Abstract
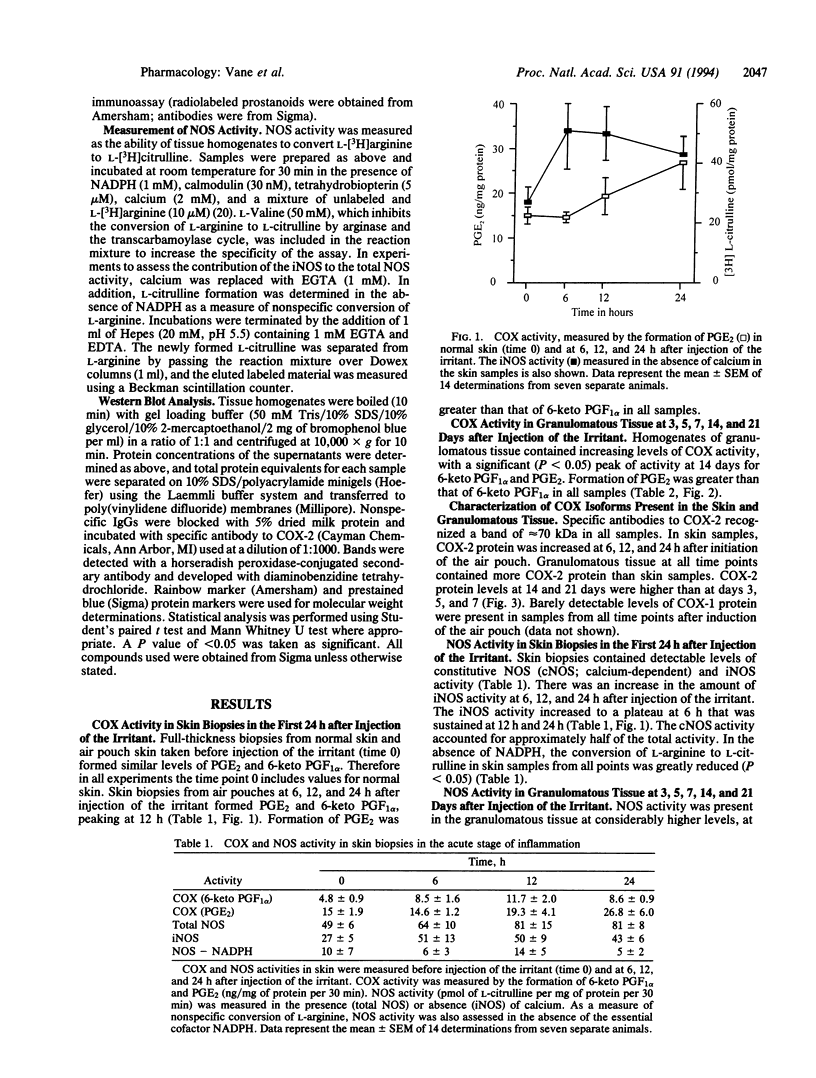
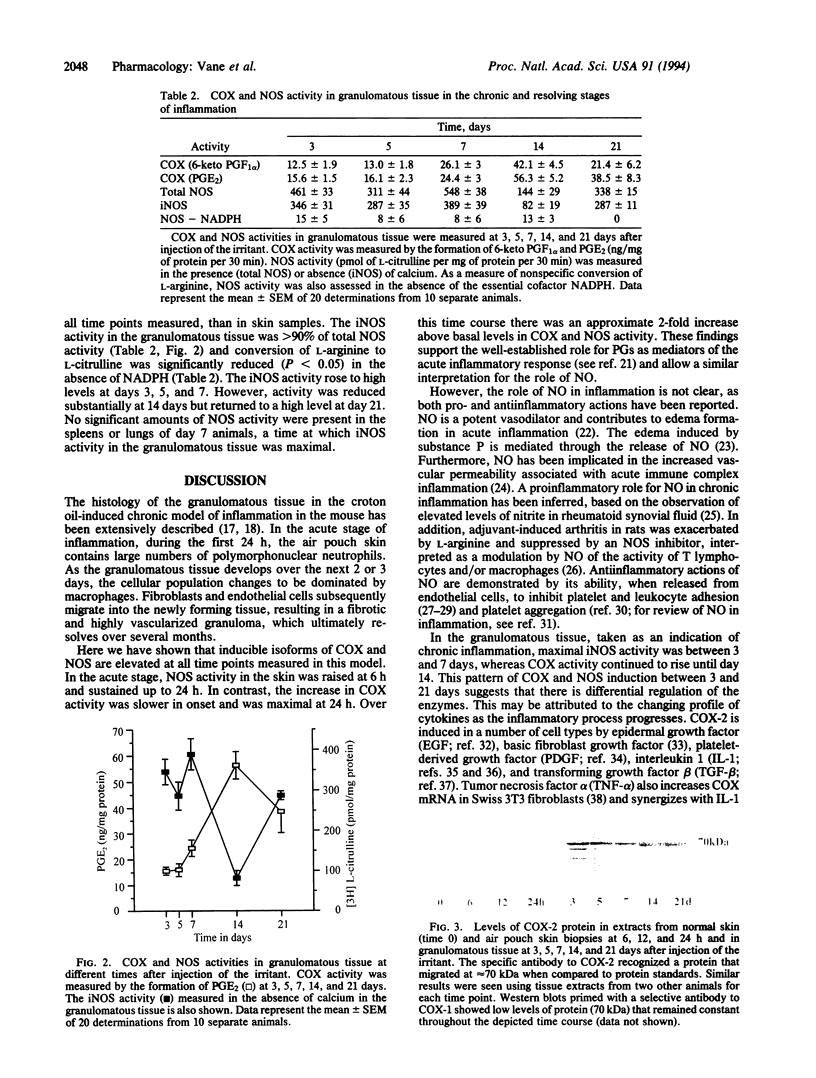
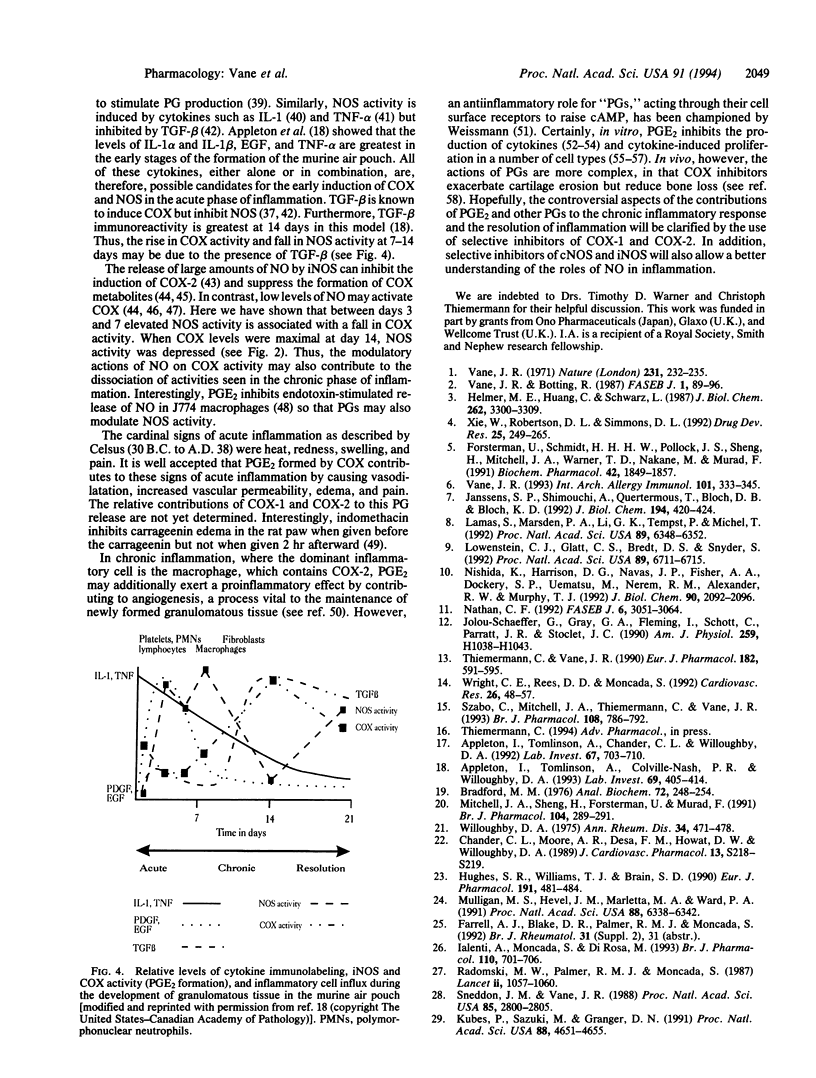
Cyclooxygenase (COX) converts arachidonic acid to prostaglandin H2, which is further metabolized to prostanoids. Two isoforms of COX exist: a constitutive (COX-1) and an inducible (COX-2) enzyme. Nitric oxide is derived from L-arginine by isoforms of nitric-oxide synthase (NOS; EC 1.14.13.39): constitutive (cNOS; calcium-dependent) and inducible (iNOS; calcium-independent). Here we have investigated inducible isoforms of COX and NOS in the acute, chronic, and resolving stages of a murine air pouch model of granulomatous inflammation. COX and NOS activities were measured in skin samples in the acute phase, up to 24 h. Activities in granulomatous tissue were measured at 3, 5, 7, 14, and 21 days for the chronic and resolving stages of inflammation. COX-1 and COX-2 proteins were assessed by Western blot. COX activity in the skin increased over the first 24 h and continued to rise up to day 14. COX-2 protein rose progressively, also peaking at day 14. COX-1 protein remained unaltered throughout. The iNOS activity increased over the first 24 h in the skin, with a further major increase in the granulomatous tissue between days 3 and 7, followed by a decrease at day 14 and a further increase at day 21. The rise in COX and NOS activities in the skin during the acute phase reinforces the proinflammatory role for prostanoids and suggests one also for nitric oxide. However, in the chronic and resolving stages, a dissociation of COX and NOS activity occurred. Thus, there may be differential regulation of these enzymes, perhaps due to the changing pattern of cytokines during the inflammatory response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Henry W. L., Jr Suppression of lymphocyte proliferation through the nitric oxide synthesizing pathway. J Surg Res. 1991 Apr;50(4):403–409. doi: 10.1016/0022-4804(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Albrightson C. R., Baenziger N. L., Needleman P. Exaggerated human vascular cell prostaglandin biosynthesis mediated by monocytes: role of monokines and interleukin 1. J Immunol. 1985 Sep;135(3):1872–1877. [PubMed] [Google Scholar]
- Appleton I., Tomlinson A., Chander C. L., Willoughby D. A. Effect of endothelin-1 on croton oil-induced granulation tissue in the rat. A pharmacologic and immunohistochemical study. Lab Invest. 1992 Dec;67(6):703–710. [PubMed] [Google Scholar]
- Appleton I., Tomlinson A., Colville-Nash P. R., Willoughby D. A. Temporal and spatial immunolocalization of cytokines in murine chronic granulomatous tissue. Implications for their role in tissue development and repair processes. Lab Invest. 1993 Oct;69(4):405–414. [PubMed] [Google Scholar]
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Muza B., Hla T., Salata K. Restoration of prostacyclin synthase in vascular smooth muscle cells after aspirin treatment: regulation by epidermal growth factor. J Lipid Res. 1985 Jan;26(1):54–61. [PubMed] [Google Scholar]
- Bailey J. M., Verma M. Analytical procedures for a cryptic messenger RNA that mediates translational control of prostaglandin synthase by glucocorticoids. Anal Biochem. 1991 Jul;196(1):11–18. doi: 10.1016/0003-2697(91)90110-f. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Deakin A. M., Follenfant R. L., Whittle B. J., Garland L. G. Role of oxygen radicals and arachidonic acid metabolites in the reverse passive Arthus reaction and carrageenin paw oedema in the rat. Br J Pharmacol. 1993 Oct;110(2):896–902. doi: 10.1111/j.1476-5381.1993.tb13897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Tiffany C. W. Tumor necrosis factor causes amplification of arachidonic acid metabolism in response to interleukin 1, bradykinin, and other agonists. J Cell Physiol. 1989 Oct;141(1):85–89. doi: 10.1002/jcp.1041410113. [DOI] [PubMed] [Google Scholar]
- Chander C. L., Moore A. R., Desa F. M., Howat D. W., Willoughby D. A. Anti-inflammatory effects of endothelin-1. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S218–S219. doi: 10.1097/00005344-198900135-00064. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Ferreri N. R., Sarr T., Askenase P. W., Ruddle N. H. Molecular regulation of tumor necrosis factor-alpha and lymphotoxin production in T cells. Inhibition by prostaglandin E2. J Biol Chem. 1992 May 5;267(13):9443–9449. [PubMed] [Google Scholar]
- Förstermann U., Schmidt H. H., Pollock J. S., Sheng H., Mitchell J. A., Warner T. D., Nakane M., Murad F. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol. 1991 Oct 24;42(10):1849–1857. doi: 10.1016/0006-2952(91)90581-o. [DOI] [PubMed] [Google Scholar]
- Goddard D. H., Grossman S. L., Newton R., Clark M. A., Bomalaski J. S. Regulation of synovial cell growth: basic fibroblast growth factor synergizes with interleukin 1 beta stimulating phospholipase A2 enzyme activity, phospholipase A2 activating protein production and release of prostaglandin E2 by rheumatoid arthritis synovial cells in culture. Cytokine. 1992 Sep;4(5):377–384. doi: 10.1016/1043-4666(92)90081-2. [DOI] [PubMed] [Google Scholar]
- Goppelt-Struebe M., Wolter D., Resch K. Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase. Br J Pharmacol. 1989 Dec;98(4):1287–1295. doi: 10.1111/j.1476-5381.1989.tb12676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Bray M. A., Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. doi: 10.1038/262401a0. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hori T., Yamanaka Y., Hayakawa M., Shibamoto S., Tsujimoto M., Oku N., Ito F. Prostaglandins antagonize fibroblast proliferation stimulated by tumor necrosis factor. Biochem Biophys Res Commun. 1991 Jan 31;174(2):758–766. doi: 10.1016/0006-291x(91)91482-r. [DOI] [PubMed] [Google Scholar]
- Hughes S. R., Williams T. J., Brain S. D. Evidence that endogenous nitric oxide modulates oedema formation induced by substance P. Eur J Pharmacol. 1990 Dec 4;191(3):481–484. doi: 10.1016/0014-2999(90)94184-y. [DOI] [PubMed] [Google Scholar]
- Ialenti A., Moncada S., Di Rosa M. Modulation of adjuvant arthritis by endogenous nitric oxide. Br J Pharmacol. 1993 Oct;110(2):701–706. doi: 10.1111/j.1476-5381.1993.tb13868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Fukuo K., Morimoto S., Koh E., Ogihara T. Nitric oxide mediates interleukin-1-induced prostaglandin E2 production by vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Jul 15;194(1):420–424. doi: 10.1006/bbrc.1993.1836. [DOI] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. H., Bienkowski M. J., Gorman R. R. Regulation of prostaglandin H synthase mRNA levels and prostaglandin biosynthesis by platelet-derived growth factor. J Biol Chem. 1989 Oct 15;264(29):17379–17383. [PubMed] [Google Scholar]
- Lowenstein C. J., Glatt C. S., Bredt D. S., Snyder S. H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta P., Sautebin L., Di Rosa M. Modulation of the induction of nitric oxide synthase by eicosanoids in the murine macrophage cell line J774. Br J Pharmacol. 1992 Nov;107(3):640–641. doi: 10.1111/j.1476-5381.1992.tb14499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. A., Sheng H., Förstermann U., Murad F. Characterization of nitric oxide synthases in non-adrenergic non-cholinergic nerve containing tissue from the rat anococcygeus muscle. Br J Pharmacol. 1991 Oct;104(2):289–291. doi: 10.1111/j.1476-5381.1991.tb12422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nishida K., Harrison D. G., Navas J. P., Fisher A. A., Dockery S. P., Uematsu M., Nerem R. M., Alexander R. W., Murphy T. J. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992 Nov;90(5):2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quill H., Gaur A., Phipps R. P. Prostaglandin E2-dependent induction of granulocyte-macrophage colony-stimulating factor secretion by cloned murine helper T cells. J Immunol. 1989 Feb 1;142(3):813–818. [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Raz A., Wyche A., Needleman P. Temporal and pharmacological division of fibroblast cyclooxygenase expression into transcriptional and translational phases. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1657–1661. doi: 10.1073/pnas.86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., Misko T. P., Masferrer J. L., Seibert K., Currie M. G., Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schini V. B., Durante W., Elizondo E., Scott-Burden T., Junquero D. C., Schafer A. I., Vanhoutte P. M. The induction of nitric oxide synthase activity is inhibited by TGF-beta 1, PDGFAB and PDGFBB in vascular smooth muscle cells. Eur J Pharmacol. 1992 Jun 17;216(3):379–383. doi: 10.1016/0014-2999(92)90434-6. [DOI] [PubMed] [Google Scholar]
- Sneddon J. M., Vane J. R. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Harbrecht B. G., Di Silvio M., Curran R. D., Jordan M. L., Simmons R. L., Billiar T. R. Endogenous nitric oxide inhibits the synthesis of cyclooxygenase products and interleukin-6 by rat Kupffer cells. J Leukoc Biol. 1993 Feb;53(2):165–172. doi: 10.1002/jlb.53.2.165. [DOI] [PubMed] [Google Scholar]
- Stadler J., Stefanovic-Racic M., Billiar T. R., Curran R. D., McIntyre L. A., Georgescu H. I., Simmons R. L., Evans C. H. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991 Dec 1;147(11):3915–3920. [PubMed] [Google Scholar]
- Stahl R. A., Thaiss F., Haberstroh U., Kahf S., Shaw A., Schoeppe W. Cyclooxygenase inhibition enhances rat interleukin 1 beta-induced growth of rat mesangial cells in culture. Am J Physiol. 1990 Sep;259(3 Pt 2):F419–F424. doi: 10.1152/ajprenal.1990.259.3.F419. [DOI] [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Thiemermann C., Vane J. R. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vane J., Botting R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J. 1987 Aug;1(2):89–96. [PubMed] [Google Scholar]
- Vane J. Control of the circulation by endothelial mediators. Inaugural G.B. West Memorial Lecture. Int Arch Allergy Immunol. 1993;101(4):333–345. doi: 10.1159/000236474. [DOI] [PubMed] [Google Scholar]
- Weissmann G. Prostaglandins as modulators rather than mediators of inflammation. J Lipid Mediat. 1993 Mar-Apr;6(1-3):275–286. [PubMed] [Google Scholar]
- Willoughby D. A., Colville-Nash P. R., Seed M. P. Inflammation, prostaglandins, and loss of function. J Lipid Mediat. 1993 Mar-Apr;6(1-3):287–293. [PubMed] [Google Scholar]
- Willoughby D. A. Heberden Oration, 1974. Human arthritis applied to animal models. Towards a better therapy. Ann Rheum Dis. 1975 Dec;34(6):471–478. doi: 10.1136/ard.34.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]




