Abstract
Objectives
Angiotensin converting enzyme inhibitors (ACEI) have been shown to decrease AGV in Marfan syndrome (MFS). We sought to compare the effect of β-blockers and ACEI on aortic growth velocity (AGV) in MFS.
Study design
We reviewed retrospectively all data from all patients with MFS seen at Arkansas Children’s Hospital between January 1, 1976 and January 1, 2013. Generalized least squares were used to evaluate AGV over time as a function of age, medication group, and the interaction between the two. A mixed model was used to compare AGV between medication groups as a function of age, medication group (none, β-blocker, ACEI), and the interaction between the two.
Results
A total of 67 patients with confirmed MFS were identified (34/67, 51% female). Mean age at first encounter was 13 ± 10 years, with mean follow-up of 7.6 ± 5.8 years. There were 839 patient encounters with a median of 10 (range 2–42) encounters per patient. AGV was nearly normal in the β-blocker group, and was less than either the ACEI or untreated groups. The AGV was higher than normal in ACEI and untreated groups (p<0.001 for both).
Conclusions
β-blocker therapy results in near-normalization of AGV in MFS. ACEI did not decrease AGV in a clinically significant manner.
Marfan syndrome (MFS) is a multi-system connective tissue disorder resulting from mutation in FBN1, the gene encoding fibrillin-1.1 MFS occurs in 1 in 3,000 live births and cardiovascular complications, especially aortopathy, are the leading cause of morbidity and premature mortality.2 Progressive aortic dilation is common with up to 80% of adults having dilation of the aortic root.3 In 1965, Wheat et al demonstrated that the use of reserpine improved survival of patients with aortic dissection.4 Subsequently, Halpern et al demonstrated that β-blocker therapy decreased myocardial contractility in two patients with MFS.5 Since that time, β-blocker therapy began to be used widely in this patient population,6 and remains the first-line therapy for the prevention of aortic complications in MFS.7. However, more recent studies have shown mixed results as to the efficacy of β-blocker therapy in these patients.8 Studies have shown decreased aortic growth rates in MFS patients taking angiotensin converting enzyme inhibitors (ACEI)9 and angiotensin-II receptor blockers.10 We sought to revisit the effects of both ACEI and β-blocker therapy on AGV in patients with MFS.
Methods
We performed a retrospective review of all patients with MFS seen at Arkansas Children’s Hospital between January 1, 1976 and January 1, 2013. Patients with MFS were identified using multiple institutional databases including those from the echocardiography and cardiac catheterization laboratories, the cardiology clinic, all cardiothoracic surgeries, and the Division of Genetics. All available clinical data were reviewed and were recorded.
Echocardiograms were performed with the patient in the supine position using commercially available ultrasound machines (Siemens Acuson Sequoia 512 with 10, 7, 5, and 3 MHz probes and Philips iE33 with 12, 8, and 5 MHz probes). Two-dimensional measurements were made in accordance with the recommendations of the American Society of Echocardiography using parasternal long-axis views of the aortic annulus, aortic sinus of Valsalva, sinotubular junction and ascending aorta.11 Measurements were made from inner edge to inner edge during ventricular systole.
The decision to initiate pharmacologic therapy was primarily based on the presence of aortic measurements above the normal range reported by Roman et al12 or accelerated progressive dilation. The selection of a pharmacologic agent and the dose were provider dependent; there were no formal algorithms. After the report from our institution by Yetman et al,9 the use of ACEI as primary therapy at our institution increased.
Anthropometric data were used to calculate the body surface area (BSA) at each patient encounter using the Dubois formula.13 A normative control comparison dataset for aortic dimension and growth rate was created by using the calculated BSA of each patient with MFS at each encounter using the formula: aortic root dimension = 24.0(BSA in m2)1/3 + 0.1(Age) – 4.3.14 This normative control dataset was then compared against actual measured aortic dimensions in the patient cohort.
Statistical Analyses
Summary statistics were expressed as frequency and percentage for categorical variables, and as mean ± standard deviation for continuous variables, except for the ages of the treatment groups, which are expressed as mean with first (Q1) and third (Q3) quartiles. To compare aortic growth velocities between medication groups, a mixed model was developed for the aortic dimension as a function of age, medication group (none, β-blocker, ACEI, or normative control), and the interaction between the two. A restricted cubic spline was used for age when fitting the mixed model with regard to the non-linear relationship between aortic dimension and age. A compound symmetry variance matrix was used to take into account the correlated measurements from the same patient. Additional mixed models were fitted for blood pressures and heart rates to assess their differences among three medication groups (none, β-blocker, or ACEI). All the data were analyzed using statistical software SAS 9.4 (SAS Institute Inc., Cary, NC). P-values < 0.05 were considered to indicate statistical significance.
Results
A total of 67 patients with confirmed MFS were identified (34/67, 51% female). The mean ± SD age at first encounter was 13 ± 10 years, with a mean followup length of 7.6 ± 5.8 years. There were 839 patient encounters with a median number of 10 (range 2–42) encounters per patient. For the patients in the untreated group, the mean age was 9.8 years (Q1: 3.9 years; Q3: 17.2 years), which was lower than either the β-blocker or ACEI groups (p<0.001).
For those treated with β-blocker therapy, the mean age was 16.9 years (Q1: 12.1 years; Q3: 22.2 years). β-blockers used included daily atenolol (45.9%), twice daily metoprolol (48.5%), and thrice daily propranolol (5.6%). The mean dose per patient was 0.95 ± 0.63 mg/kg (total daily dose 47.3 ± 23.5mg). The mean systolic (109 ± 16 mmHg) and diastolic (67 ± 8 mmHg) blood pressures were not different from patients who were not treated with medication. The mean heart rate in the β-blocker group (78 ± 19 bpm) was significantly lower compared with those who were untreated (90 ± 24 bpm, p=0.001).
For those treated with ACEI therapy, the mean age was 16.8 years (Q1: 10.7 years; Q3: 24.4 years). ACEIs used included daily lisinopril (12.9%), twice daily enalapril (85.5%), and thrice daily captopril (1.6%). The mean ACEI dose per patient was 0.22 ± 0.1 mg/kg (total daily dose 12.7 ± 6.9 mg). The mean systolic (113 ± 16 vs. 106 ± 20 mmHg, p=0.005) and diastolic (68 ± 10 vs. 64 ± 10 mmHg, p=0.005) blood pressures were significantly higher in the ACEI group compared with the untreated group. The mean heart rate in the ACEI group (83 ± 17 bpm) was significantly lower compared with the untreated group (90 ± 24 bpm, p=0.003). The systolic and diastolic blood pressures were higher in the ACEI group compared with the β-blocker group (p=0.015 and p=0.019, respectively), and there was no difference in heart rate (p=0.696).
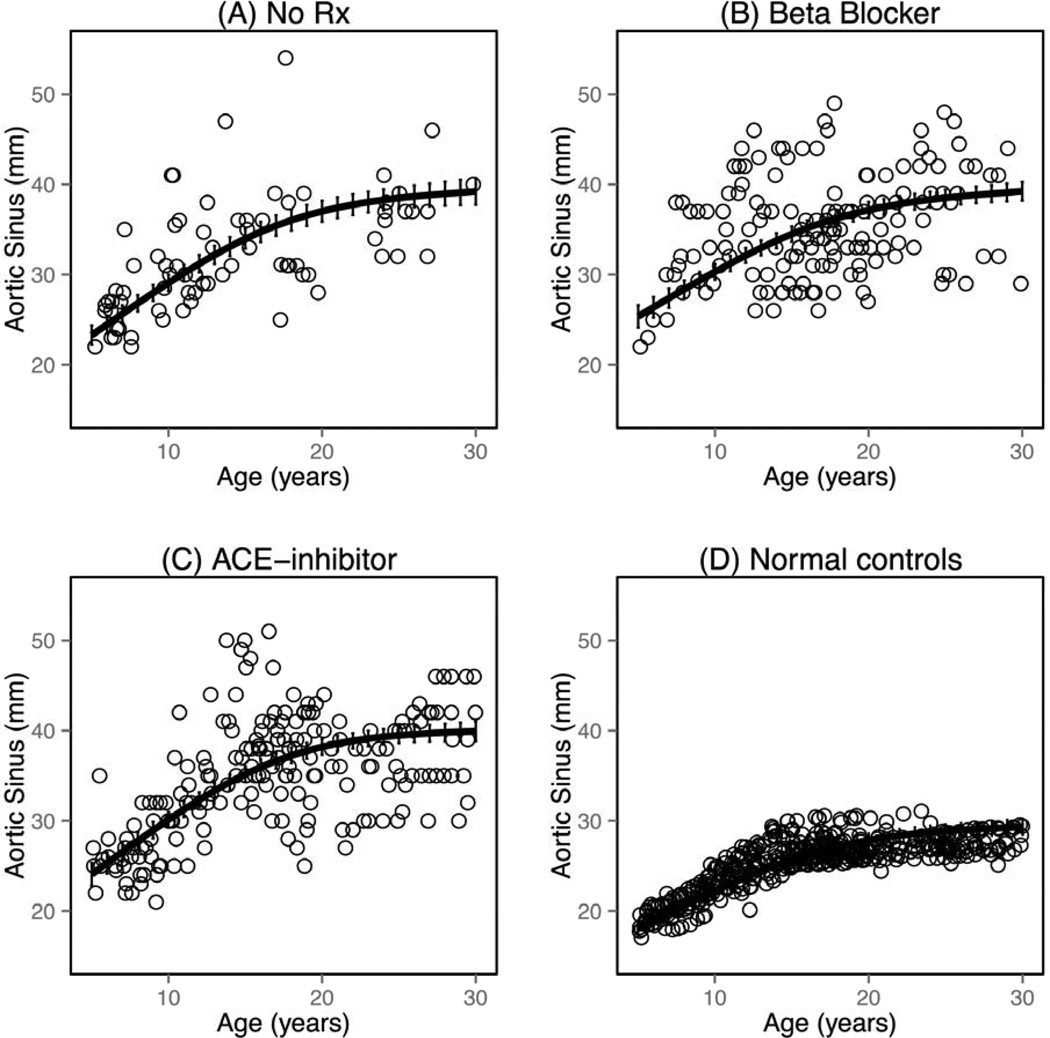
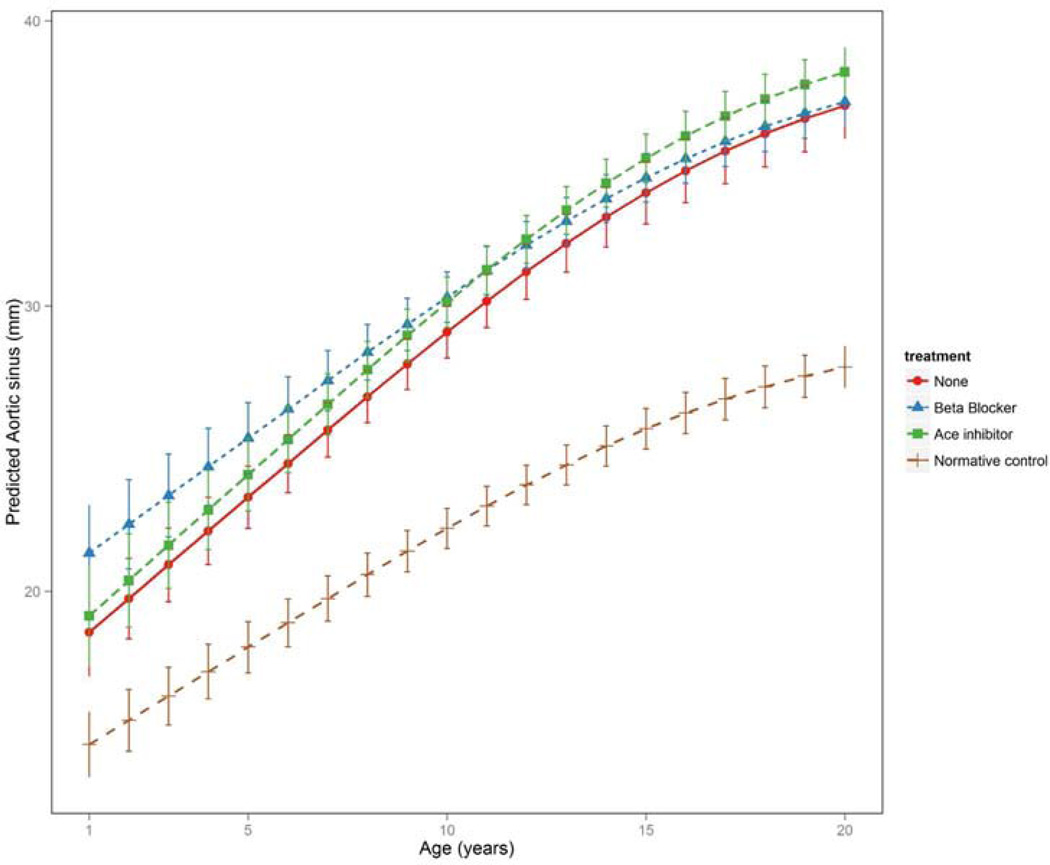
The aortic size and AGV for the three largest groups (none, ACEI, and β-blocker) were determined (Figure 1) and intergroup comparisons were made, as well as comparisons with the calculated, expected normative control aortic root dimensions (Figure 2). The aortic dimensions were significantly larger in all groups compared with the normative control dataset (p<0.001). At younger ages, the aortic dimensions in the β-blocker group were significantly larger than the ACEI and untreated groups (p<0.01; Figure 1). There was no difference between the ACEI and untreated groups with regard to aortic dimensions at younger ages (p=0.636). The aortic dimensions and growth velocities varied between the four groups. Aortic growth velocity was significantly attenuated in the β-blocker, and nearly approximated that in the normative control dataset. The attenuation of the AGV in the ACEI group was less than that of the β-blocker group compared with those not treated with medication.
Figure 1.
Aortic growth in in patients with Marfan syndrome: (A) without treatment; (B) treated with beta-blocker; and (C) treated with ACE-inhibitor. (D) Aortic growth in the predicted, normative control dataset for the cohort.
Figure 2.
Comparison of aortic growth velocities over time in treated and untreated groups and in the predicted, normative control dataset.
A total of 14 patients underwent 17 surgeries. Aortic root replacement was performed in 11/14 patients (79%). The median age at aortic root replacement was 17 years (range 9–35 years). Two patients experienced aortic dissections: one treated with metoprolol experienced acute dissection of the entire thoracic aorta and survived to surgery; the other was treated with enalapril and found to have an asymptomatic dissection of the aortic root. Of the 11 patients who underwent aortic surgery, pharmacologic treatment prior to surgery included: ACEI in 5; β-blocker in 4; and no therapy in 2. One patient in the study group died approximately 2 years after aortic root replacement. Further details of the death could not be obtained.
Discussion
The present study demonstrates a significant attenuation of the AGV in pediatric patients with MFS treated with β-blocker therapy. In fact, the growth velocity was almost identical to the predicted normal growth velocity for the cohort. On average, the patients in the β-blocker group began with a larger aortic dimension compared with the patients in the ACEI and untreated groups, and this difference resolved over time, a finding that may suggest that the early initiation of β-blocker therapy should be considered in patients with MFS. This is a significant finding because aortic wall tension increases as the aortic diameter increases, which results in acceleration of aortic dilation at larger diameters.15 The expected result would then be that the AGV should be faster in the β-blocker group; however, the opposite was found in the β-blocker group indicating a definite mitigating effect on the AGV in patients with MFS.
Prior investigators have demonstrated decreases in AGV in patients with MFS treated with β-blocker.16–18 Recently, Mueller et al demonstrated a decrease in AGV in patients with MFS who were treated with either β-blockers or angiotensin II receptor blockers.19 However, other studies have suggested that β-blocker therapy does not alter the AGV or clinical outcomes in patients with MFS.20, 21 Gersony et al conducted a meta-analysis of six studies available at the time,22 and concluded that β-blocker therapy was ineffective in patients with MFS.
In the original studies used to conduct their meta-analysis, most of the original authors arrived at different conclusions than Gersony et al. Silverman et al showed that death was twice as common in the untreated versus the β-blocker treated group.23 Those patients treated with β-blocker therapy lived longer (p=0.01). Legget et al reported that aortic complications were more common in the β-blocker treatment group; however, the aortic complication group also had significantly larger aortas at the initiation of the study (p<0.0001).21 Further, in that study patients were dichotomized, without clear delineation as to why, to those who had β-blocker therapy for ≥1 year versus those who had therapy for < 1 year. Similar to the work of Silverman et al, Roman et al reported that in 113 patients with MFS those who had aortic complications had larger aortic size at presentation (p<0.005), were significantly older (p<0.01), and had significantly faster AGV (p<0.05); however, β-blocker therapy use only trended toward being more common in the complication group (86% vs 66%, p=non-significant).15 Those authors stated that the trend toward increased β-blocker use in the group with complications was because they started out with larger aortas, which represents a significant selection bias.15 Salim et al reported that the aorta is dilated at young ages in MFS and showed that the AGV was faster in those who were untreated.24 In that study, the 5 patients treated with β-blocker therapy who underwent surgical intervention had larger aortas at the time of enrollment. In their randomized trial of propranolol therapy in MFS, Shores et al reported in the propranolol group a decreased rate of aortic dilation, fewer deaths, and significantly fewer patients who reached one or more predetermined clinical end-points.17 Finally, Tahernia reported three patients who were treated with β-blocker therapy and three who were not.25 With a mean follow-up of 3.3 years, the β-blocker group had a mean AGV of 0.2 mm/year. The three untreated patients had a mean follow-up of 3 years with a mean AGV of 1.4 mm/year.
In the consideration of studies evaluating AGV, it is imperative to consider the size of the aorta at the time of the initiation of pharmacotherapy. In accordance with the law of Laplace, when wall thickness and blood pressure are stable, wall tension in the aorta increases as the aortic diameter increases,26 resulting in acceleration of aortic dilation at larger diameters.15 To make a truly meaningful comparison between therapeutic groups, either both groups have to begin with the same diameters or the group that is shown to have a decreased growth rate must have begun at a larger diameter, thus showing a diminution of AGV. If the aorta is larger in one group than the other, those patients are beginning the study at a different point in the aortic disease process than the group with the smaller diameter. The present study is not subject to this concern because those patients treated with β-blocker therapy began, on average, with a larger aortic diameter than either the ACEI group or the untreated group and were still demonstrated to have a lower AGV.
The difference in results between the β-blocker and ACEI groups in the present study seem to support the hypothesis that a decrease in myocardial contractility is the explanation for decreased AGV in patients with MFS treated with β-blockers. In the present study, there were no clinically significant differences in blood pressure or heart rate between the β-blocker and ACEI groups; however, the AGV in the β-blocker group was significantly less than either the ACEI or untreated groups. However, other explanations cannot be ruled out, such as heretofore-unknown pleiotropic effects of β-blockers.
Yetman et al demonstrated that there was a decrease in the AGV and improvement in the aortic distensibility in patients with MFS treated with ACEI versus those treated with β-blocker.9 The present study does not corroborate those findings. In the study by Yetman et al, the subject ages and the doses of both medications were similar to those in the present study. The length of follow-up was shorter in that prior study (3.0 ± 0.2 years) compared with the present study (7.6 ± 5.8 years).
The present study is limited by its retrospective nature. The lack of a true control group also is a limitation. However, a previously published and validated formula for the determination of predicted aortic size was used in combination with the anthropometric data from the present study cohort to create a predicted “normal” aortic growth curve, which allowed for comparison of the study measurements against a unique dataset specific to the study cohort. The patients in the non-treatment group were younger than those in either the β-blocker or ACEI group. An older population would be expected to have an increased AGV, which if anything would blunt any effect of the medication on slowing AGV.
These findings suggest that β-blocker therapy may be more beneficial. Early introduction of β-blocker therapy in patients with MFS should be considered particularly even prior to the demonstration of aortic dilation.
Acknowledgments
The authors wish to than Bruce S. Alpert, MD (previously at the University of Tennessee College of Medicine; currently a case reviewer for the health care industry), for his thoughtful review and comments on the manuscript.
Supported in part by National Center for Advancing Translational Sciences/National Institutes of Health (1 UL1 RR029884).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, Stetten G, Meyers DA, Francomano CA. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 2.Murdoch JL, Walker BA, Halpern BL, Kuzma JW, McKusick VA. Life expectancy and causes of death in the Marfan syndrome. N Engl J Med. 1972;286:804–808. doi: 10.1056/NEJM197204132861502. [DOI] [PubMed] [Google Scholar]
- 3.Mueller G, Stark V, Steiner K, von Kodolitsch Y, Rybczynski M, Weil J, Mir T. Impact of age and gender on cardiac pathology in children and adolescents with Marfan syndrome. Pediatr Cardiol. 2013;34:991–998. doi: 10.1007/s00246-012-0593-0. [DOI] [PubMed] [Google Scholar]
- 4.Wheat MWJ, Palmer RF, TD B, Seelman RC. Treatment of dissecting aneurysms of the aorta without surgery. J Thorac Cardiovasc Surg. 1965;50:364–373. [PubMed] [Google Scholar]
- 5.Halpern BL, Char F, Murdoch JL, Horton WB, McKusick VA. A prospectus on the prevention of aortic rupture in the Marfan syndrome with data on survivorship without treatment. Johns Hopkins Med J. 1971;129:123–129. [PubMed] [Google Scholar]
- 6.Ose L, McKusick V. Prophylactic use of propranolol in the Marfan syndrome to prevent aortic dissection. Birth defects original article series. 1977;13:163. [PubMed] [Google Scholar]
- 7.Keane MG, Pyeritz RE. Medical management of Marfan syndrome. Circulation. 2008;117:2802–2813. doi: 10.1161/CIRCULATIONAHA.107.693523. [DOI] [PubMed] [Google Scholar]
- 8.Danyi P, Elefteriades JA, Jovin IS. Medical therapy of thoracic aortic aneurysms: are we there yet? Circulation. 2011;124:1469–1476. doi: 10.1161/CIRCULATIONAHA.110.006486. [DOI] [PubMed] [Google Scholar]
- 9.Yetman AT, Bornemeier RA, McCrindle BW. Usefulness of enalapril versus propranolol or atenolol for prevention of aortic dilation in patients with the Marfan syndrome. Am J Cardiol. 2005;95:1125–1127. doi: 10.1016/j.amjcard.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz H. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N Engl J Med. 2008;358:2787–2795. doi: 10.1056/NEJMoa0706585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64:507–512. doi: 10.1016/0002-9149(89)90430-x. [DOI] [PubMed] [Google Scholar]
- 13.Dubois D, Dubois E. A formula to estimate the approximate surface area if height and weight are known. Arch Int Med. 1916;17:863–871. [Google Scholar]
- 14.Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980;62:1054–1061. doi: 10.1161/01.cir.62.5.1054. [DOI] [PubMed] [Google Scholar]
- 15.Roman MJ, Rosen SE, Kramer-Fox R, Devereux RB. Prognostic significance of the pattern of aortic root dilation in the Marfan syndrome. J Am Coll Cardiol. 1993;22:1470–1476. doi: 10.1016/0735-1097(93)90559-j. [DOI] [PubMed] [Google Scholar]
- 16.Rossi-Foulkes R, Roman MJ, Rosen SE, Kramer-Fox R, Ehlers KH, O'Loughlin JE, Davis JG, Devereux RB. Phenotypic features and impact of beta blocker or calcium antagonist therapy on aortic lumen size in the Marfan syndrome. Am J Cardiol. 1999;83:1364–1368. doi: 10.1016/s0002-9149(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 17.Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan's syndrome. N Engl J Med. 1994;330:1335–1341. doi: 10.1056/NEJM199405123301902. [DOI] [PubMed] [Google Scholar]
- 18.Ladouceur M, Fermanian C, Lupoglazoff JM, Edouard T, Dulac Y, Acar P, Magnier S, Jondeau G. Effect of beta-blockade on ascending aortic dilatation in children with the Marfan syndrome. Am J Cardiol. 2007;99:406–409. doi: 10.1016/j.amjcard.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 19.Mueller GC, Stierle L, Stark V, Steiner K, von Kodolitsch Y, Weil J, Mir TS. Retrospective analysis of the effect of angiotensin II receptor blocker versus β-blocker on aortic root growth in paediatric patients with Marfan syndrome. Heart. 2014;100:214–218. doi: 10.1136/heartjnl-2013-304946. [DOI] [PubMed] [Google Scholar]
- 20.Selamet Tierney ES, Feingold B, Printz BF, Park SC, Graham D, Kleinman CS, Mahnke CB, Timchak DM, Neches WH, Gersony WM. Beta-blocker therapy does not alter the rate of aortic root dilation in pediatric patients with Marfan syndrome. J Pediatr. 2007;150:77–82. doi: 10.1016/j.jpeds.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Legget ME, Unger TA, O'Sullivan CK, Zwink TR, Bennett RL, Byers PH, Otto CM. Aortic root complications in Marfan's syndrome: identification of a lower risk group. Heart. 1996;75:389–395. doi: 10.1136/hrt.75.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gersony DR, McClaughlin MA, Jin Z, Gersony WM. The effect of beta-blocker therapy on clinical outcome in patients with Marfan's syndrome: a meta-analysis. Int J Cardiol. 2007;114:303–308. doi: 10.1016/j.ijcard.2005.11.116. [DOI] [PubMed] [Google Scholar]
- 23.Silverman DI, Burton KJ, Gray J, Bosner MS, Kouchoukos NT, Roman MJ, Boxer M, Devereux RB, Tsipouras P. Life expectancy in the Marfan syndrome. Am J Cardiol. 1995;75:157–160. doi: 10.1016/s0002-9149(00)80066-1. [DOI] [PubMed] [Google Scholar]
- 24.Salim MA, Alpert BS, Ward JC, Pyeritz RE. Effect of beta-adrenergic blockade on aortic root rate of dilation in the Marfan syndrome. Am J Cardiol. 1994;74:629–633. doi: 10.1016/0002-9149(94)90762-5. [DOI] [PubMed] [Google Scholar]
- 25.Tahernia AC. Cardiovascular anomalies in Marfan's syndrome: the role of echocardiography and beta-blockers. South Med J. 1993;86:305–310. [PubMed] [Google Scholar]
- 26.Nathan D, Xu C, Plappert T, Desjardins B, Gorman J, Bavaria J, Gorman R, Chandran K, Jackson B. Increased ascending aortic wall stress in patients with bicuspid aortic valves. Ann Thorac Surg. 2011;92:1384–1389. doi: 10.1016/j.athoracsur.2011.04.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson CW. Control of a field outbreak of dissecting aneurysms and laying hen studies with reserpine; Conference on the Use of the Tranquilizing and Antihypertensive Agent, Serpasil; 1959. pp. 29–35. [Google Scholar]
- 28.Prokop EK, Palmer RF, Wheat MW. Hydrodynamic forces in dissecting aneurysms In-vitro studies in a tygon model and in dog aortas. Circ Res. 1970;27:121–127. doi: 10.1161/01.res.27.1.121. [DOI] [PubMed] [Google Scholar]
- 29.Beaven DW, Murphy EA. Dissecting aneurysm during methonium therapy; a report on nine cases treated for hypertension. Br Med J. 1956;1:77–80. doi: 10.1136/bmj.1.4958.77. [DOI] [PMC free article] [PubMed] [Google Scholar]