Abstract
Background:
Many studies reported that follicle size has an essential role in developmental potential of oocytes. Also, the brilliant cresyl blue (BCB) test is one of the most important criteria in selection of more competent oocytes.
Objective:
Selection of developmentally competent bovine oocytes.
Materials and Methods:
A total of 1730 bovine cumulus oocyte complexes (COCs) were recovered from the ovaries by follicles isolation and classified into 3 categories according to the diameters of the follicles (small, <3 mm; medium 3-6 mm and large >6 mm). Oocytes were exposed to the BCB stain, diluted in Dulbecco's phosphate-buffered saline, modified with 0.4% bovine serum albumin (BSA) for 90 min. Oocytes with or without blue coloration of the cytoplasm were designated as BCB+ and BCB-, respectively.
Results:
The BCB+ and control oocytes originated from large and medium follicles exhibited a higher (p<0.0001) cleavage and blastocyst rate than BCB- oocytes. Furthermore, the BCB+ oocytes from large and medium follicles had the highest (p<0.0001) proportion of blastocyst than other treatment groups. In contrast, the BCB- oocytes from small follicles had the lowest (p<0.0001) proportion of blastocyst than other treatment groups. Interestingly, the percentage of the BCB+ oocytes from the large and medium ovarian follicles was significantly higher (p<0.0001), than the BCB+ oocytes from the small follicles.
Conclusion:
Current results confirmed that each BCB+ oocyte could not lead to perfect embryo development and the BCB test is not sufficient enough for the identification of oocytes that are competent for in vitro embryo development.
Key Words: Bovine, Oocyte, Development
Introduction
Mammalian immature oocytes are routinely selected for in vitro fertilization (IVF) on the basis of the visual assessment of morphological features, such as the thickness and compactness of the cumulus investment and the homogeneity of the ooplasm, this may reduce the yield of transferable embryos, as some oocytes with apparently normal morphology are in the early stages of degeneration (1).
Consequently, morphological evaluation alone is insufficient to distinguish competent oocytes that have the ability to bring about full-term pregnancy (2). On the other hand, only 30-40% of the zygotes obtained after in vitro maturation and fertilization (IVF) will reach the blastocyst stage in culture (3). This is probably due to the quality of the oocytes at the beginning of maturation, and indicates the need for the development of other approaches. Different selection criteria have been used to predict the quality of these oocytes and to improve the embryo development such as oocyte diameter, brilliant cresyl blue (BCB) test and the follicle diameter (4-6). With the urgent need for establishing non-invasive option for embryo selection, the BCB test has been successfully used to differentiate oocytes (7-9).
The BCB stain is an electron acceptor, which can be used to semi-quantitate the level of glucose-6-phosphate dehydrogenize (G6PDH) activity in the oocytes, by modification of a visual color (10). G6PDH is known to be a component of the pentose phosphate pathway (PPP), which provides ribose phosphate for nucleotide synthesis and the formation of fatty acids. Moreover, the BCB test is based on the capability of G6PDH to convert the BCB stain from blue to colorless (active G6PDH: BCB-, inactive G6PDH: BCB+) (11). Therefore, the oocytes that have completed the growth phase are blue (BCB+) because the G6PDH activity is too low for stain reduction. Also, the growing oocytes become colorless (BCB-) due to G6PDH activity (12). Studies in small ruminants, pigs and heifer have shown that oocytes stained with BCB (BCB+) to be generally larger and more competent in maturation and developmental rate, than those unstained (BCB-) (5, 8, 11-13).
Other authors have reported that the percentage of BCB+ oocytes developing to the morula and blastocyst stage were significantly higher than the control and BCB- oocytes (8, 9). Also, follicle size has been the other important criterion used in selecting competent oocytes (14, 15). Likewise, the follicle size from which the oocytes are obtained characterizes the developmental stage of the follicle and the maturational stages of the oocyte within that follicle (16). Crozet et al described a positive relationship between follicular diameter, oocyte diameter, meiotic competence and embryonic development in goats (14). In other words, there is a relationship between follicle size and oocyte competence; the competence increases as the follicle enlarges (17). Oocytes from bovine follicles greater than 6 mm in diameter produce blastocysts in vitro at substantially greater rates than those from 2-6 mm follicles (17).
Furthermore, follicles smaller than 2 mm yield oocytes capable of fertilization, but lack the ability to cleave beyond the 8-cell stage (15). Although, previous study evaluated the relationship between follicle size and oocyte selection using the BCB test, cleavage and blastocyst rate were not included (8). Due to the lack of reports about the effects of follicle size and BCB test in selection of developmentally competent bovine oocytes together following in vitro embryo production, therefore, the present study was conducted to use the BCB test as a selection criterion of developmentally competent bovine oocytes with every follicular origin (large, medium or small).
Materials and methods
This prospective study was done in IVF and Embryo Transfer Laboratory at Razi University of Kermanshah, Iran (2011). The ethics committees of Razi University of Kermanshah approved this study.
Chemicals
All plastic ware used in the present experiments were obtained from Falcon, USA, while all chemicals and media were purchased from Sigma, USA.
Oocyte collection
The number of 85 bovine ovaries were obtained from a local slaughterhouse and transported to the laboratory (within 2hr after slaughter) at 33-35oC in saline containing 50 IU/ml of penicillin and 50 µg/ml of streptomycin. Ovaries were washed three times in warm saline (8). The follicles were classified into 3 categories: small (<3 mm), medium (3-6 mm) and large (>6 mm) according to their diameters. The COCs from those follicles were aspirated using 21-guage needles attached to 10-mL syringe.
A total of 1730 bovine COCs were recovered from different follicle diameters and used for the investigation. Of these, 640 were used for the production of control embryos. The medium used for recovery was TCM-199 with 25 mM HEPES supplemented with 50 IU/ml heparin, 50 µg/ml gentamicin and 4 mg/ml BSA. Only the oocytes with three or more complete layers of unexpanded cumulus cells and homogeneous cytoplasm were used.
BCB test
Immediately after collecting COCs from different follicle diameters, the number of 1090 COCs was washed three times in Dulbecco’s phosphate-buffered saline modified by the addition of 0.4% BSA (A-3311; mDPBS). Then the COCs were exposed to 26 µM of BCB (B-5388) diluted in mDPBS for 90 min at 38.5oC in humidified air atmosphere (8). After exposure to BCB, COCs from different follicle diameters were washed three times in mDPBS and classified into two groups, depending on their cytoplasm coloration: 616 oocytes with a blue cytoplasm (BCB+) and 474 oocytes without a blue cytoplasm (BCB-).
In vitro maturation (IVM)
The classified COCs were washed three times in the maturation medium. The maturation medium was TCM-199 supplemented with 0.23 mmol/L sodium pyruvate, 0.02 IU/ml porcine follicular stimulating hormone (p-FSH), 1 µg/ml 17β estradiol, 50 ng/ml epidermal growth factor (EGF), 10% (v/v) fetal calf serum (FCS) and 50 µg/ml gentamycin (8). COCs were placed in groups of 10-12 into 50 µl droplets of maturation medium under mineral oil. Maturation proceeded for 24 hr at 38.5oC in an environment of 5% CO2 in humidified air atmosphere.
Sperm preparation
Bovine spermatozoa were squeezed out from the caudal epididymis into 2 mL sperm tyrods albumin lactate pyruvate (TALP) medium (18). The motility of the sperm cells was evaluated under an inverted microscope and separated motile sperm fraction by swim-up. The top 1.5 ml of medium was then collected after incubation for 45 min at 38.5oC and 5% CO2. The pooled medium containing spermatozoa was washed twice (700 g for 5 min) with sperm TALP medium. The final pellet of spermatozoa was resuspended in the fertilization medium to a concentration of 20×106 spermatozoa/ml.
IVF
After IVM, The COCs were washed three times in the fertilization medium (TALP, 6) and transferred in groups of 10-12 to 45 µl of fertilization droplets. Insemination was carried out by adding 20×106 spermatozoa/ml, 2 µg/ml heparin, and PHE (penicillamine, 20 µM; hypotaurin, 10 µM; epinephrine, 1 µM) (8). Oocytes were coincubated with spermatozoa for 22-24 hr at 38.5oC and 5% CO 2 in humidified air atmosphere.
In vitro culture (IVC)
Presumptive zygotes were denuded by pipetting using a small-bore pipette in synthetic oviductal fluid (SOF) +HEPES After washing, the zygotes were cultured in groups of 10-15 zygotes in 50 µl droplet of SOF modified by Takahashi and First at 38.5oC in a humidified atmosphere of 5% CO2, 5% O2 and 90% N2 (19). Cleavage was assessed after 48 hr of culture, and the numbers of embryos developing to the blastocyst stages were assessed on day 8. To prevent toxic accumulation of ammonium as a result of amino acid degradation, SOF medium was replaced every 48 hr. In this study, a two-culture system was used. The first system (SOF culture 1) medium for the first 48 hr, then, the medium was replaced by the second system (SOF culture 2) for the remaining 6 days of culture (20).
Experimental design
The developmental competence of the selected bovine oocytes by BCB test from different follicle diameters was evaluated. The follicles were aspirated at 33oC and divided according to their diameter into 3 groups- small follicles: <3 mm; medium follicles: 3-6 mm; and large follicles: >6 mm and the BCB test was used. The COCs were exposed to BCB staining (26 µM BCB in mDPBS) and oocytes were classified as BCB+ or BCB- and without exposure to BCB (control). The cleavage and blastocyst rate were recorded for each follicle category.
Statistical analysis
The experiment was conducted as complete randomized design. Data has been collected from nine experiment’s treatment in four replicates. Percentage values were logarithmic transformed and data analyzed by GLM procedure, followed by the LS means comparison by SAS statistical software (SAS Institute Inc., Cary, NC). Differences with a probability value of 0.05 or less was considered significant.
Results
In table I the percentages of oocytes selected by BCB test is set out. Regardless of follicle diameters, there were no significant differences in proportion of BCB+ and BCB- oocytes (56.34% and 43.66%, respectively). Table II shows the oocyte selection by the BCB test from different follicle diameters. The mean proportion of COCs classified as BCB+ in small, medium and large follicles were 45.14%, 64.56% and 59.30%, respectively (no significant difference between follicles group). The mean proportion of BCB+ oocytes (64.56%) of medium follicles was higher (p=0.0054) than those of the BCB- oocytes (35.43 %).
Table I.
Oocyte selection by the BCB test
| Method of definition |
Parameters
|
|
|---|---|---|
| No. of oocytes | Each group % | |
| BCB+ | 616 | 56.34±6.32 |
| BCB- | 474 | 43.66±6.32 |
Note: Data expressed as mean ± SE
Mean Standard Error of Group: 0.08
BCB: Brilliant cresyl blue
BCB+: Oocytes with a blue cytoplasm
BCB-: Oocytes without a blue cytoplasm
Table II.
Oocyte selection by the BCB test from different follicle diameters
| Follicle size | Method of definition |
Parameters
|
||
|---|---|---|---|---|
| No. of oocytes | Each group % | Total % | ||
| Large | BCB+ | 175 | 59.30 ± 5.04ab | 15.85 ± 1.65 |
| BCB- | 138 | 40.70 ± 5.04bc | 12.60 ± 1.22 | |
| Medium | BCB+ | 278 | 64.56 ± 5.46a | 25.82 ± 2.51 |
| BCB- | 158 | 35.43 ± 5.46c | 14.33 ± 2.59 | |
| Small | BCB+ | 163 | 45.14 ± 8.47abc | 14.84 ± 4.11 |
| BCB- | 178 | 54.85 ± 8.47ab | 16.54 ± 1.72 | |
Note: Data expressed as mean ± SE.
Mean Standard Error of Group: 0.08
Mean Standard Error of Total: 0.10
BCB: Brilliant cresyl blue
BCB+: Oocytes with a blue cytoplasm
BCB-: Oocytes without a blue cytoplasm
Different letters indicate statistical difference within each column (p<0.05).
For large and small follicles, the percentage of oocytes classified as BCB+ and BCB- was not significantly different (59.30%, 45.14% and 40.70%, 54.85%, respectively), but, in those BCB status, this difference was high numerically in large follicles. The percentages of cleavage and development to the blastocyst stage of oocytes selected by BCB from different follicles diameter has been shown in Table III. There were no significant differences between control and BCB+ oocytes in cleavage rate two days after IVF in all follicle diameters.
Table III.
Developmental competence of in vitro matured and in vitro fertilized oocytes selected by BCB from different follicle diameters
| Follicle size | Method of definition |
Parameters
|
|||
|---|---|---|---|---|---|
| No. of oocytes | Percentage cleaved | Percentage blast 1 / cleaved | |||
| Large | Control | 122 | 84.80 ± 1.06a | 19.75 ± 0.98b | |
| BCB+ | 175 | 85.70 ± 1.50a | 26.65 ± 1.51a | ||
| BCB- | 138 | 41.46 ± 0.50b | 10.66 ± 2.12d | ||
| Medium | Control | 353 | 82.43 ± 0.46a | 19.15 ± 1.45b | |
| BCB+ | 278 | 83.51 ± 0.50a | 25.69 ± 1.21a | ||
| BCB- | 158 | 42.45 ± 5.51b | 13.25 ± 1.28cd | ||
| Small | Control | 165 | 78.17 ± 3.68a | 11.23 ± 1.91cd | |
| BCB+ | 163 | 82.93 ± 1.20a | 14.76 ± 0.35c | ||
| BCB- | 178 | 39.31 ± 3.34b | 5.84 ± 0.30e | ||
Note: Data expressed as mean ± SE
Mean Standard Error of cleave: 0.01
Mean Standard Error of blast: 0.04
BCB: Brilliant cresyl blue
BCB+: Oocytes with a blue cytoplasm
BCB-: Oocytes without a blue cytoplasm
Different letters indicate statistical difference within each column (p<0.05).
Blastocyst
Conversely, significant differences (p<0.0001) were recorded between BCB+ and BCB- oocytes in cleavage rate in all follicle diameters. Furthermore, the cleavage rate was significantly low (p<0.0001) for BCB- oocytes originated from all follicle diameters. After seven days of culture, BCB+ oocytes exhibited a higher (p<0.0001) blastocyst rate than BCB- oocytes among all follicle diameters. Also, the percentage of blastocyst for BCB+ oocytes from large (26.65%) and medium (25.69%) follicles was respectively higher (p=0.0014, p=0.0022) than control (19.75%, 19.15%, respectively). There were no significant differences in blastocyst rate in BCB+ oocytes from large and medium follicles.
The proportion of blastocyst from control and BCB+ oocytes originated from large and medium follicles were higher than (p<0.05) control and BCB+ oocytes originated from small follicles.
There were no significant differences in blastocyst rate in BCB- oocytes from large and medium follicles but significant differences were respectively (p=0.0190, p=0.0007) observed with BCB- oocytes from small follicles (10.66%, 13.25% vs. 5.84%, respectively). In other way, the BCB- oocytes originated from small follicles had the lowest (p<0.05) proportion of blastocyst than other treatment groups. In contrast, the BCB+ oocytes from large and medium follicles had the highest (p<0.05) proportion of blastocyst than other treatment groups.
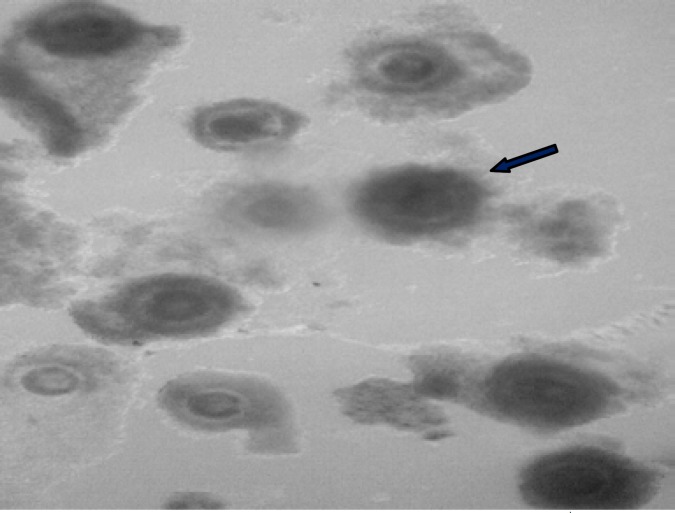
Figure 1.
Immature bovine oocytes following BCB exposure. Arrow indicates BCB+ oocytes.
Discussion
Studies confirm that the BCB test is a good marker in pre-selection procedures of developmentally competent oocytes (8, 9). Regardless of follicle diameters, the percentage of BCB+ oocytes (56.3%) obtained in the present study, employing 26 mM BCB, indicates that they had finished their growth phase and could be used for IVM/ IVF. This is in agreement with the result of Alm et al in cow oocytes, who observed that 57.9% of oocytes had a blue coloration after staining with BCB (21). Similar results reported in buffalo (53.9%); heifer (66%) (, ).
Nevertheless, the percentage of BCB+ oocytes obtained in the present study was lower than percentages observed in pigs (81%) and cattle oocytes (70%) (, ). In contrast, in prepubertal goat oocytes the percentage of BCB+ and BCB- oocytes was 30.1% and 69.9%, respectively (11). It is a hypothesis that these differences were caused by the selection criteria after BCB staining vary among laboratories. The current results show the difference in cleavage rate in BCB+ oocytes (range 85.70%-82.93%) in all follicle diameters and BCB- oocytes (rang 41.46-39.31%) whereas these differences were significant. Similar findings were observed in other reports (23, 24). However, this is in contrast with the previous studies which found no difference in cleavage rate, based on BCB status (21, 25).
In the present study, BCB+ oocytes exhibited a higher blastocyst rate than BCB- oocytes among all follicle diameters. These observations are in agreement with those of previous studies, in which the percentage of BCB+ oocytes developing to the blastocyst stage were significantly higher than BCB- oocytes (7, 8, 26). These findings highlighting the ability of the BCB stain to be able to differentially select the developmentally competent oocytes. Despite higher blastocyst production with BCB+ oocytes than with BCB- oocytes, no difference was found between BCB+ and control oocytes with bovine and equine oocytes (24, 27).
Nevertheless, those findings are different from the other reports and the ones of the present study in large and medium follicles (5, 22). There is no clear reason for such discrepancy among studies. It may be associated with different morphological criteria used to select oocytes before exposing to BCB or control group. As current study demonstrates, there are no significant differences in blastocyst rate between BCB+ and control oocytes recovered from small follicles. Meanwhile, in the most studies, regardless of follicle diameters, those comparisons have been done and this could be another possible reason. Among ovarian populations, the oocytes that complete their growth show full competence for the resumption of meiosis and the completion of meiotic maturation. As a result of insufficient cytoplasmic maturation, BCB- oocytes are not able to fully develop (28).
The higher developmental competence of BCB+ oocytes, which are largely obtained from fully-grown follicles, can be referred to the better cytoplasmic maturation of these oocytes during the final phases of folliculogenesis (24). Previous studies demonstrated that BCB+ oocytes contained higher number of mitochondrial DNA copies and had a greater diameter and larger cytoplasm volume in comparison with BCB- oocytes (5). These factors have positive impact on fertilization and blastocyst rates (29). Spikings et al reported that BCB- oocytes are delayed in the onset of expression of proteins in comparison with BCB+ oocytes (30). Moreover, BCB- oocytes had lower transcript level of genes involved in mitochondrial biosynthesis, suggesting that this may be one of the reasons for their low developmental competence compared to BCB+ and control oocytes (12).
In the previous reports mentioned before, oocytes were obtained regardless of follicle diameters, resulting mostly, in the release of oocytes from small follicles (<2.0 mm), oocytes that are not competent for development. On the other hand, the present results demonstrated that the BCB+ oocytes from small follicles have lower blastocyst rate than BCB+ oocytes from medium and large follicles. Therefore, the developmental competence of oocytes is dependent on the size of follicle from which the oocyte is obtained (31). Moreover, the size of follicles is the most important criteria for oocyte selection (17).
Various studies demonstrated that oocytes derived from large follicles are developmentally more competent than those derived from smaller follicles following IVF (6, 32). According to the results of the present study, oocytes isolated from 3-6 mm and >6 mm follicles consistently had a higher blastocyst rate than oocytes from <3 mm follicles in those BCB status (BCB+ and BCB- oocytes). Also, the rate of blastocyst, in that BCB status following IVF was slightly, but not significantly, greater for oocytes originating from large (26.65%) than those from medium follicles (25.69%). This was confirmed in the other studies (17, 6).
There is a positive relationship between follicle and oocyte diameter (32). Previous studies have indicated that oocytes from adult cows acquire developmental competence while the follicles grow from small to antrum size (33). The follicle must reach a diameter of at least 2-3 mm before the oocyte reaches a satisfactory developmental competence to blastocyst stage (34). It may be associated with that bovine oocytes from 2-mm follicles have not yet completed growth and RNA synthesis (34).
The greater developmental competence of oocytes originating from large follicles (>6 mm) is probably due to differentiation that occurred at the more advanced stage of follicular development. There are several changes have been reported in large follicles like expression of LH receptors by granulosa cells, decrease of IGF-binding protein and increase of IGF-I within the follicular fluid and increased expression of growth factors such as TGF-β, activin and inhibin. These happen simultaneously with ultrastructure changes within the oocyte and cumulus cells and with further growth of the oocyte (33).
These may partly explain the better fertilization and development results observed for the oocytes from large and medium follicles in the present study. Based on the results each BCB+ oocyte could not lead to perfect embryo development and the BCB test is not sufficient enough for the identification of oocytes that are competent for in vitro embryo development.
Acknowledgments
Authors would like to thank Mrs. L. Soltani for her technical assistance. In addition, the authors would like to thank the Razi University for financial support.
Conflict of interest
None of the authors have any conflict of interest to declare.
Note
This article extracted from M.Sc. thesis. (Golshan Azimi)
References
- 1.Gordon I. Laboratory Production of Cattle Embryos (Biotechnology in Agriculture no. 27) 2nd Ed. Cambridge UK: CAB International/Cambridge University Press; 2003. Recovering the bovine oocyte; pp. 79–111. [Google Scholar]
- 2.De Loos Van Maurik F, Van Beneden P, Kruip T. Structural aspects of bovine oocyte maturation in vitro. Mol Reprod Dev. 1992;31:208–214. doi: 10.1002/mrd.1080310308. [DOI] [PubMed] [Google Scholar]
- 3.Katska-Ksiazkiewicz L, Rynska B, Bochenek M, Opiela J, Jurkiewicz J. In vitro production of bovine embryos using flow-cytometrically sexed sperm. Arch Tierz Dummerstorf. 2006;49:133–140. [Google Scholar]
- 4.Anguita B, Jimenez-Macedo AR, Izquierdo D, Mogas T, Paramio MT. Effect of oocyte diameter on meiotic competence, embryo development, p34 (cdc2) expression and MPF activity in prepubertal goat oocytes. Theriogenology. 2007;67:526–536. doi: 10.1016/j.theriogenology.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Pujol M, López-Béjar M, Paramio MT. Developmental competence of heifer oocytes selected using the Brilliant Cresyl Blue (BCB) test. Theriogenology. 2004;61:735–744. doi: 10.1016/s0093-691x(03)00250-4. [DOI] [PubMed] [Google Scholar]
- 6.Marchal R, Vigneron C, Perreau C, Bali-Papp A, Mermillod P. Effect of follicular size on meiotic and developmental competence of porcine oocytes. Theriogenology. 2002;57:1523–1532. doi: 10.1016/s0093-691x(02)00655-6. [DOI] [PubMed] [Google Scholar]
- 7.Mirshamsi SM, Karami-Shabankareh H. Selection of developmentally competent sheep zygotes using the brilliant cresyl blue (BCB) test, after IVF. Small Ruminant Res. 2012;105:250–254. [Google Scholar]
- 8.Karami-Shabankareh H, Mirshamsi SM. Selection of developmentally competent sheep oocytes using the brilliant cresyl blue test and the relationship to follicle size and oocyte diameter. Small Ruminant Res. 2012;105:244–249. [Google Scholar]
- 9.Mirshamsi SM, KaramiShabankareh H, Ahmadi-Hamedani M, Soltani L, Hajariana H, et al. Combination of oocyte and zygote selection by brilliant cresyl blue (BCB) test enhanced prediction of developmental potential to the blastocyst in cattle. Anim Reprod Sci. 2013;136:245–251. doi: 10.1016/j.anireprosci.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Tian W, Braunstein LD, Pang J, Stuhlmeier KM, Xi QC, Tian X, et al. Importance of glucose-6-phosphate dehydrogenase activity for cell growth. Biol Chem. 1998;273:10609–10617. doi: 10.1074/jbc.273.17.10609. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-González E, López-Bejár M, Velilla E, Paramio MT. Selection of prepubertal goat oocytes using the brilliant cresyl blue test. Theriogenology. 2002;57:1397–1409. doi: 10.1016/s0093-691x(02)00645-3. [DOI] [PubMed] [Google Scholar]
- 12.Opiela J, Lipinski D, Słomski R, Katska-Ksiazkiewicz L. Transcript expression of mitochondria related genes is correlated with bovine oocyte selection by BCB test. Anim Reprod Sci. 2010;118:188–193. doi: 10.1016/j.anireprosci.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Roca J, Martinez E, Vazquez JM, Lucas X. Selection of immature pig oocytes for homologous in vitro penetration assays with the brilliant cresyl blue test. Reprod Fertil Dev. 1998;10:479–485. doi: 10.1071/rd98060. [DOI] [PubMed] [Google Scholar]
- 14.Crozet N, Dahirel M, Gall L. Meiotic competence of in vitro grown goat oocytes. J Reprod Fertil. 2000;118:367–373. [PubMed] [Google Scholar]
- 15.Pavlok A, Lucas-Hahn A, Niemann H. Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Mol Reprod Dev. 1992;31:63–67. doi: 10.1002/mrd.1080310111. [DOI] [PubMed] [Google Scholar]
- 16.Lequarre AS, Vigneron C, Ribaucour F, Holm P, Donnay I, Dalbies-Tran R, et al. Influence of antral follicle size on oocyte characteristics and embryo development in the bovine. Theriogenology. 2005;63:841–859. doi: 10.1016/j.theriogenology.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Longergan P, Monaghan P, Rizoz D, Boland MP, Gordon I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization and culture in vitro. Mol Reprod Dev. 1994;37:48–53. doi: 10.1002/mrd.1080370107. [DOI] [PubMed] [Google Scholar]
- 18.Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod. 1988;38:1171–1180. doi: 10.1095/biolreprod38.5.1171. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y, First NL. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology. 1992;37:963–978. doi: 10.1016/0093-691x(92)90096-a. [DOI] [PubMed] [Google Scholar]
- 20.Kafilzadeh F, Karami-Shabankareh H, Soltani L. Effect of various concentrations of Minimal Essential Medium vitamins (MEM vitamins) on development of sheep oocytes during in-vitro maturation. Iran J Reprod Med. 2012;10:93–98. [PMC free article] [PubMed] [Google Scholar]
- 21.Alm H, Torner H, Löhrke B, Viergutz T, Ghoneim IM, Kanitz W. Bovine blastocyst development rate in vitro is influenced by selection of oocytes by brillant cresyl blue staining before IVM as indicator for glucose-6-phosphate dehydrogenase activity. Theriogenology. 2005;63:2194–2205. doi: 10.1016/j.theriogenology.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 22.Heleil B, Fayed M. Developmental competence of buffalo oocytes from follicles of different diameters selected by brilliant cresyl blue staining. Global Vet. 2010;4:176–184. [Google Scholar]
- 23.Opiela J, Kątska-Książkiewicz L, Lipiński D, Słomski R, Bzowska M, Ryńska B. Interactions among activity of glucose-6-phosphate dehydrogenase in immature oocytes, expression of apoptosis-related genes Bcl-2 and Bax and developmental competence following IVP in cattle. Theriogenology. 2008;69:546–555. doi: 10.1016/j.theriogenology.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Mohammadi-Sangcheshmeh A, Held E, Ghanem N, Rings F, Salilew-Wondim D, et al. G6PDH-activity in equine oocytes correlates with morphology, expression of candidate genes for viability and preimplantative in vitro development. Theriogenology. 2011;76:1215–1226. doi: 10.1016/j.theriogenology.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Torner H, Ghanem N, Ambros C, Hölker M, Tomek W, Phatsara C, et al. Molecular and subcellular characterisation of oocytes screened for their developmental competence based on glucose-6-phosphate dehydrogenase activity. Reproduction. 2008;135:197–212. doi: 10.1530/REP-07-0348. [DOI] [PubMed] [Google Scholar]
- 26.Mirshamsi M, Karami-Shabankareh H, Soltani L. Brilliant cresyl blue (BCB) scoring system as an efficient tool for zygotes selection. Iran J reprod Med. 2011;9 (Suppl.):65. [Google Scholar]
- 27.Mota GB, Batista RITP, Serapiao RV, Boite MC, Viana JHM, Torres CAA, et al. Developmental competence and expression of the MATER and ZAR1 genes in immature bovine oocytes selected by brilliant cresyl blue. Zygote. 2009;18:209–216. doi: 10.1017/S0967199409990219. [DOI] [PubMed] [Google Scholar]
- 28.Susor A, Tomek W, Alm H, Kubelka M, Pavlok A, Torner H. Inhibition of final maturation of bovine oocytes in vitro differed by G6PDH-status. Proceedings of the Conference on Reproductive and Developmental Biology; 2007. p. 26. [Google Scholar]
- 29.El Shourbagy SH, Spikings EC, Freitas M, St John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006;131:233–245. doi: 10.1530/rep.1.00551. [DOI] [PubMed] [Google Scholar]
- 30.Spikings EC, Alderson J, St John JC. Regulated mitochondrial DNA replication during oocyte maturation is essential for successful porcine embryonic development. Biol Reprod. 2007;76:327–335. doi: 10.1095/biolreprod.106.054536. [DOI] [PubMed] [Google Scholar]
- 31.Vatzias G, Hagen DR. Effects of porcine follicular fluid and oviduct-conditioned media on 398 maturation and fertilization of porcine oocytes in vitro. Biol Reprod. 1999;60:42–48. doi: 10.1095/biolreprod60.1.42. [DOI] [PubMed] [Google Scholar]
- 32.Romaguera R, Casanovas A, Morato R, Izquierdo D, Catala M, et al. Effect of follicle diameter on oocyte apoptosis, embryo development and chromosomal ploidy in prepubertal goats. Theriogenology. 2010;74:364–373. doi: 10.1016/j.theriogenology.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Hendriksen PJM, Vos PLAM, Steenweg WNM, Bevers MM, Dieleman SJ. Bovine follicular development and its effect on the in vitro competence of oocytes. Theriogenology. 2000;53:11–20. doi: 10.1016/s0093-691x(99)00236-8. [DOI] [PubMed] [Google Scholar]
- 34.Hyttel P, Fair T, Callesen H, Greve T. Oocyte growth, capacitation and final maturation in cattle. Theriogenology. 1997;47:23–32. [Google Scholar]