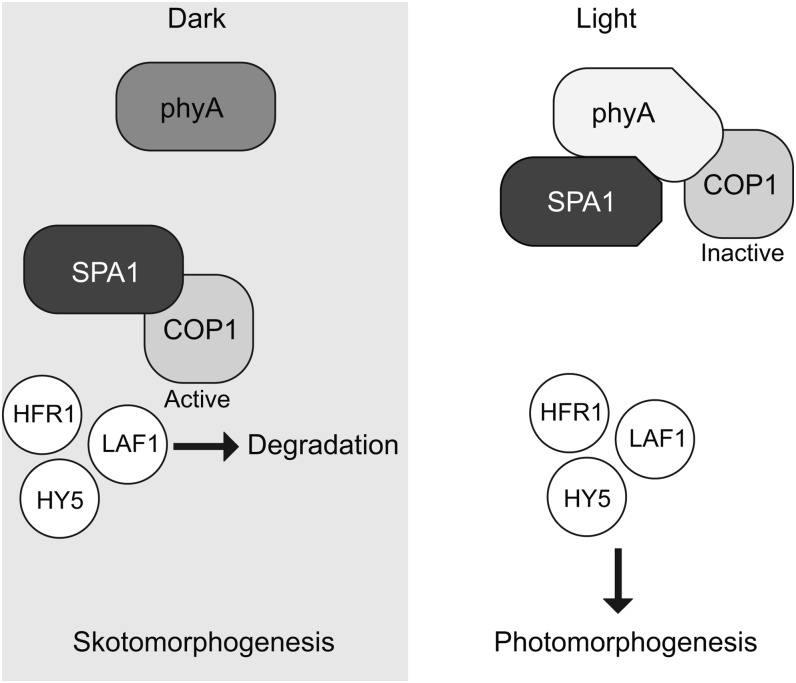
In the dark, seedling photomorphogenesis is repressed via CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1). COP1 forms a complex in the nucleus with SUPPRESSOR OF phyA-105 (SPA) proteins and functions as an E3 ubiquitin ligase, marking positive regulators of photomorphogenesis for degradation. When seedlings are exposed to light, phytochrome (phy) photoreceptors promote photomorphogenesis by inhibiting the action of the COP1-SPA complex, thereby allowing photomorphogenesis to proceed. A fundamental question remaining about this process, however, is how the phys inactivate COP1-SPA (reviewed in Li et al., 2011). New work from Sheerin et al. (2015) reveals that phyA and phyB directly interact with SPA proteins and that this interaction likely is related to COP1-SPA inactivation.
Sheerin et al. screened for interactors of phyA using a unique yeast two-hybrid screen conducted in the presence of phycocyanobilin to generate photoactive phyA protein. This screen identified SPA1 as binding the far-red-light-absorbing Pfr form of phyA, which is generally considered the biologically active form. SPA1 did not bind the red-light-absorbing Pr form of phyA. The authors confirmed this interaction in planta by fluorescence lifetime imaging microscopy and found that SPA1 and phyA colocalized in the nucleus to foci termed nuclear bodies. In addition, phyA and SPA2 interacted in nuclear bodies, as did phyB and SPA1.
Nuclear bodies contain a number of light signal transduction components, and it is increasingly clear that they are important sites of action in light signaling, possibly even serving as hubs for several pathways (reviewed in Van Buskirk et al., 2012). Sheerin and coworkers found that whereas phyA presence in nuclear bodies is dependent on light, that of SPA1 was independent of light and SPA proteins were not necessary for recruitment of phyA to nuclear bodies.
The authors went on to examine the influence of phyA on COP1/SPA protein interactions and function. They demonstrated that upon light activation, the presence of phyA inhibited COP1 interaction with SPA proteins and that phyB also inhibited COP1/SPA1 interaction. Furthermore, LONG HYPOCOTYL IN FAR-RED1 (HFR1), a transcription factor that promotes photomorphogenesis and is a target of COP1/SPA, was stabilized upon light exposure with timing similar to that of phyA accumulation in nuclear bodies. This stabilization suggests that phyA suppresses the COP1-SPA complex-mediated targeting of HFR1 for degradation.
Together, the results of Sheerin et al. suggest that exposure to light induces phyA localization to nuclear bodies, where it directly interacts with SPA proteins to inhibit the activity of the COP1/SPA complexes, thereby promoting photomorphogenic development (see figure). This work represents an exciting step forward in our understanding of phytochrome-mediated promotion of photomorphogenesis, as well as of signaling mechanisms involving COP1, which might serve as an integrator of signals from various types of photoreceptors.
A model for light-induced inactivation of COP1-SPA by phyA. phyA interacts with SPA proteins in the light to alter the interaction between COP1 and SPA proteins, thereby inactivating COP1 and releasing its proteasome-mediated inhibition of photomorphogenesis. (Reprinted from Sheerin et al. [2015], Figure 7.)
References
- Li J., Li G., Wang H., Wang Deng X. (2011). Phytochrome signaling mechanisms. The Arabidopsis Book 9:e0148, doi/10.1199/tab.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerin D.J., Menon C., zur Oven-Krockhaus S., Enderle B., Zhu L., Johnen P., Schleifenbaum F., Stierhof Y.-D., Huq E., Hiltbrunner A. (2015). Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell 27: 10.1105/tpc.115.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk E.K., Decker P.V., Chen M. (2012). Photobodies in light signaling. Plant Physiol. 158: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]