et al, 1995), suggesting that action selection based on action–outcome contingencies is uniquely regulated by the supplementary motor cortex. Pronounced projections from the basolateral amygdala to rodent M2 could be critical to goal-directed action selection since the basolateral amygdala is necessary for sensitivity to instrumental contingency degradation (Kita and Kitai, 1990; Balleine et al, 2003). Future research should resolve the precise role(s) of GABA-mediated M2 neuroplasticity in outcome-based decision-making, since harnessing cortico–striatal–amygdalar contributions to outcome-oriented action selection could provide an outlet for novel therapeutic interventions aimed at optimizing goal-directed decision-making.
FUNDING AND DISCLOSURE
This work was supported by Children's Healthcare of Atlanta, the Children's Neuroscience Research Center, NIH MH101477, T32GM008602, and the Brain and Behavior Research Foundation where Dr Gourley is the Foundation's Katherine Deschner Family Investigator. The Yerkes Center is supported by the Office of Research Infrastructure Programs/OD P51OD11132. The Emory Viral Vector Core is supported by P30NS055077. The authors declare no conflicts of interest.
Acknowledgments
We thank Mr Alonzo Whyte for assistance, Ms Kelsey Zimmermann and Dr Donald Rainnie for feedback, and Dr Kerry Ressler for advice and for generously providing the Gabra1-tm1Geh mice used here.
References
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci. 2003;23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer JJ, Park DH, Joh TH, Pickel VM. Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Res. 1984;302:267–275. doi: 10.1016/0006-8993(84)90239-7. [DOI] [PubMed] [Google Scholar]
- Chen Y-C, Thalar D, Nixon PD, Stern CE, Passingham RE. The functions of the medial premotor cortex. II. The timing and selection of learned movements. Exp Brain Res. 1995;102:461–473. doi: 10.1007/BF00230650. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Rescorla RA.1986Associative structures in instrumental learningIn: Bower Gordon H (ed)Psychology of Learning and Motivation Vol 20Academic Press, Inc.: London [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Ostlund SB, Balleine BW. Sensitivity to instrumental contingency degradation is mediated by the entorhinal cortex and its efferents via the dorsal hippocampus. J Neurosci. 2002;22:10976–10984. doi: 10.1523/JNEUROSCI.22-24-10976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy LM, Noble B, Allen AG, Gourley SL. Developmentally divergent effects of Rho-kinase inhibition on cocaine- and BDNF-induced behavioral plasticity. Behav Brain Res. 2013;243:171–175. doi: 10.1016/j.bbr.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Dickinson A. Contemporary Animal Learning Theory. Cambridge University Press: Cambridge; 1980. [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron. 2006;51:495–507. doi: 10.1016/j.neuron.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of the orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Howell JL, Rios M, DiLeone RJ, Taylor JR. Prelimbic cortex bdnf knock-down reduces instrumental responding in extinction. Learn Mem. 2009;16:756–760. doi: 10.1101/lm.1547909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of goal-directed action within mouse prefrontal cortex. Eur J Neurosci. 2010;32:1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Gordon J, Taylor JR. Cytoskeletal determinants of stimulus-response habits. J Neurosci. 2013;33:11811–11816. doi: 10.1523/JNEUROSCI.1034-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Zimmermann KS, Ressler KJ, DiLeone RJ, Taylor JR. The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur J Neurosci. 2013;38:2382–2388. doi: 10.1111/ejn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, Dileone RJ, et al. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci USA. 2012;109:20714–20719. doi: 10.1073/pnas.1208342109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Costa RM. Premotor cortex is critical for goal-directed action. Front Comp Neurosci. 2013;7:110. doi: 10.3389/fncom.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond LJ. The effect of contingency upon the appetitive conditioning of free-operant behavior. J Exp Anal Behav. 1980;34:297–304. doi: 10.1901/jeab.1980.34-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen K, Baker RE, Spijker S, Rosahl T, van Pelt J, Brussaard AB. Impaired dendritic spine maturation in GABAA receptor alpha1 subunit knock out mice. Neuroscience. 2003;122:699–705. doi: 10.1016/s0306-4522(03)00477-9. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Amygdala-specific reduction of alpha1-GABAA receptors disrupts the anticonvulsant, locomotor, and sedative, but not anxiolytic, effects of benzodiazepines in mice. J Neurosci. 2010;30:7139–7151. doi: 10.1523/JNEUROSCI.0693-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines RM, Davies PA, Moss SJ, Maguire J. Functional regulation of GABAA receptors in nervous system pathologies. Curr Opin Neurobiol. 2012;22:552–558. doi: 10.1016/j.conb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton EA, Wheeler MG, Gourley SL. Early-life cocaine interferes with BDNF-mediated behavioral plasticity. Learn Mem. 2014;21:253–257. doi: 10.1101/lm.033290.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killcross S, Coutoureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid projection to the frontal cortex and the striatum in the rat. J Comp Neurol. 1990;298:40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Korpi ER, O'Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABA(A) receptor alpha1 subunit knockout mice. J Pharmacol Exp Ther. 2002;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:1370–1381. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Arguello AA, Wells AM, Hodges MA, Fuchs RA. Contribution of a mesocorticolimbic subcircuit to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2014;39:660–669. doi: 10.1038/npp.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex B, Hauber W. Disconnection of the entorhinal cortex and dorsomedial striatum impairs the sensitivity to instrumental contingency degradation. Neuropsychopharmacology. 2010;35:1788–1796. doi: 10.1038/npp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67:821–833. doi: 10.1016/j.neuron.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Lim B, Poo MM. Cocaine exposure in utero alters synaptic plasticity in the medial prefrontal cortex of postnatal rats. J Neurosci. 2009;29:12664–12674. doi: 10.1523/JNEUROSCI.1984-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex in rats, monkeys, and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balline BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmier JB, Linwick D. Conditional choice-unique outcomes establish expectancies that mediate choice behavior. Integr Physiol Behav Sci. 2001;36:173–181. doi: 10.1007/BF02734091. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- Rosen G, Williams AG, Capra JA, Connolly MT, Cruz B, Lu L, et al. The mouse brain library @ www.Mbl.Org. International Mouse Genome Conference; 14, 166 2000.
- Schwabe L, Dickinson A, Wolf OT. Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Exp Clin Psychopharmacol. 2011;19:53–63. doi: 10.1037/a0022212. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress and multiple memory systems: from ‘thinking' to ‘doing'. Trends Cogn Sci. 2013;17:60–68. doi: 10.1016/j.tics.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Skilbeck KJ, Johnston GAR, Hinton T. Stress and GABAA receptors. J Neurochem. 2010;112:1115–1130. doi: 10.1111/j.1471-4159.2009.06539.x. [DOI] [PubMed] [Google Scholar]
- Sonner JM, Cascio M, Xing Y, Fanselow MS, Kralic JE, Morrow AL, et al. Alpha1 subunit-contain GABA type A receptors in forebrain contribute to the effects of inhaled anesthetics on conditioned fear. Mol Pharmacol. 2005;68:61–68. doi: 10.1124/mol.104.009936. [DOI] [PubMed] [Google Scholar]
- Swanson AM, Shapiro LP, Whyte AJ, Gourley SL. Glucocorticoid receptor regulation of action selection and prefrontal cortical dendritic spines. Commun Integr Biol. 2013;6:e26068. doi: 10.4161/cib.26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Shima K, Mushiake H. Multiple cortical motor areas and temporal sequencing of movements. Brain Res Cogn Brain Res. 1996;1-2:117–122. doi: 10.1016/s0926-6410(96)00047-x. [DOI] [PubMed] [Google Scholar]
- Trapold MA. Are expectancies based upon different positive reinforcing events discriminably different. Learn Motiv. 1970;1:129–140. [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH. The role of the murine motor cortex in action duration and order. Front Integr Neurosci. 2009;3:23. doi: 10.3389/neuro.07.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Huang Z, Ding L, Deel ME, Arain FM, Murray CR, et al. Altered cortical GABAA receptor composition, physiology, and endocytosis in a mouse model of a human genetic absence epilepsy syndrome. J Biol Chem. 2013;288:21458–21472. doi: 10.1074/jbc.M112.444372. [DOI] [PMC free article] [PubMed] [Google Scholar]