Abstract
Being key health care professional, physicians, pharmacist and nurses have immense responsibility in reporting adverse drug reaction (ADR). Therefore, the study objective was to evaluate the knowledge, attitude and practices (KAP) toward pharmacovigilance and ADRs of postgraduate students of our institute. A cross-sectional questionnaires based study was carried out in postgraduate students of the clinical department at tertiary care hospital attached with Govt. Medical College, Vadodara, Gujarat (India). A total of 22 questionnaires about KAP toward ADRs and pharmacovigilance were developed and peer viewed of all questionnaires by expert faculties from our institute. We were contacted directly to postgraduate students of respective clinical department; questionnaires were distributed and taken back after 30 min. The filled KAP questionnaires were analyzed in question wise and their percentage value was calculated by using Microsoft Excel spreadsheet. Postgraduate residents (n = 101) from different clinical departments were enrolled in the study. Average 34.83% correct and 64.08% incorrect knowledge about ADRs and pharmacovigilance and an average 90.76% students were agreed to reporting ADRs is necessary, mandatory and increased patient's safety. Only 7.92% of postgraduate doctors were reported ADR at institute or ADR reporting center. We concluded that postgraduate students have a better attitude toward reporting ADRs, but have lack of knowledge and poor practices of ADRs. The majority of postgraduate students were felt ADR reporting and monitoring is very important, but few had ever reported ADRs because of lack of sensitization and knowledge of pharmacovigilance and ADR.
Keywords: Adverse drug reaction, attitude and practices study, knowledge, pharmacovigilance, postgraduate student
INTRODUCTION
Adverse drug reactions (ADRs) are global problems and affects majority both children and adults causing both morbidity and mortality[1,2,3,4] and also a major impact on public health.[5] The success of a pharmacovigilance program depends upon the active involvement of the healthcare professionals such as doctors, pharmacist, nurses.[6,7] Being the key healthcare professionals, providing information on suspected ADRs is as much a moral duty for the DOCTOR as other aspects of patient care.[8] Spontaneous ADR reporting is important to monitor known and unknown adverse effects of medicines.[9] Furthermore, spontaneous reporting of ADRs has played a most important role in the detection of serious and unusual ADRs during marketing of the drug in actual practicing in the market. This has led to the withdrawal of many drugs in the past such as rofecoxib, cisapride, terfenadine, etc.[7] To transform the pharmacovigilance activity into practices for enhancing the safety of patient and more ADR monitoring center are being set up across the country under pharmacovigilance program of India (PvPI).[10]
It can also help to prevent the occurrence of new medicine tragedies and can improve the safety profile of pharmaceutical products.[11] ADR reporting does not currently appear to be considered a part of routine professional practice by health care professional.[12]
The ADR reporting rate in India is below 1% compared to the worldwide rate of 5%.[13] One of the reasons for low reporting rate in India may be a lack of knowledge and sensitization towards pharmacovigilance and ADR amonghealth care professional. The study also showed that the average cost involved in treating these ADRs was INR 900/- (USD 15$) per patient.[14]
With adequate knowledge and practices of pharmacovigilance and ADR reporting in India, there will be not only increasing reporting of ADR, but also reducing incidence rate as well as health care cost of patient and also banned harmful drug to the patient in actual clinical practices.
Pharmacovigilance program of India
The PvPI was launched with a broad objective in patient safety for more than one billion people of India. In July, 2010, the Central Drug Standard Control organization, New Delhi has initiated a nationwide pharmacovigilance program under aegis of Ministry of health and Family welfare, Government of India with All India Institute of Medical Sciences (AIIMS), New Delhi as a National Coordinating Center (NCC) to monitor ADR.[15]
For more effective way to implementation of this program, recently NCC shifts from AIIMS, New Delhi to the Indian Pharmacopoeia Commission, Ghaziabad, (UP) in April, 2011 under aegis of Uppsala Monitoring Center-World Health Organization (UMC-WHO). The advantage of pharmacovigilance program includes the detection of medicines of substandard quality as well as prescribing pattern and administration errors.
The (UMC, WHO), Sweden is maintaining the international database of ADR reports received ADRs report data from several national pharmacovigilance centers of different countries. However, still, it is estimated that only 6–10% of all ADRs are reported in all over the world. Although, India is one of participating in national pharmacovigilance program, but its contribution to UMC database is very little. Now a days, participation is increased but not up to mark. This program is essential due to the absence of a vibrant ADR monitoring system and also lack of a reporting culture among health care professional in India.[16]
Therefore, the study was planned and primary objective was to evaluate the knowledge, attitude and practices (KAP) toward pharmacovigilance and ADRs reporting in postgraduate students of tertiary care hospital, Vadodara, Gujarat because postgraduate students are the resident doctor to observe the patient 24 h while the patient is admitted in the hospital.
MATERIALS AND METHODS
Study setting
The study was conducted at SSG Hospital attached with Govt. Medical College, Vadodara, Gujarat, a tertiary care teaching hospital in West India between months of July- and August-2014.
Type of study
It was a cross-sectional, anonymous, KAP questionnaire study.
Sample size
Convenient sampling method was used in which all postgraduate students who are pursuing postgraduation in clinical subjects were enrolled in the study.
Before study, The KAP questionnaires toward pharmacovigilance and ADRs were developed and peer viewed of all questions by expert faculties from pharmacology and different clinical department of our institute. The questionnaires were semi-structured, predesigned, pretested and validated used for data collection as a research tool.[17] Few changes were made as per our study requirement and the finalized KAP questionnaires consisted of 22 questions: Q. 1–10, 15, 20, 22, Q.11–14, Q.16–19, 21 was KAP aspects of pharmacovigilance and ADRs reporting, respectively.
Process
All study participants were contacted directly in their respective department, explained the purpose of the study and distributed the questionnaires, given 30 min to fill them and hand it back. Any clarification needed in understanding the questionnaires and additional time to filled form was provided. Those postgraduate students were busy at that moment was requested to return back the duly filled form within 1-week. The KAP survey questionnaire was analyzed, question-wise and their percentage value was calculated with the help of Microsoft excel spread sheet in MS Office 2007.
RESULTS
In our study, postgraduate resident doctors (n = 101) were enrolled from different clinical department. Out of them 1st year (n = 40), 2nd year (n = 30), 3rd year (n = 31) were filled form sent back it [Table 1].
Table 1.
Demographic details of postgraduate students (n=101)
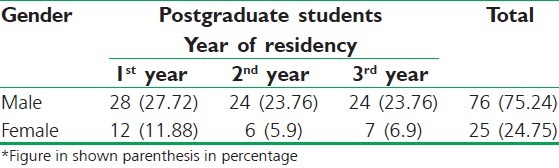
Out of the total (n = 101) postgraduate students, 76 males, and 25 females residents doctors filled questionnaires form and sent back.
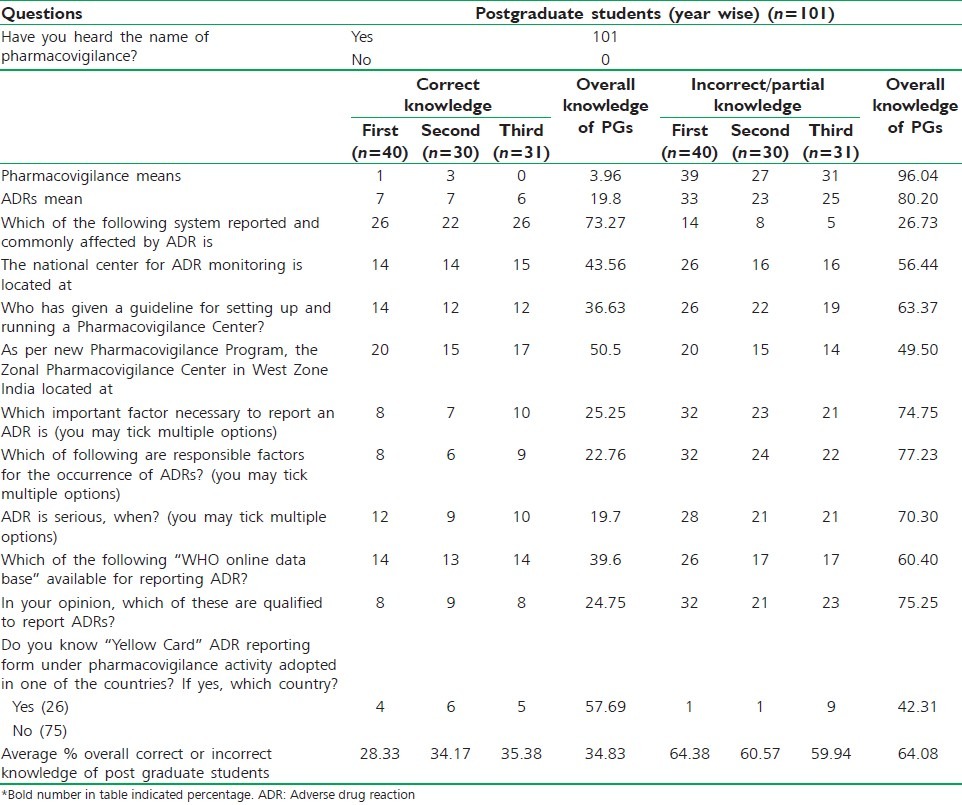
An average 28.33%, 34.17%, 35.38% of 1st, 2nd and 3rd year postgraduate students respectively, have correct knowledge about the pharmacovigilance and ADRs [Table 2]. Remaining 64.38% 1st year, 60.57% 2nd year, 59.94% 3rd year have incorrect/no knowledge. Overall average 34.83% and 64.08% student does of all year have correct and incorrect/no knowledge about pharmacovigilance and ADRs. Only 26 (25.74%) heard about “yellow card” ADR reporting system, of them only 15 (14.85%) students know the correct answer.
Table 2.
Correct and incorrect knowledge of postgraduate students about pharmacovigilance and ADRs (year wise) (n=101)
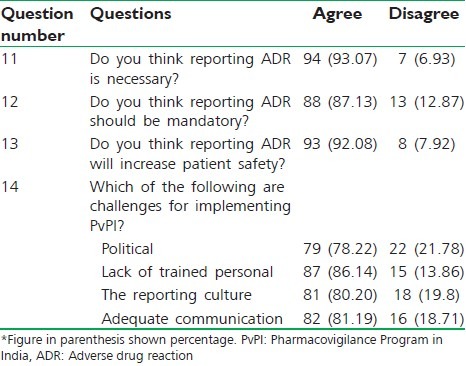
As shown in Table 3, 93.07%, 87.13%, 92.08% of postgraduate students agreed that reporting ADRs is necessary, mandatory, increased safety of patient, respectively. About 86.14% of postgraduate students agreed that lack of training of ADR reporting is challenging factor for implementing pharmacovigilance program in India.
Table 3.
Postgraduate students attitude toward pharmacovigilance and ADRs reporting (n=101)
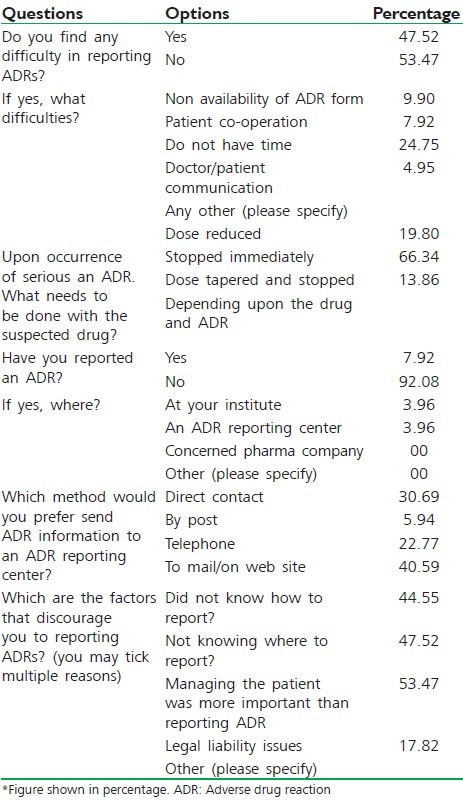
In Table 4, 47.52% of students were find difficulties during reporting ADRs, of them 24.75% of students do not have time to report ADRs. 66.34% of postgraduate students have practices like stop drug immediately when serious ADRs occurred. Only 7.02% student report ADRs and 92.08% do not report ADRs in any way. 40.59% of students were preferred to report ADRs via mail/on web site. 53.47% of postgraduate doctors believed that managing patient more important than reporting ADRs, whereas 44.55% and 47.52% of postgraduate student do not know how to report or where to report, respectively.
Table 4.
Practices of postgraduate students toward ADRs (n=101)
DISCUSSION
Reporting ADRs is an essential component of pharmacovigilance program. Spontaneous reporting system is important method for reporting ADR and also new ADR of new drug.
In the present study, we observed that postgraduate resident doctors were a lack of correct knowledge about ADRs reporting and pharmacovigilance in individual year (overall average correct knowledge 34.83%, incorrect knowledge 64.08%, [Table 2]). A study conducted by Ramesh and Parthasarathi[18] that stated doctors were less aware or lack of knowledge of national and international pharmacovigilance program. In some other study or the literature noted, a lack of time and knowledge about ADRs is often considered to be a cause of underreporting.[19,20,21]
In the present study, 93.07%, 87.13% and 92.08% of postgraduate students agreed that reporting ADRs is necessary, mandatory, increased safety of patient, respectively. In another study[22] found that ADR reporting was considered to be important by 97.3% of the respondents. The need to improve patient safety (28.8%) and the detection of new ADRs (24.6%) were the common reasons cited for reporting. Furthermore, 86.14% of postgraduate students agreed that lack of training and trained doctors is most challenges factor for implementing pharmacovigilance program in India. In other study[22] results had shown the good attitude for ADR reporting among postgraduate students but real scenario, no practices of ADRs reporting. A study at Mumbai,[23] showed that high knowledge but poor practices for ADRs reporting in doctors. But in the present study, not only poor practices but little knowledge of ADR reporting was found [Tables 2 and 4].
In the present study shown that while right attitude for ADR reporting existed among most PGs, but the actual practice of ADR reporting was lacking. Another study conducted at Mysore,[24] and Muzzafarnagar[25] has shown high Knowledge but poor practice for ADR among prescribers. In contrast our study has found not only poor practice, but also inadequate knowledge regarding ADR reporting. The average knowledge score was low (34.08%) and that indicating that there is still require to educate and sensitize about knowledge and importance of ADR reporting and pharmacovigilance among the doctors who are in training phase (postgraduate resident doctors).
An interesting observation was that 12.87% of the respondents did not think that reporting ADRs was mandatory, 7.92% of students disagree with ADR reporting helps to improve patient safety and 17.82% of students were thought towards legal liability issue. The study done at Spain,[26] where the major problem, reporting of ADRs were identified to be difficulty in diagnosis of ADRs, lack of knowledge regarding the ADR reporting system, clinical workload on the doctors, a concern for patient confidentiality and possible legal implications of reporting.
The result of the present study showed that the major factors that discourages the doctors of reporting ADRs were did not know how (44.55%), where (47.52%) to report and managing patient more important than reporting ADRs (53.57%) and less to legal liability issue (17.82%). In one study[17] in residents, found that lack of knowledge on how (68%) and where (70%) to report the ADRs were the major factors that discouraged reporting. In this study, greater percentage of residents responded that they did not know how to report it.
In the present study, 47.50% of postgraduate doctors found difficulties to report ADRs, of them non availability of form (9.90%), do not have time (24.55%), doctor-patient communication (4.95%), patient co-operation (7.92%). In another study,[22] lack of easy access to ADR reporting form (49.2%) was major factor for discouraged reporting.
The study conducted by Chatterjee et al.[23] which stated that clinical negligibility or underreporting of ADRs from clinicians due to lack of time and no or little knowledge about types of reactions to be reported.
Even as ADR reporting was considered to be important by a large majority of the participants but the actual practices of ADR reporting was very low. In our study, 7.92% of the respondents stated that they had reported an ADR previously. Similarly, the study at Mumbai[22] also cited similar findings of under-reporting of ADR to any of the national ADR monitoring centers (2.9%) in spite of 90% of the respondents considering it important.
A study from Northern India,[27] reported that the KAP regarding ADR monitoring was low and the knowledge scores needed an improvement and update KAP about ADR and pharmacovigilance. A survey among medical residents in France[28] showed that the majority of them had a lower knowledge regarding pharmacovigilance. A study from Italy[29] reported that doctors had little information concerning ADRs and ADR reporting systems. A study from India[30] also identified that the awareness about pharmacovigilance program and the knowledge of ADR reporting were very low among the doctors. In our study, similar results were found out. These findings suggest the need for interventions to improve the KAP of the healthcare professionals.
CONCLUSION
We concluded from this study, the postgraduate resident doctors had a relatively better attitude but lack of knowledge and practices towards ADRs and pharmacovigilance. The majority of the PGs are felt ADR reporting and monitoring to be important, but only a few had ever reported an ADR. Lack of motivation and training toward ADR reporting and pharmacovigilance discourages them from reporting. The findings of the study suggest that there is need for continuous education and sensitization regarding pharmacovigilance and ADR reporting system for residents and improving the ongoing pharmacovigilance activities in our hospital.
Limitation of study
Reporting ADRs is moral duty and other aspect of patient care but present study, we were enrolled only postgraduate resident doctors, not other health care professionals like actually practicing doctor (private as well as government), nurses who are continuous keep in touch with the patient because study already conducted by other author. We are evaluating knowledge of pharmacovigilance and ADRs in individual year wise students (e.g. 1st year, 2nd year, 3rd year) and in other study they evaluate cumulative knowledge of all students' neither means nor separately year-wise.
Recommendation
The study results suggest that there is establishment of one separate department of pharmacovigilance under pharmacology department and continuous keep in touch with clinical doctors, also watch drug reaction. There is also organizing sensitization workshop on pharmacovigilance and ADR at least 2 times in a year.
ACKNOWLEDGMENT
I am very thankful to all postgraduate students for their participated in study and help to successful and meaningful completion of this study.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 2.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ. 2004;329:15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oshikoya KA. Adverse drug reaction in children: Types, incidence and risk factors. Niger J Paediatr. 2006;33:29–35. [Google Scholar]
- 4.Martínez-Mir I, García-López M, Palop V, Ferrer JM, Rubio E, Morales-Olivas FJ. A prospective study of adverse drug reactions in hospitalized children. Br J Clin Pharmacol. 1999;47:681–8. doi: 10.1046/j.1365-2125.1999.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin Pharmacol. 2009;11(9):14. doi: 10.1186/1472-6904-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad SR. Adverse drug event monitoring at the Food and Drug Administration. J Gen Intern Med. 2003;18:57–60. doi: 10.1046/j.1525-1497.2003.20130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969-2002: The importance of reporting suspected reactions. Arch Intern Med. 2005;165:1363–9. doi: 10.1001/archinte.165.12.1363. [DOI] [PubMed] [Google Scholar]
- 8.Faich GA. Adverse-drug-reaction monitoring. N Engl J Med. 1986;314:1589–92. doi: 10.1056/NEJM198606123142427. [DOI] [PubMed] [Google Scholar]
- 9.Meyboom R, Osslen S, Thorogood M. Teaching pharmacovigilance. In: Mann RD, Andrews EB, editors. Pharmacovigilance. Chichester: John Wiley and Sons; 2002. pp. 505–8. [Google Scholar]
- 10.Pharmacovigilance programme of India 2010. CDSCO, Ministry of Health and Family Welfare, Government of India. 2010. [Last accessed on 2014 Nov 22]. Available from: http://www.cdsco.nic.in/pharmacovigilance.htm .
- 11.Hartigan-Go K. Pharmacovigilance and the pursuit of rational drug use: The Philippines experience. Uppsala Rep. 2001;14S:1–4. [Google Scholar]
- 12.Green CF, Mottram DR, Brown AM, Rowe PH. Attitudes of hospital pharmacists to adverse drug reactions and the ‘yellow card’ scheme: A qualitative study. Int J Pharm Pract. 1999;7:247–55. [Google Scholar]
- 13.Amrita P, Kharbanda B. Knowledge, attitude and skills of nurses of Delhi towards adverse drug reaction reporting. Indian J Pharm Pract. 2012;5:45–51. [Google Scholar]
- 14.Ramesh M, Pandit J, Parthasarathi G. Adverse drug reactions in a south Indian hospital – Their severity and cost involved. Pharmacoepidemiol Drug Saf. 2003;12:687–92. doi: 10.1002/pds.871. [DOI] [PubMed] [Google Scholar]
- 15.Pharmacovigilance Programme in India (PvPI)-Indian scenario. [Last accessed on 2014 Dec 07]. Available from: http://www.ipc.gov.in/PvPI/Pv_home.html .
- 16.Feely J, Moriarty S, O'Connor P. Stimulating reporting of adverse drug reactions by using a fee. BMJ. 1990;300:22–3. doi: 10.1136/bmj.300.6716.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radhakrishnan R, Vidyasagar S, Varma DM. An educational intervention to assess knowledge attitude practice of pharmacovigilance among Health care professionals in an Indian tertiary care teaching hospital. Int J Pharm Tech Res. 2011;3:678–92. [Google Scholar]
- 18.Ramesh M, Parthasarathi G. Adverse drug reactions reporting: Attitudes and perceptions of medical practitioners. Asian J Pharm Clin Res. 2009;2:10–4. [Google Scholar]
- 19.Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2009;32:19–31. doi: 10.2165/00002018-200932010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Eland IA, Belton KJ, van Grootheest AC, Meiners AP, Rawlins MD, Stricker BH. Attitudinal survey of voluntary reporting of adverse drug reactions. Br J Clin Pharmacol. 1999;48:623–7. doi: 10.1046/j.1365-2125.1999.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasford J, Goettler M, Munter KH, Müller-Oerlinghausen B. Physicians' knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epidemiol. 2002;55:945–50. doi: 10.1016/s0895-4356(02)00450-x. [DOI] [PubMed] [Google Scholar]
- 22.Desai CK, Iyer G, Panchal J, Shah S, Dikshit RK. An evaluation of knowledge, attitude, and practice of adverse drug reaction reporting among prescribers at a tertiary care hospital. Perspect Clin Res. 2011;2:129–36. doi: 10.4103/2229-3485.86883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee S, Lyle N, Ghosh S. A survey of the knowledge, attitude and practice of adverse drug reaction reporting by clinicians in eastern India. Drug Saf. 2006;29:641–2. doi: 10.2165/00002018-200629070-00009. [DOI] [PubMed] [Google Scholar]
- 24.Gupta P, Udupa A. Adverse drug reaction reporting and pharmacovigilance: Knowledge, attitudes and perceptions among resident doctors. J Pharm Sci Res. 2011;3:1064–9. [Google Scholar]
- 25.Ghosh S, Ali S, Chhabra L, Prasad C, Gupta A. Investigation of attitudes and perception of medical practitioners on adverse drug reaction reporting - A pilot study. Pharma Res. 2010;3:1–9. [Google Scholar]
- 26.Vallano A, Cereza G, Pedròs C, Agustí A, Danés I, Aguilera C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60:653–8. doi: 10.1111/j.1365-2125.2005.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehan HS, Vasudev K, Tripathi CD. Adverse drug reaction monitoring: Knowledge, attitude and practices of medical students and prescribers. Natl Med J India. 2002;15:24–6. [PubMed] [Google Scholar]
- 28.Graille V, Lapeyre-Mestre M, Montastruc JL. Drug vigilance: Opinion survey among residents of a university hospital. Therapie. 1994;49:451–4. [PubMed] [Google Scholar]
- 29.Cosentino M, Leoni O, Banfi F, Lecchini S, Frigo G. Attitudes to adverse drug reaction reporting by medical practitioners in a Northern Italian district. Pharmacol Res. 1997;35:85–8. doi: 10.1006/phrs.1996.0138. [DOI] [PubMed] [Google Scholar]
- 30.Bharathan B, Raju N. A Survey about the Knowledge, Attitude and Practice of Adverse Drug Reaction Reporting Among Doctors in Bangalore City. Sixth Annual Conference of the Society of Pharmacovigilance (India); November 11-12, 2006; Kurupanidhi College of Pharmacy, Bangalore, India. [Google Scholar]


