Abstract
Context:
Identification of sex from skeletal remains is one of the important forensic considerations. Discriminant function analysis is increasingly used to determine the sex from skeleton.
Aims:
To develop discriminant function to determine sex from mandible in a Central Indian population.
Settings and Design:
This was a prospective study done at the Department of Anatomy.
Materials and Methods:
The mandibles used in the present study were from the museum specimens. Only 82 adult mandibles (55 male and 27 female) that had been preserved were selected. Ten mandibular parameters were measured.
Statistical Analysis Used:
Statistical analysis was conducted using Statistical Package for Social Sciences (SPSS) for Windows, version 16. The level of statistical significance was set at P < 0.05.
Results:
Using stepwise discriminant function analysis, only six variables were selected as the best discriminant between sexes, with the projection length of corpus mandibulae being the most dimorphic. It was observed that sex classification accuracy of the discriminant functions ranged from 57.3 to 80.5% for the individual variables, 81.7% for the stepwise method, and 85.4% for the direct method.
Conclusion:
The results of the study show that mandibles can be used for determining sex and the results are comparable with other similar studies. The studied mandibular variables showed sexual dimorphism with an accuracy comparable with other skeletal remains, next to cranium and pelvis.
Keywords: Discriminant function, forensic, identification, mandible, sex determination
Introduction
Identification of sex from skeletal remains is one of the important forensic considerations because it eliminates approximately half the population from the view of examiner.[1] Sex determination is done either by assessing the morphological features or by doing osteometric measurements.[2] The accuracy of sex estimation depends mainly on the degree of sexual dimorphism exhibited by the skull, pelvis, and long bones.[3] Assessment of sex by morphological features is subjective, and many subtle peculiarities may be missed or misinterpreted by an inexperienced examiner. As a result, more reliance is placed on osteometric measurements and statistical techniques. The metric approach or statistical techniques using quantitative analysis had been performed on other skeletal elements such as scapula, patella, calcaneus, or fragmentary skeletal remains.[4,5,6,7,8,9,10]
Discriminant function analysis is increasingly used to determine the sex from skeleton. The method is a reliable one, reduces the examiner's subjective opinion, and is reproducible. But the results obtained from discriminant function analysis for determination of sex are population specific and, thus, the same result cannot be applied to other geographical areas due to population differences.[1] Therefore, there is a need for development of population-specific discriminant function. The present study is an attempt to develop discriminant function to determine sex from the mandible in a Central Indian population.
Materials and Methods
The mandibles used in the present study were from the museum specimens of Department of Anatomy, Government Medical College. The mandibles were in different states of preservation. Only 82 adult mandibles of known sex (55 male and 27 female) that had been preserved were selected. For the present study, 10 mandibular measurements were taken to determine the sex. The measurements consisted of the following:
Bicondylar breadth: It is a measure of the straight distance between two condylia lateralia (BB). It was measured with a sliding caliper Figure 1
Coronoid breadth of the lower jaw: It is a measure of the distance between two coronia (CB). It was measured with a sliding caliper Figure 2
Bigonial breadth: It is a measure of the straight distance between two gonia (BGB). It was measured with a sliding caliper
Projection length of corpus of mandible: It is a measure of the straight distance from the posterior margin of the chin to the tangent drawn at the two gonia (PLCM). It was measured with a scale
Symphyseal height: It is a measure of the straight distance between infradentale and the lowest point on the lower margin of the mandible at the level of symphysion (SH). It was measured with a sliding caliper Figure 3
Height of the mandibular corpus: It is a measure of the distance from the alveolar margin to the lower margin of the mandible in the level of mental foramen perpendicular to the base (HMC). It was measured with a sliding caliper Figure 4
Corpus thickness of mandibular body: It is a measure of the maximum thickness in the plane of foramina mentale perpendicular to the longitudinal axis of the body (CTMB). It was measured with a sliding caliper
Gonion condylar height: It is the distance between the highest points on the mandibular capitulum measured by drawing a perpendicular to the line extending from the base of mandible (GCH). It was measured with a scale
Minimum breadth of ramus: It is a measure of the minimum breadth of the ramus taken at right angle to the height (MNBR). It was measured with a sliding caliper Figure 5
Mandibular arch length: It is a measure of the distance between gonion and the lowest point on the symphysis menti from the outer surface (MAL). It was measured with a thread.
Figure 1.

Measurement of bicondylar breadth using sliding caliper
Figure 2.

Measurement of coronoid breadth using sliding caliper
Figure 3.

Measurement of symphyseal height using sliding caliper
Figure 4.

Measurement of height of the mandibular corpus using sliding caliper
Figure 5.
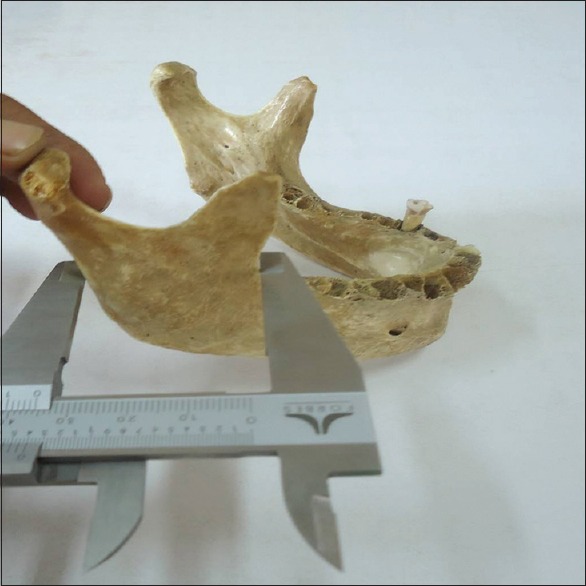
Measurement of minimum breadth of ramus using sliding caliper
A manual spreading caliper [Forbes Mumbai 0-200 mm/0.8″ (0.02 mm/0.001″)] with fine adjustments) was used. All measurements were done in centimeters and recorded to the nearest millimeter. All parameters were measured on both sides of the mandible, but because there was no statistically significant difference between left and right sides, only the measurements taken on the right side were included for analysis. Statistical analysis was conducted using Statistical Package for Social Sciences (SPSS) for Windows, version 16. The level of statistical significance was set at P < 0.05. Firstly, the general descriptive statistics for the mandibular measurements were obtained. Student's t-test was used to establish whether significant differences existed (P < 0.05) between each male and female measurement. Then the demarking point for each variable was calculated. The demarking point is the average of the mean values for each sex. Secondly, sexual dimorphism ratios were calculated to assess the general pattern of dimorphism. The sexual dimorphism ratio was calculated as: (male mean/female mean) × 100. Finally, stepwise and direct discriminant function analyses were -obtained to find one or more functions that can discriminate between the sexes.
Results
Table 1 summarizes the descriptive statistics for both sexes. The table also shows the sexual dimorphism ratio and independent sample t-test for male and female samples. It is observed that males were larger in all dimensions than females, exhibiting sexual dimorphism. However, the t-test showed high significance (P < 0.001) for the variables BGB, CTMB, GCH, and MNBR than the other variables. The sexual dimorphism indices for all variables were greater than 100 and indicate that males had greater mandibular measurements than females.
Table 1.
Descriptive statistics (in cm) and sexual dimorphism ratio of the mandible

For all variables, the within-group correlation matrices were generated and are shown in Table 2. Variables showing strong and positive correlations are shown in the table with asterisks. Variables CB and BB (0.654), BB and CB (0.654), BB and BGB (0.59), MAL and PLCM (0.66), HMC and SH (0.83), SH and HMC (0.83), and PLCM and MAL (0.66) exhibited strong and positive correlation.
Table 2.
Within-group correlation matrices for the analyzed variables

Stepwise discriminant function analysis (function 1) was developed for all variables and is presented in Table 3. The variable PLCM was found to be most dimorphic followed by CTMB, HMC, CB, SH, and BB. Accordingly, using these six variables, another discriminant function (function 2) was developed and is presented in Tables 4 and 5.
Table 3.
Stepwise discriminant function analysis for sex determination from mandible

Table 4.
Unstandardized and standardized discriminant function coefficients, structure matrix, centroids, and constant of best six variables

Table 5.
Eigen value, canonical correlation, Wilks' lambda, Chi-square, and significance level for the six best variables

Direct discriminant function analysis of all variables (function 3) was generated and is shown in Tables 6 and 7. The canonical correlation of 0.74 and Wilks' lambda of 0.451 were found when all variables were used with high significance (P < 0.001). Multivariate and cross-validation classification using “leave-one-out” classification method was used for all the calculations. Table 8 shows the classification accuracy of the original and cross-validated samples for functions 1, 2, and 3.
Table 6.
Unstandardized and standardized discriminant function coefficients, structure matrix, centroids, and constant for direct discriminant function

Table 7.
Eigen value, canonical correlation, Wilks' lambda, Chi-square, and significance level for the direct discriminant function

Table 8.
Classification accuracy of the original and cross-validated samples in various functions

By using stepwise analysis (function 1), it was noted that BB alone can classify the sex in 75.6% cases, BGB in 70.7% cases, SH in 64.6% cases, CTMB in 72% cases, GCH in 78% cases, MNBR in 79.3% cases, and MAL can classify the sex in 80% cases. Direct analysis for the best six variables (function 2) showed an average accuracy of 81.7%. On direct analysis by using all variables (function 3), it was possible to identify the sex in 85.4% cases. The average accuracy for cross-validated sex classification for function 3 was 82.9%, and this value is nearly similar to the accuracy obtained by using all 10 variables. Thus, the accuracy obtained by using single variable would be less than the accuracy obtained by combined use of all variables or by the direct analysis of the best six variables.
In case of a damaged or incomplete mandible, sex can be determined by using single variable by comparing the specific dimension of the mandible with the demarking point [Table 3]. While using demarking point, a higher value indicates male and a lower value indicates female. Thus, if a single variable is used, the sex determination accuracy varies from 57.3% (PLCM) to 80.5% (MAL).
Discussion
Skull and pelvis are the exclusively studied bones for determination of sex. Although mandible is a part of skull, it is not investigated as vigorously as the rest of the cranium.[11] Sex differences in the mandible have been described based on traditional morphological and features or statistical analysis of metrical system. However, in recent times, Franklin et al., have tried to utilize the principles of geometric morphometric method and data were analyzed using specific software and three-dimensional configuration.[12] While the study appears modern and valuable, it requires highly technical and expensive morphometric equipment, and therefore, the results are less helpful at most of the forensic or anthropologic centers. Consequently, it is imperative to use the conventional morphological or anthropometric measurements to arrive at a conclusion.
Many morphological features such as robustness of the mandible, ramus flexure, gonial eversion, square shape of chin, etc., had been described by many researchers, but unlike skull, determination of sex from isolated mandible poses problems even for an experienced examiner.[11,12,13,14] Few studies describing the discriminant function analysis of mandibles are available.[2,15,16,17,18,19] But due to population specificity of discriminant function, the results obtained in one area cannot be applied to other area.
Considering the Indian population, some studies were done to determine sex from various skeletal elements with different degrees of accuracy, such as cranium,[20] sternum,[21] clavicle,[22] hip bone,[23] humerus,[24] radius,[25] ulna,[26] femur,[27] tibia,[28] fibula,[29] and tarsal bones.[30] However, discriminant function for the determination of sex from mandible has not been derived specifically for this region.
In the present study, 10 mandibular variables were examined. All the mandibular measurements exhibited sexual dimorphism. But the variables BGB, CTMB, GCH, and MNBR showed statistically significant difference (P < 0.001). To ensure the reliability and validity of the measurements, intra-observer errors were assessed and they showed good reliability. Considering the sexual dimorphism ratios, the variables GCH, MNBR, SH, CTMB, HMC, BGB, MAL, and BB showed high index value, with GCH being the highest with a value of 122.02. By the stepwise method, six best variables were selected. These variables were PLCM, CTMB, HMC, CB, SH, and BB, with their respective Wilks' lambda being 0.976, 0.936, 0.929, 0.901, 0.867, and 0.807, respectively. Amongst these variables, PLCM was found to be most dimorphic. The accuracy of sexing a mandible while using single variable varies from 57.3% (PLCM) to 80.5% (MAL). Combined use of best six variables yields an accuracy of 81.7%, while direct discriminant function analysis using all variable gives 85.4% accuracy.
Inclusion of demarking point is one of the features of the present study. It can be noticed from the calculation of mean and mean ± standard deviation of the variables that the minimum and maximum ranges of males were higher than those of females [Table 1]. Therefore, statistically one can fix whether the given sample is of male or female by comparing with the stated dimension and referring the demarking point. This parameter is important from a forensic or archaeological point of view, especially if the presented mandible is incompleteone, mutilated, or badly preserved.
The results obtained in the present study are comparable with other studies. Hanihara had used four mandibular variables and noted 85% accuracy while studying the Japanese mandibles.[15] While studying the mandibles of American Whites and Blacks with eight mandibular measurements, Giles found that sexing of mandible was possible in 84% cases.[14] While using Japanese cranium (including mandible), Iscan et al., studied 11 variables and found an accuracy of 84.1% (cranium and mandible).[16] While using three variables of South African Whites mandible, Steyn et al., found 81.5% accuracy.[17] While utilizing 18 mandibular measurements from two Croatian archaeological sites, Vodanovic et al., found 92.06% accuracy.[2] Dayal et al., studied six mandibular measurements of South African Blacks and noted that average accuracy for sexing varies from 80 to 85%.[18] While studying the indigenous South African mandibles, Franklin et al., employed nine linear measurements obtained from mathematically transformed three-dimensional landmark data and concluded that sex classification accuracy of the discriminant functions ranged from 70.7 to 77.3% for the univariate method, 81.8% for the stepwise method, and from 63.6 to 84% for the direct method.[19]
Conclusion
The uniqueness of the craniofacial features is well known, and comparison of the antemortem and postmortem skull configurations may contain sufficiently distinctive patterns for personal identification, even in badly burnt bodies.[9,10]
The study has resulted in development of population-specific data for Central Indian population. The results of the present study are promising, and the studied mandibular variables showed sexual dimorphism with an accuracy comparable with other skeletal remains, next to cranium and pelvis. Measurements of the variables PLCM, CTMB, HMC, CB, SH, and BB showed best sexual dimorphism and can be used for sex determination in Central Indian population with an accuracy rate that varies from 81.7 to 85.4%.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Ahmed AA, Mohammed HA, Hassan MA. Sex determination from cranial measurements in recent northern Sudanese. Khartoum Med J. 2011;4:539–47. [Google Scholar]
- 2.Vodanovic M, Dumancic J, Demo Z, Mihelic D. Determination of sex by discriminant function analysis of mandibles from two Croatian archaeological sites. Acta Stomatol Croat. 2006;40:263–77. [Google Scholar]
- 3.Krogman WM, Iscan MY. The human skeleton in forensic medicine. 2nd ed. Illinois: Charles C Thomas; 1986. [Google Scholar]
- 4.Bainbridge D, Genovese-Taraza S. A study of sex differences in the scapula. J Roy Anthropol Institute. 1956;86:109–34. [Google Scholar]
- 5.Di Vella G, Campobasso CP, Dragone M, Introna F., Jr Skeletal sex determination by scapular measurements. Boll Soc Ital Biol Sper. 1994;70:299–305. [PubMed] [Google Scholar]
- 6.Introna F, Jr, Di Vella G, Campobasso CP. Sex determination by discriminant analysis of patella measurements. Forensic Sci Int. 1998;95:39–45. doi: 10.1016/s0379-0738(98)00080-2. [DOI] [PubMed] [Google Scholar]
- 7.Steele DG. The estimation of sex on the basis of the talus and calcaneus. Am J Phys Anthropol. 1976;45:581–8. doi: 10.1002/ajpa.1330450323. [DOI] [PubMed] [Google Scholar]
- 8.Introna F, Jr, Di Vella G, Campobasso CP, Dragone M. Sex determination by discriminant analysis of calcanei measurements. J Forensic Sci. 1997;42:725–8. [PubMed] [Google Scholar]
- 9.Brogdon BG. Radiological identification of individual remains. In: Thali MJ, Viner MD, Brogdon BG, editors. Brogdon's Forensic Radiology. 2nd ed. Boca Raton, Florida: CRC Press; 2010. [Google Scholar]
- 10.Campobasso CP, Dell'Erba AS, Belviso M, Di Vella G. Craniofacial identification by comparison of antemortem and postmortem radiographs: Two case reports dealing with burnt bodies. Am J Forensic Med Pathol. 2007;28:182–6. doi: 10.1097/PAF.0b013e31806195cb. [DOI] [PubMed] [Google Scholar]
- 11.Krogman WM. The human skeleton in forensic medicine. I. Postgrad Med. 1955;17:A-48. passim. [PubMed] [Google Scholar]
- 12.Franklin D, O'Higgins P, Oxnard CE. Sexual dimorphism in the mandible of indigenous South Africans: A geometric morphometric approach. S Afr J Sci. 2008;104:101–6. [Google Scholar]
- 13.Loth SR, Henneberg M. Mandibular ramus flexure: A new morphologic indicator of sexual dimorphism in the human skeleton. Am J Phys Anthropol. 1996;99:473–85. doi: 10.1002/(SICI)1096-8644(199603)99:3<473::AID-AJPA8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Giles E. Sex determination by discriminant function analysis of the mandible. Am J Phys Anthropol. 1964;22:129–35. doi: 10.1002/ajpa.1330220212. [DOI] [PubMed] [Google Scholar]
- 15.Hanihara K. Sex diagnosis of Japanese skulls and scapulae by means of discriminant functions. J Anthropol Soc Nippon. 1959;67:191–7. [Google Scholar]
- 16.Ýþcan MY, Yashino M, Kato S. Sexual dimorphism in modern Japanese crania. Am J Hum Biol. 1995;7:459–64. doi: 10.1002/ajhb.1310070407. [DOI] [PubMed] [Google Scholar]
- 17.Steyn M, Iºcan MY. Sexual dimorphism in the crania and mandibles of South African whites. Forensic Sci Int. 1998;98:9–16. doi: 10.1016/s0379-0738(98)00120-0. [DOI] [PubMed] [Google Scholar]
- 18.Dayal MR, Spocter MA, Bidmos MA. An assessment of sex using the skull of black South Africans by discriminant function analysis. Homo. 2008;59:209–21. doi: 10.1016/j.jchb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Franklin D, O'Higgins P, Oxnard CE, Dadour I. Discriminant function sexing of the mandible of indigenous South Africans. Forensic Sci Int. 2008;179:84e1–5. doi: 10.1016/j.forsciint.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Patil KR, Mody RN. Determination of sex by discriminant function analysis and stature by regression analysis: A lateral cephalometric study. Forensic Sci Int. 2005;147:175–80. doi: 10.1016/j.forsciint.2004.09.071. [DOI] [PubMed] [Google Scholar]
- 21.Jit I, Jhingan V, Kulkarni M. Sexing the human sternum. Am J Phys Anthropol. 1980;53:217–24. doi: 10.1002/ajpa.1330530206. [DOI] [PubMed] [Google Scholar]
- 22.Jit I, Singh S. The sexing of the adult clavicles. Indian J Med Res. 1966;54:551–71. [PubMed] [Google Scholar]
- 23.Raju PB, Singh S. Sexual dimorphism in hip bone. Indian J Med Res. 1979;69:846–52. [PubMed] [Google Scholar]
- 24.Singh S, Singh SP. Identification of sex from the humerus. Indian J Med Res. 1972;60:1061–6. [PubMed] [Google Scholar]
- 25.Singh G, Singh SP, Singh S. Identification of sex from the radius. J Indian Acad Forensic Sci. 1974;13:10–3. [Google Scholar]
- 26.Singh S, Singh G, Singh SP. Identification of sex from the ulna. Indian J Med Res. 1974;62:731–5. [PubMed] [Google Scholar]
- 27.Singh SP, Singh S. The sexing of adult femora-demarking points for Varanasi zone. J Indian Acad Forensic Sci. 1972;11:1–6. [Google Scholar]
- 28.Singh G, Singh S, Singh SP. Identification of sex from tibia. J Anat Soc India. 1975;24:20–4. [Google Scholar]
- 29.Singh G, Singh SP. Identification of sex from the fibula. J Indian Acad Forensic Sci. 1976;15:29–34. [Google Scholar]
- 30.Singh S, Singh SP. Identification of sex from the tarsal bones. Acta Anat (Basel) 1975;93:568–73. doi: 10.1159/000144534. [DOI] [PubMed] [Google Scholar]


