Abstract
Microbial alkaline protease has become an important industrial and commercial biotech product in the recent years and exerts major applications in food, textile, detergent, and pharmaceutical industries. By immobilization of microbes in different entrapment matrices, the enzyme produced can be more stable, pure, continuous, and can be reused which in turn modulates the enzyme production in an economical manner. There have been reports in support of calcium alginate and corn cab as excellent matrices for immobilization of Bacillus subtilis and Bacillus licheniformis, respectively. This study has been carried out using calcium alginate, κ-carrageenan, agar-agar, polyacrylamide gel, and gelatin which emphasizes not only on enzyme activity of immobilized whole cells by different entrapment matrices but also on their efficiency with respect to their reusability as first attempt. Gelatin was found to be the best matrix among all with highest enzyme activity (517 U/ml) at 24 h incubation point and also showed efficiency when reused.
Keywords: Bacillus licheniformis, calcium alginate, cell immobilization, entrapment matrices, gelatin, microbial alkaline protease
INTRODUCTION
Protease enzyme plays the pivotal role in the hydrolysis of the peptide bonds that link amino acids to one another while forming a polypeptide chain. Amidst all the different types of proteases, alkaline protease is active from neutral to alkaline pH and is of maximal industrial interest. From the viewpoint of biotechnology, alkaline protease has its importance in pharmaceutical and medicinal industry, food industry, peptide synthesis in brewing and baking industry, detergent industry, leather and textile industry, and wastewater treatment.[1,2,3]
Micro-organisms were found to be an excellent source for production of alkaline protease than plants and animals as they exhibit better advantages such as broad biochemical diversity, simplicity in genetic manipulation, and feasibility in mass culture. Among many alkaline protease producing microbes, bacteria of Bacillus genus are active producers of extracellular alkaline proteases. They are industrially very useful specifically as commercial alkaline protease producers because of their high pH and thermal stability.[2,3]
Due to the wide range of industrial application of alkaline protease, different measures have been taken to reduce the production cost and increase its industrial utility. Immobilization of enzymes and whole cells has been an efficient tool for this purpose for years as it offers numerous advantages over free enzyme like increase in production rate, operational stability, possibility in reuse of enzyme, and recovery of final product free of enzyme contamination.[1,2,4] Depending on the materials used for entrapment, whole cells show modifications in increased mechanical, chemical, thermal resistance, and stability in changes in pH.[2,5] There have been reports where different Bacillus species like alkalophylic and thermophylic Bacillus have been immobilized in various substances like calcium alginate, polyacrylamide gel, gelatin, chitosan, corn cab, κ-carrageenan, etc.[2,3,4] Calcium alginate has been reported as a better matrix for immobilization of Bacillus subtilis by Mahto and Bose[2] and corn cab for B. licheniformis as reported by Maghsoodi et al.[3]
In this present study, we have reported the use of different entrapment matrices such as calcium alginate, κ-carrageenan, agar-agar, polyacrylamide gel, and gelatin to immobilize B. licheniformis isolated from poultry farm soil of 24 Parganas (West Bengal) for better production of alkaline protease and its reusability as first comparative approach. B. licheniformis is a mesophylic soil bacterium, mainly found in the poultry farm soil,[6] and also in feathers and breast of birds mainly hen, sparrow, and ducks. Besides, the prospect of reusability of the enzyme has also been investigated. Calcium alginate has been earlier successfully used to immobilize rifamycin oxidase in the form of Chryseobacterium species whole cells[5] and Candida antarctica Lipase B.[7]κ-carrageenan is a naturally occurring polysaccharide isolated from marine red algae.[8] It has been in extensive use for immobilization worldwide. It has been effectively employed in immobilization of Halobacterium sp. JS1 cells for the production of haloalkaliphilic protease[9] and lipase enzyme from Burkholderia cepacia.[8] Agar-agar is a polysaccharide having strong gel forming ability and shows no reactivity with protein.[10] It has been effectively used for immobilization of thermostable α-amylase extracted from soybean seeds[10] and pectinase isolated from B. licheniformis KIBGE-IG21.[11] Polyacrylamide gels have been in use for immobilization and entrapment for decades. It has a special characteristic of having all its charges embedded in the interior providing a negatively charged three dimensional network for enzyme entrapment.[12] It has also been used for immobilization of Halobacterium sp. JS1 whole cells[9] as well as alkaline phosphatase obtained from the Escherichia coli.[12] Recent studies has shown that gelatin cross linked with glutaraldehyde has been a good prospect for immobilizing enzymes like hyperthermostable β-amylase isolated from B. subtilis DJ5.[13] Earlier gelatin was used along with polyacrylamide for entrapment and pure invertase enzyme.[14]
MATERIALS AND METHODS
Materials
Media required for bacterial growth, Luria-Bertani (LB) broth and LB agar as well as Casein were procured from HiMedia. All the rest of the reagents and chemicals were brought from SRL.
Sample collection and bacterial culture
Soil samples were collected from the poultry farm of 24 Parganas region (West Bengal), crushed into powder and sieved to get rid of large pieces and sticks. 1% (w/v) soil sample was prepared by mixing it properly in 9 ml of double distilled autoclaved water. The soil solution was then serially diluted. The last dilution (1 × 10− 8) was chosen for culture. 100 μl of the solution was used for preparing a spread plate in LB agar. The plates were incubated at 37°C for 24 h.
Screening and identification of isolates
For successful screening of the microbes, the colonies grown on LB agar were then grown in two selective media, viz. Brilliance™ Bacillus cereus agar and HiCrome Bacillus agar.[15] Subsequently microscopic and biochemical analysis as par Bergey's manual[16] was done for further identification of the isolated microbe.
Protease production and enzyme assay
After screening and identification of the microbes, a loop full of colonies were transferred into 100 ml of LB broth in aseptic condition and grown for 24 h. 10 ml of the 24 h old LB broth culture was then transferred aseptically to a 250 ml flask containing a complex media having 6% glucose, 2% soybean meal, 0.04% CaCl2 and 0.02% MgCl2.[3] The culture was incubated for 48 h at 37°C under shaking condition at 150 rpm. Incubation was followed by centrifugation of the culture at 10,000 g for 10 min at 4°C. The cell pellet obtained was washed thoroughly with sterile 2% KCl solution followed by double distilled water. The cell mass was then suspended in sterile 0.85% saline solution.[5] This cell suspension was used later as inoculum for immobilization. The supernatant was further used for protease assay by following modified Folin and Ciocalteu method.[17]0.65% casein solution in 800 μl of 50 mm phosphate buffer (pH 9) was added as substrate to 200 μl of supernatant. The reaction mixture was incubated at 75°C for 10 min followed by addition of 5% trichloroacetic acid to stop the enzymatic reaction. The reaction mixture was centrifuged at 10,000 g for 15 min.[2] Absorbance was measured at 660 nm.[3] One unit enzyme activity was defined as the amount of enzyme that liberated 1 μg tyrosine/min under assay condition. The enzyme activity was measured using tyrosine solutions as standard.[18]
Immobilization of whole cells
Calcium alginate
The cell suspension was added to 2% sodium alginate solution in 1:1 ratio and vortexed for thorough mixing. The obtained solution was then added drop-wise to 30 ml of 0.2 M CaCl2 solution from a fair height. Colorless transparent gel beads of 2-5 mm were formed. They were allowed to stand for 30–60 min in the CaCl2 solution, followed by thorough washing in double distilled water and stored at −11°C for a maximum of 1-week.[5,7]
κ-carrageenan
Ten milliliter of 4% (w/v) κ-carrageenan solution was prepared by heating at around 80°C.[8] It was then cooled to around 45°C; 5 ml of cell suspension was added to it and mixed thoroughly by vortexing. This mixture was then added drop-wise to 2% of cold KCl solution for formations of beads.[19] The beads were then washed thoroughly in double distilled water stored in the refrigerator.
Agar-agar
A 4% (w/v) agar-agar solution was prepared in 25 mM sodium acetate buffer (pH 5.5) by heating at a temperature of 50°C.[10] After cooling to room temperature, 5 ml of cell suspension was mixed thoroughly with 10 ml of the agar-agar solution.[19] The solution was then poured on a flat bottom petridish and allowed to solidify. After solidification, the agar-agar block containing the cell suspension immobilized in it was cut into small equal sized cubes and washed properly with double distilled water and stored in fresh sterile double distilled water at 4°C for further studies.
Polyacrylamide gel
2.85 g of acrylamide, 0.15 g of bisacrylamide 10 mg ammonium phosphate and 1 ml of tetramethylethylenediamine was added to 10 ml of 0.2M sterile phosphate buffer (pH 7.0). To this solution, prechilled cell suspension was mixed at the ratio of 1:1. The solution was mixed thoroughly and poured onto a petridish and allowed to polymerize. The firm gel was then cut into equal sized small cubes and transferred to 0.2 M sterile phosphate buffer (pH 7.0) and kept in the refrigerator for 1 h for curing. The cubes were then washed properly and stored in double distilled water at 4°C.[20]
Gelatin
Ten milliliter of the solution was prepared by heating 10% (w/v) gelatin in 0.1 M phosphate buffer (pH 6.9) at 50°C.[13] Cell suspension was added to the gelatin solution at the ratio of 1:2, mixed and poured onto petridish followed by overlaying it with 10 ml of 5% glutaraldehyde and left for solidification at room temperature.[19] The gel was then cut into small equal sized cubes and washed thoroughly with double distilled water to remove the excess glutaraldehyde.[20] The cubes were then stored in the buffer at 4°C for future use.
Enzyme production and assay by immobilized whole cells
Instead of LB broth immobilized whole cells were added to 50 ml of complex media having 6% glucose, 2% soybean meal, 0.04% CaCl2 and 0.02% MgCl2 and incubated in shaking condition at 150 rpm for 48 h at room temperature. Rest of the extraction and enzyme assay procedure has already been discussed above. To study the reusability of immobilized whole cells, after incubation, the cell beads were washed and added to fresh complex media.
Statistical analysis
All the data obtained regarding the assay of enzyme production, and the reusability of immobilized whole cells are confirmed by carrying out independent experiments in triplicate and presented as mean ± standard error of the mean.[21]
RESULTS AND DISCUSSION
Screening and identification of the soil isolates
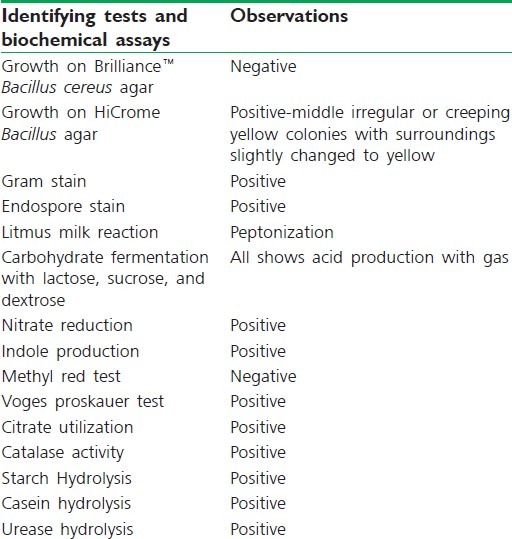
The soil isolates grown on LB agar were grown on Brilliance™ B. cereus agar and HiCrome Bacillus agar. Gram staining [Figure 1] and other biochemical assays [Figure 2] according to Bergey's manual[16] was used in identifying the soil isolate, namely B. licheniformis [Table 1] which was at par with Němečková et al.[15] and Vigneshwaran et al.,[6] respectively.
Figure 1.
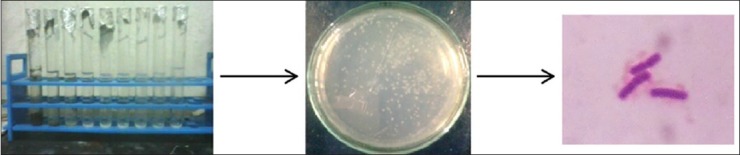
Isolation of Bacillus licheniformis from soil and its Gram staining
Figure 2.

Biochemical assays. (a) Nitrate reduction test. (b) Indole production test. (c) Methyl red test. (d) Citrate utilization test
Table 1.
Microscopic and biochemical assays for identification of soil isolates
Production of alkaline protease by the immobilized whole cells
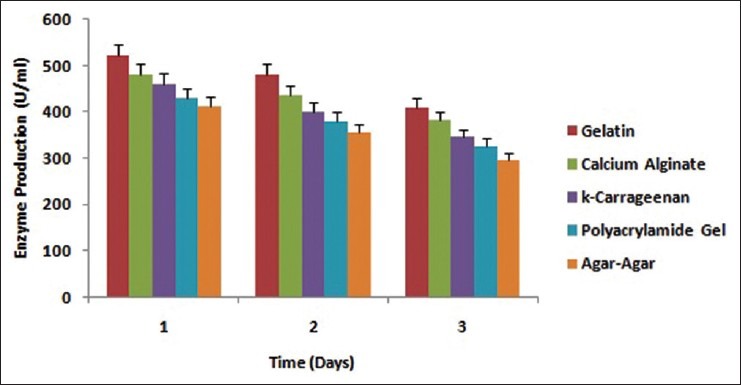
Immobilization of B. licheniformis was done with various entrapment matrices like calcium alginate, κ-carrageenan, agar-agar, polyacrylamide gel and gelatin for production of alkaline protease [Figure 3] with the sole interest to find out the best entrapment matrices with respect to enzyme activity as well as its reusability. Experimental studies reveal that there was a gradual increase in enzyme production with the immobilized whole cells which was at its maximum at 24 h incubation point. Comparing the enzyme production with the free cells, the yield of enzyme was more in case of immobilized cells of all the matrices. Excess incubation showed a decline in enzyme production [Figure 4]. When the same cells entrapped in matrices were washed and replenished with fresh media, a rise in enzyme production was observed. However, when the same approach was taken for free cells reverse results were obtained. This difference in results unveils the fact that immobilized whole cells are highly suitable for reuse [Figure 5]. Nevertheless, the production of the enzyme by reused immobilized whole cells after 24 h incubation was less than the fresh immobilized whole cells. With respect to the production of alkaline protease and reusability of immobilized whole cells, gelatin manifested better immobilizing ability than calcium alginate which has already been established as a better entrapment matrix for Bacillus sp. by different research groups.[1,2,4,5]
Figure 3.
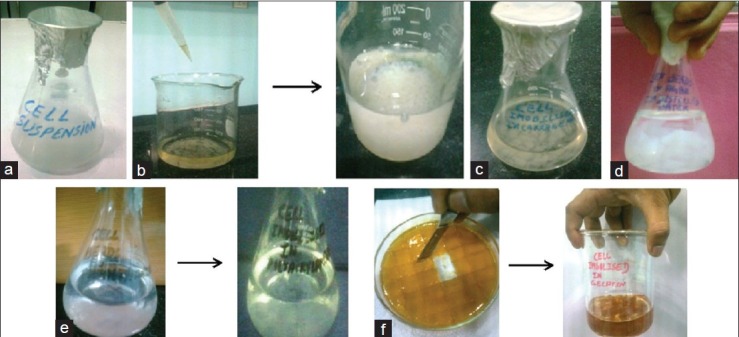
Immobilization ofBacillus licheniformiss whole cells. (a) Cell Suspension. (b) Formation of calcium alginate cell beads. (c) Cell immobilized in κ-carrageenan. (d) Cell immobilized in agar-agar. (e) Immobilization of cells in polyacrylamide gel. (f) Formation of gelatin cell beads
Figure 4.
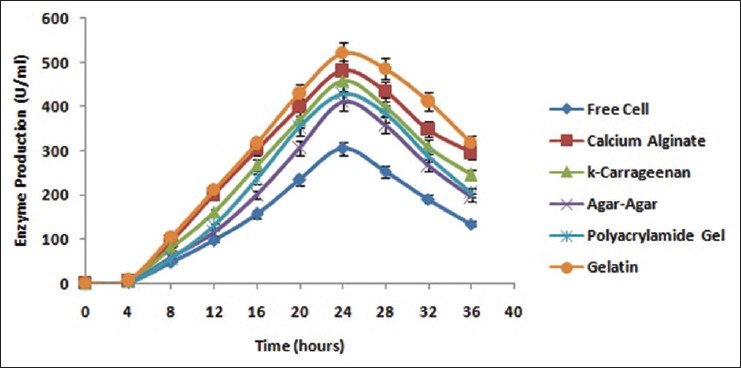
Time course profile of production of alkaline protease by Bacillus licheniformis immobilized in different entrapment matrices
Figure 5.
Decrease in production of alkaline protease of reused immobilized Bacillus licheniformis whole cells
CONCLUSION
The present study emphasizes on the production of alkaline protease by immobilized cells using various entrapment matrices like calcium alginate, κ-carrageenan, agar-agar, polyacrylamide gel, and gelatin. It also focuses on the degree of reusability of the immobilized cells compared to the free cells. The experimental data obtained indicate gelatin as a promising matrix for immobilization of B. licheniformis and optimum production of alkaline protease and its reusability when compared to calcium alginate. κ-carrageenan exhibited moderate ability, and agar-agar showed least ability towards immobilization of B. licheniformis and production of alkaline protease.
Footnotes
Source of Support: The authors are grateful to the home institute for providing space and resources to carry out this work
Conflict of Interest: Nil.
REFERENCES
- 1.Anwar A, Qader SA, Raiz A, Iqbal S, Azhar A. Calcium alginate: A support material for immobilization of proteases from newly isolated strain of Bacillus subtilis KIBGE-HAS. World Appl Sci J. 2009;7:1281–6. [Google Scholar]
- 2.Mahto RB, Bose KJ. Production of alkaline protease from Bacillus subtilis by different entrapment techniques. J Biochem Tech. 2012;4:498–501. [Google Scholar]
- 3.Maghsoodi V, Kazemi A, Nahid P, Yaghmaei S, Sabzevari MA. Alkaline protease production by immobilized cells using B. licheniformis. Sci Iran C. 2013;20:607–10. [Google Scholar]
- 4.Adinarayana K, Ellaiah P. Production of alkaline protease by immobilized cells of alkalophilic Bacillus sp. J Sci Ind Res. 2003;62:589–92. [Google Scholar]
- 5.Jobanputra AH, Karode BA, Chincholkar SB. Calcium alginate as supporting material for the immobilization of rifamycin oxidase from Chryseobacterium species. Biotechnol Bioinform Bioeng. 2011;1:529–35. [Google Scholar]
- 6.Vigneshwaran C, Shanmugam S, Kumar TS. Screening and characterization of keratinase from Bacillus licheniformis isolated from namakkal poultry farm. Researcher. 2010;2:89–96. [Google Scholar]
- 7.Zhang S, Shang W, Yang X, Zhang S, Zhang X, Chen J. Immobilization of lipase using alginate hydrogel beads and enzymatic evaluation in hydrolysis of ρ-nitrophenol butyrate. Bull Korean Chem Soc. 2013;34:2741–6. [Google Scholar]
- 8.Jegannathan KR, Seng CE, Ravindra P. Immobilization of lipase in κ-carrageenan by encapsulation – An environmental friendly approach. J Environ Res Dev. 2009;4:431–9. [Google Scholar]
- 9.Vijayanand S, Hemapriya J, Selvin J, Kiran S. Operational stability and reusability of Halobacterium sp. JS1 cells immobilized in various matrices for haloalkaliphilic protease production. Int J Microbiol Res. 2012;3:1–6. [Google Scholar]
- 10.Prakash O, Jaiswal N. Immobilization of a thermostable-Amylase on agarose and agar matrices and its application in starch stain removal. World Appl Sci J. 2011;13:572–7. [Google Scholar]
- 11.Rehman HU, Aman A, Zohra RR, Qader SA. Immobilization of pectin degrading enzyme from Bacillus licheniformis KIBGE IB-21 using agar-agar as a support. Carbohydr Polym. 2014;102:622–6. doi: 10.1016/j.carbpol.2013.11.073. [DOI] [PubMed] [Google Scholar]
- 12.González-Sáiz JM, Pizarro C. Polyacrylamide gels as support for enzyme immobilization by entrapment. Effect of polyelectrolyte carrier, pH and temperature on enzyme action and kinetics parameters. Eur Polym J. 2001;37:435–44. [Google Scholar]
- 13.Poddar A, Jana SC. Immobilization of hyperthermostable β-amylase from Bacillus subtilis DJ5 into gelatin film by glutaraldehyde crosslinking. Int J Pharm Bio Sci. 2011;2:77–86. [Google Scholar]
- 14.Emregul E, Sungur S, Akbulut U. Polyacrylamide-gelatine carrier system used for invertase immobilization. Food Chem. 2006;97:591–7. [Google Scholar]
- 15.Němečková I, Solichová K, Roubal P, Uhrová B, Šviráková E. Methods for detection of Bacillus sp. B. cereus, and B. licheniformis in raw milk. Czech J Food Sci. 2011;29:555–60. [Google Scholar]
- 16.Holt JG, Krieg NR, Sneath PH, Staley JT, Williams ST. Gram-positive cocci. In: Hensyl WR, editor. Bergey's Manual of Determinative Microbiology. 9th ed. 1994. pp. 527–58. [Google Scholar]
- 17.Folin O, Ciocalteu V. On tyrosine and tryptophan determinations in proteins. J Biol Chem. 1927;73:627–50. [Google Scholar]
- 18.Hadj-Ali NE, Agrebi R, Ghorbel-Frikha B, Nasri M. Biochemical and molecular characterization of a detergent stable alkaline serine-protease from a newly isolated Bacillus licheniformis NH1. Enzyme Microb Technol. 2007;40:515–23. [Google Scholar]
- 19.Veelken M, Pape H. Production of tylosin and nikkomycin by immobilized Streptomyces cell. Eur J Appl Microb Biotechnol. 1982;15:206–10. [Google Scholar]
- 20.Adinarayana K, Jyothi B, Ellaiah P. Production of alkaline protease with immobilized cells of Bacillus subtilis PE-11 in various matrices by entrapment technique. AAPS PharmSciTech. 2005;6:E391–7. doi: 10.1208/pt060348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Show S, Banerjee S, Chakraborty I, Sikdar M. In vitro comparison between antibacterial activity of Catharanthus roseus and Nyctanthes arbortristis on antibiotic resistant Staphylococcus aureus strain. Indo Am J Pharm Res. 2014;4:1487–93. [Google Scholar]


