Significance
Cancer cells compete for space and nutrients against healthy cells and other cancer cells but also cooperate by secreting growth factors. Clones that do not produce growth factors, however, have a proliferation advantage because they can use the factors produced by neighboring cells without the cost of producing them. Therefore, the cooperative production of growth factors by tumor cells should collapse. What maintains cooperation within the tumor? Here, we use evolutionary game theory to explain how heterogeneity can persist, and we use experiments with pancreatic cancer cells to test the predictions of the theory. Cancer is a process of clonal selection, and studying cancer cell populations using methods and concepts from evolutionary biology can reveal potential evolutionarily stable therapies.
Keywords: game theory, tumor, evolution
Abstract
The extensive intratumor heterogeneity revealed by sequencing cancer genomes is an essential determinant of tumor progression, diagnosis, and treatment. What maintains heterogeneity remains an open question because competition within a tumor leads to a strong selection for the fittest subclone. Cancer cells also cooperate by sharing molecules with paracrine effects, such as growth factors, and heterogeneity can be maintained if subclones depend on each other for survival. Without strict interdependence between subclones, however, nonproducer cells can free-ride on the growth factors produced by neighboring producer cells, a collective action problem known in game theory as the “tragedy of the commons,” which has been observed in microbial cell populations. Here, we report that similar dynamics occur in cancer cell populations. Neuroendocrine pancreatic cancer (insulinoma) cells that do not produce insulin-like growth factor II (IGF-II) grow slowly in pure cultures but have a proliferation advantage in mixed cultures, where they can use the IGF-II provided by producer cells. We show that, as predicted by evolutionary game theory, producer cells do not go extinct because IGF-II acts as a nonlinear public good, creating negative frequency-dependent selection that leads to a stable coexistence of the two cell types. Intratumor cell heterogeneity can therefore be maintained even without strict interdependence between cell subclones. Reducing the amount of growth factors available within a tumor may lead to a reduction in growth followed by a new equilibrium, which may explain relapse in therapies that target growth factors.
Cancer is a process of clonal selection within the body on the time scale of an individual’s lifetime (1–4): Tumor cells that reproduce more rapidly increase in frequency at the expense of neighboring healthy cells, even if this process is deleterious for the organism. For the same reason, cell subclones that have a proliferative advantage within the tumor are expected to drive other subclones to extinction. However, intratumor cell heterogeneity is commonly observed (5, 6). Despite the implications for cancer progression, diagnosis, and treatment (7–9), the mechanistic basis for this heterogeneity remains unclear (3, 9).
One possible answer comes from the observation that cancer cells not only compete for space and resources but also cooperate by sharing molecules with paracrine functions, such as growth factors. Because growth factors diffuse in the ECM, their effects are not limited to producer cells and can be considered a form of cooperation between cells (10). Heterogeneity can be maintained in case of strict interdependence between cell subclones because individual subclones are unable to proliferate autonomously but can complement each other’s deficiency (10, 11), and hence coexist. An example of such a scenario has recently been reported in a mouse model of mammary cancer (12), where luminal cells secrete the oncogenic factor Wnt1 and basal cells carry a cancer-driving mutation in the Hras gene (11).
This kind of cooperation between subclones (11) is analogous to mutualism in ecology (10); yet, like mutualism in ecology, a strict interdependence is not always the case. More commonly, a mutation may simply impair the production of a growth factor, and thus create a nonproducer cell. This mutant “defector” cell will still be able to “free-ride” on the growth factors produced by its cooperative neighboring cells; hence, this subclone would be expected to have a higher fitness and take over the population. Although this kind of interaction has been studied extensively in bacteria (13), where it has implications for the evolution of resistance to antibiotics (14), and in yeast (15), it has received little attention in cancer research due, in part, to a lack of adequate experimental systems.
Here, we have analyzed the dynamics of cooperation and defection for the production of insulin-like growth factor II (IGF-II) in an experimental cancer system in vitro. IGF-II is an ideal growth factor to study cooperation and defection among cancer cells because it is up-regulated in many cancer types and has been shown to stimulate cell growth and to protect cells from apoptosis (16–19). We used β-tumor cell lines derived from insulinomas of Rip1Tag2 mice (20) [henceforth called “producer” cells (+/+)] and from the same transgenic mice carrying a homozygous deletion of the IGF-II gene (16) [“nonproducer” cells (−/−)] to investigate whether, in the absence of interdependence between subclones, cell heterogeneity can be maintained or nonproducer cells drive producer cells to extinction. As we shall see, cooperation and stable heterogeneity are observed, a result that we analyzed by resorting to evolutionary game theory (21).
Results
IGF-II Is an Intratumor Public Good.
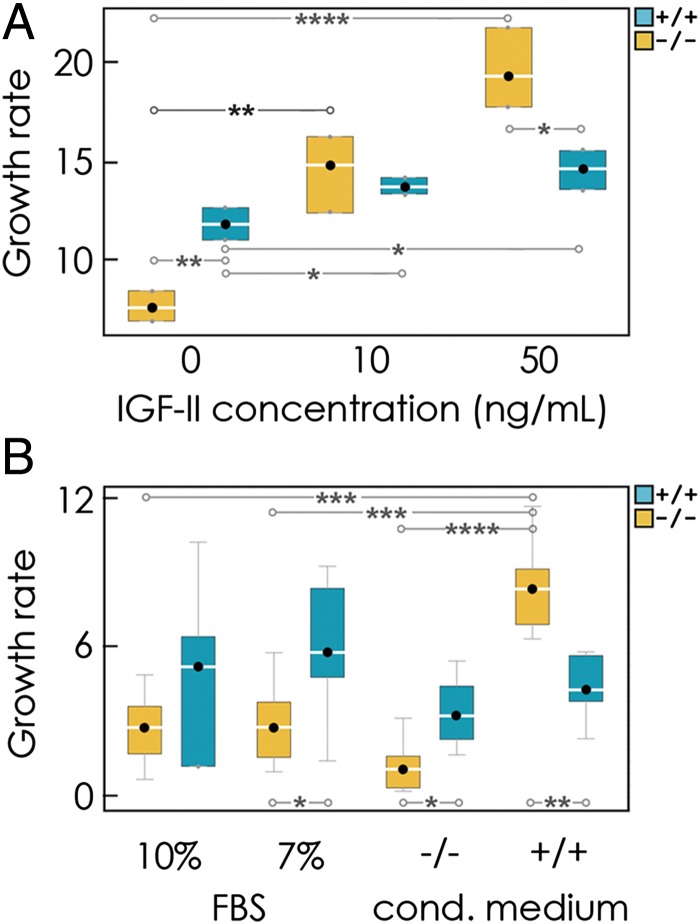
We first determined experimentally the growth rates of individual cultures. Pure cultures of −/− cells grew more slowly than pure +/+ cultures in the absence of exogenous IGF-II (Fig. 1A). This finding is not surprising, because IGF-II is known to enhance cell proliferation and survival. The growth rates of +/+ cultures were only marginally affected by exogenous IGF-II (Fig. 1A), suggesting that the IGF-II produced by the +/+ cells themselves is enough to sustain their proliferation. The growth rates of −/− cultures, on the other hand, increased at higher concentrations of IGF-II; more specifically, proliferation is a sigmoid function of IGF-II concentration (Fig. S1). Notably, at high concentrations of IGF-II, −/− cultures exhibited higher growth rates than pure +/+ cultures (Fig. 1A), indicating a cost for producing IGF-II.
Fig. 1.
IGF-II is a nonlinear public good. (A) Growth rates of producer (+/+) and nonproducer (−/−) cells in vitro (relative to day 1) at different concentrations of exogenous IGF-II in the growth medium. (B) Growth rates of +/+ and −/− cells in vitro (relative to the day with the minimum number of cells) with medium containing FBS (7% or 10%) or in conditioned (cond.) medium from −/− or +/+ cultures. Box plots show the median and the 25% and 75% quartiles (upper and lower fences, respectively). Asterisks show significant P values in a t test: *P < 0.05; **P < 0.005; ***P < 0.0005; ****P < 0.00005.
If −/− cells can benefit from soluble IGF-II in the growth medium, they can arguably also benefit from the presence of IGF-II produced by +/+ cells. To verify whether this is the case, we measured the growth rates of pure cultures in conditioned medium derived from −/− and +/+ pure cultures. Indeed, we observed that the growth rates of −/− cells improved when cultured in the presence of conditioned medium from +/+ cultures, whereas medium from −/− cultures had no significant effect (Fig. 1B). This finding suggests that a factor secreted by the +/+ cells is responsible for the increased growth of the −/− cells. Because the two cell lines differ in the production of IGF-II, this factor is arguably IGF-II itself.
The critical result was that −/− cells grew better than +/+ cells in medium conditioned by +/+ cells (Fig. 1B). This finding suggested that −/− cells would also outperform +/+ cells in mixed cultures if enough +/+ cells were cocultured. One could expect, therefore, that nonproducer cells would increase in frequency over time, because they are able to free-ride on the IGF-II produced (at a cost) by their +/+ cooperative neighbors. Similar observations have been made in bacteria (13, 14) and in yeast (15), a problem that is generally referred to as the “tragedy of the commons” in game theory (22): Because of the individual incentive to free-ride on other group member’s contributions, a nonproducer has a fitness advantage that will eventually lead to the demise of the population because of the lack of public good. We experimentally tested this hypothesis by observing how mixed cultures of +/+ and −/− cells change over time in vitro.
In the presence of 10% FBS (the concentration generally used for maintaining β-tumor cells in culture), we observed a rapid decline in the frequency of +/+ cells in mixed populations. At lower serum concentrations, however, we observed the opposite: The +/+ genotype increased in frequency. At intermediate levels of serum, on the other hand, there was no clear winner: The two cell types coexisted (Fig. 2). Only when starting the culture with low initial frequencies of +/+ cells did they go extinct. The coexistence of −/− and +/+ cells is of particular interest for our understanding of intratumor cell heterogeneity. What maintains a mixed population? Why does this heterogeneity disappear at low and high levels of serum?
Fig. 2.
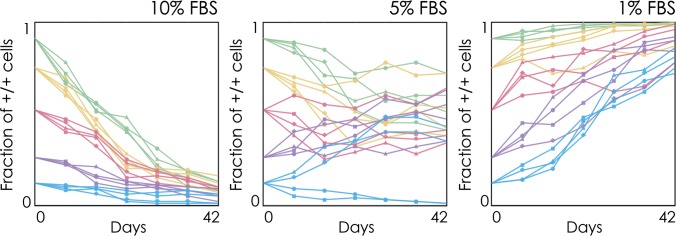
Long-term dynamics of IGF-II production in vitro. Observed changes in the frequency of IGF-II–producing cells (+/+) in mixed populations of +/+ cells and −/− cells seeded at varying ratios and under different concentration of FBS in the culture medium; the cost/benefit ratio of IGF-II increases with the amount of FBS.
Nonlinear Benefits Maintain Heterogeneity.
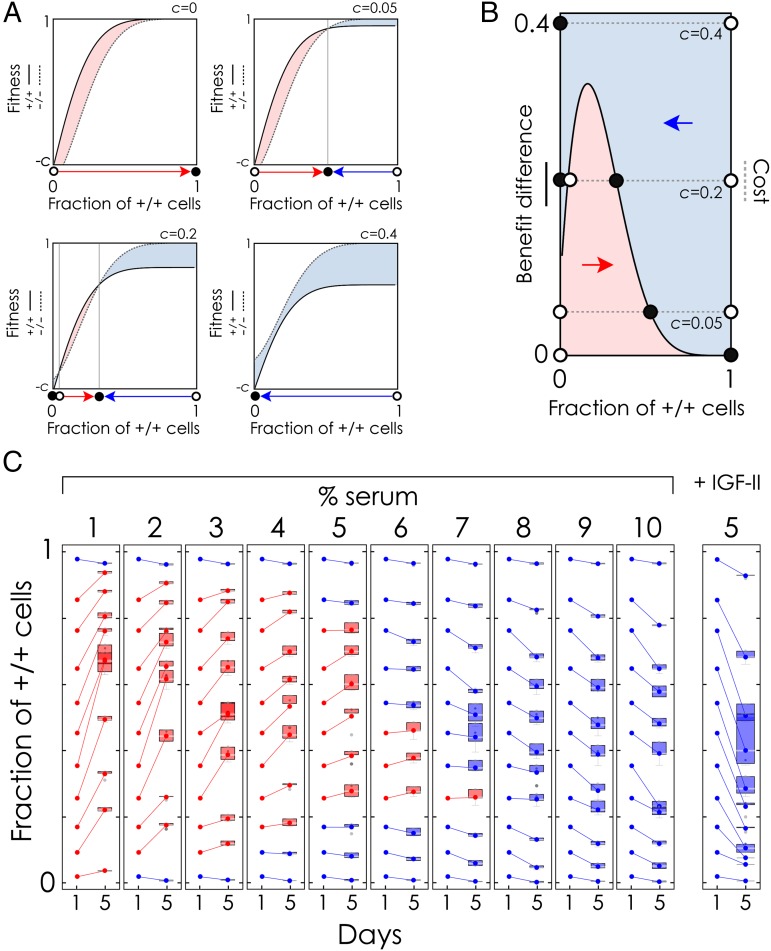
To decipher the growth dynamics observed in the coculture experiments, we resorted to evolutionary game theory (21). A +/+ cell pays a cost for producing the growth factor, which a −/− cell does not pay, yet the +/+ cell has a higher benefit because of the extra growth factor produced by itself. When the extra benefit offsets the cost, +/+ cells are predicted to increase in frequency, whereas they should decline in frequency when the cost is higher than the extra benefit (Fig. 3A) If this negative frequency-dependent benefit is nonlinear (because of synergistic effects and diminishing returns), clonal selection will lead to an increase of −/− cells when there are too many or too few +/+ cells, but not at intermediate frequencies. In other words, because IGF-II acts as a nonlinear public good (23), clonal selection can lead to a stable coexistence of +/+ and −/− cells if the cost of producing IGF-II is not too high (Fig. 3B).
Fig. 3.
Varying the cost/benefit ratio of IGF-II production changes the outcome of competition between producer and nonproducer cells. (A) Fitness is a nonlinear function of the fraction of +/+ cells; the fitness of +/+ cells depends also on the cost c of producing IGF-II. The dynamics depend on the relative fitness of the two types. Circles denote equilibria (●, stable; ○, unstable), and arrows show the direction of the dynamics (h = 0.2, s = 10, n = 30). (B) Alternative view of the dynamics of mixed populations. Equilibria occur where the difference in benefit between +/+ and −/− (i.e., the additional benefit for a +/+ cell due to its own production of IGF-II) equals c. (C) Experimentally observed changes in the frequency of +/+ cells in vitro after 5 d of coculture for different initial frequencies and different amounts of serum (a measure of the cost/benefit ratio of producing IGF-II), and with additional exogenous IGF-II (100 ng/mL). Error bars indicate the 25% and 75% quartiles.
If this interpretation is correct, we expect to observe that +/+ cells will go extinct in mixed populations when the cost/benefit ratio of producing IGF-II is too high. In contrast, at lower cost/benefit ratios, we should observe coexistence of the two cell types. Although the cost of IGF-II production is constant, we can change the benefit provided by endogenous IGF-II by varying the amount of serum in the medium: Because serum contains additional nutrients and growth factors (24) (including IGF-II), more serum reduces the relative benefit of the IGF-II produced by the +/+ cells, and thus increases its cost/benefit ratio. Likewise, reducing the concentration of serum in the medium increases the benefit of endogenously produced IGF-II, thus reducing its cost/benefit ratio. The bistability predicted by the theory (the existence of an internal unstable equilibrium, Fig. 3 A and B) would also explain why, given the same amount of FBS, +/+ cells sometimes went extinct when starting from low frequencies (Fig. 2).
We tested this prediction by experimentally measuring the instant growth rates of mixed populations (Fig. 3C). As expected, we observed that +/+ cells declined in frequency at high serum concentrations but increased at lower concentrations if the frequency of +/+ cells was neither too high nor too low. Below a critical threshold, as well as at very high frequencies of +/+ cell seeding, +/+ cell numbers decreased, leading to the bistable system predicted by the theory (Fig. 3B). The instant growth rates were also consistent with the prediction that the equilibrium fraction of +/+ cells should decline with the cost/benefit ratio of producing IGF-II (hence with serum concentration), whereas the critical initial fraction of +/+ cells required for the population to reach a stable coexistence with the −/− cells should increase. When the cost/benefit ratio was too high (high serum concentrations), there was no internal equilibrium and the +/+ cells went extinct. Although we did not observe complete extinction of the −/− type at low serum concentrations (Figs. 2 and 3C), the +/+ type could go to fixation if the cost of the growth factor was low enough, and in this case, there would be no social dilemma (23).
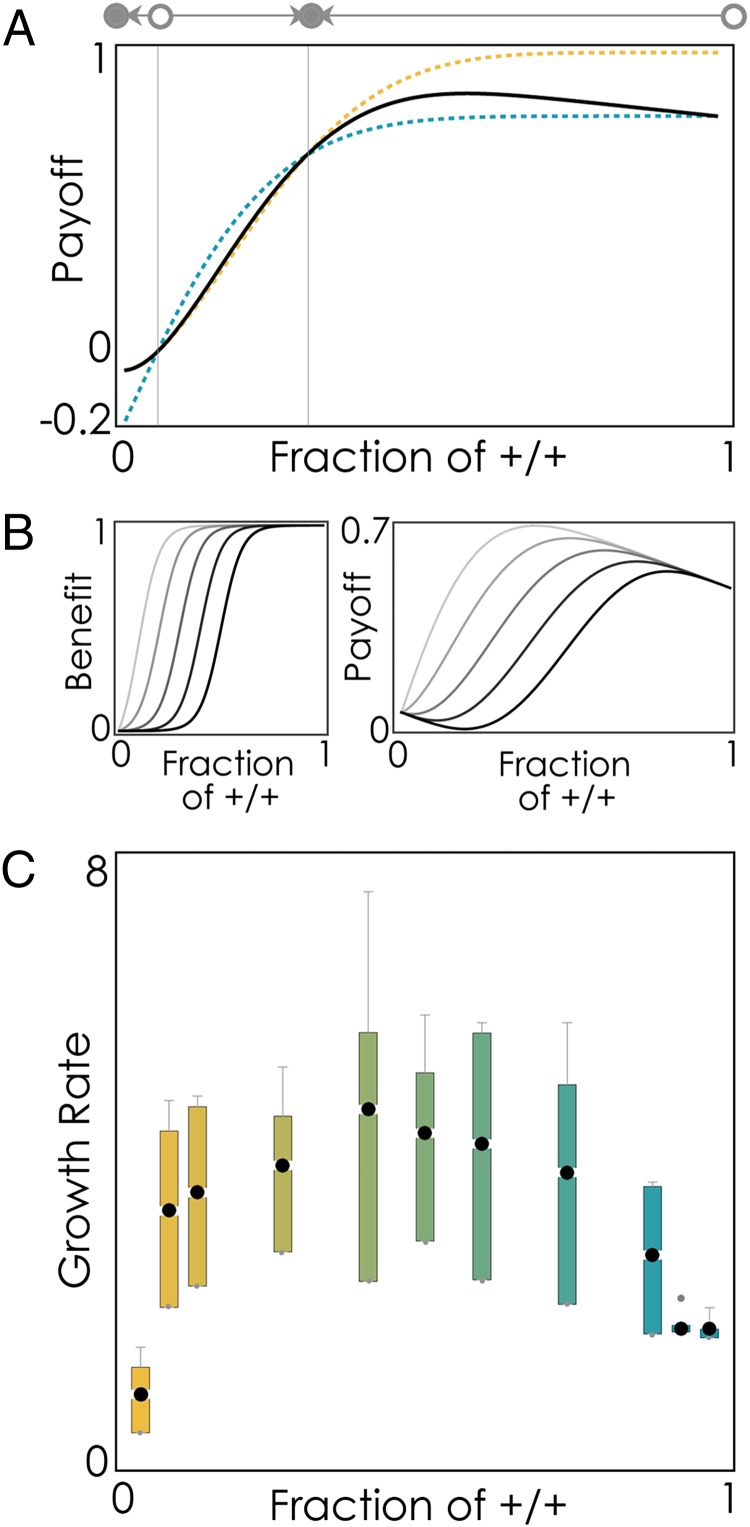
Adding exogenous IGF-II changed the dynamics in a similar way to increasing the amount of serum. An alternative interpretation could be that adding IGF-II reduced the amount of growth factor that must be produced by the cells to generate a certain benefit. In other words, adding exogenous growth factors reduced the value (h) of the inflection point of the benefit function (Fig. S2), thus reducing the equilibrium fraction of +/+ cells. Theory also predicted that the maximum growth rate should be observed at intermediate frequencies of producers (23), a prediction that was confirmed in our observations of the growth rates of mixed populations in vitro (Fig. 4).
Fig. 4.
Growth rates peak at intermediate frequencies of producers. (A) Predicted fitness of the two cell types (dotted blue curve, +/+; dotted yellow curve, −/−) and the average fitness of the population (solid black line) as a function of the frequency of +/+ cells. Circles show the equilibria (●, stable; ○, unstable), and arrows show the direction of the dynamics (h = 0.2, s = 30, c = 0.2, n = 10). (B) Benefit of growth factors and the corresponding predicted average tumor fitness (payoff) as a function of the frequency of +/+ cells in the population for given values of h (the position of the threshold: light to dark curves; h = 0.1 to 0.5, s = 20, c = 0.4, n = 10). (C) Observed growth rates of mixed cultures in vitro as a function of the fraction of +/+ cells. Boxes show the mean and the 25% and 75% quartiles (upper and lower fences, respectively).
Dynamics in Planar Heterogeneous Networks.
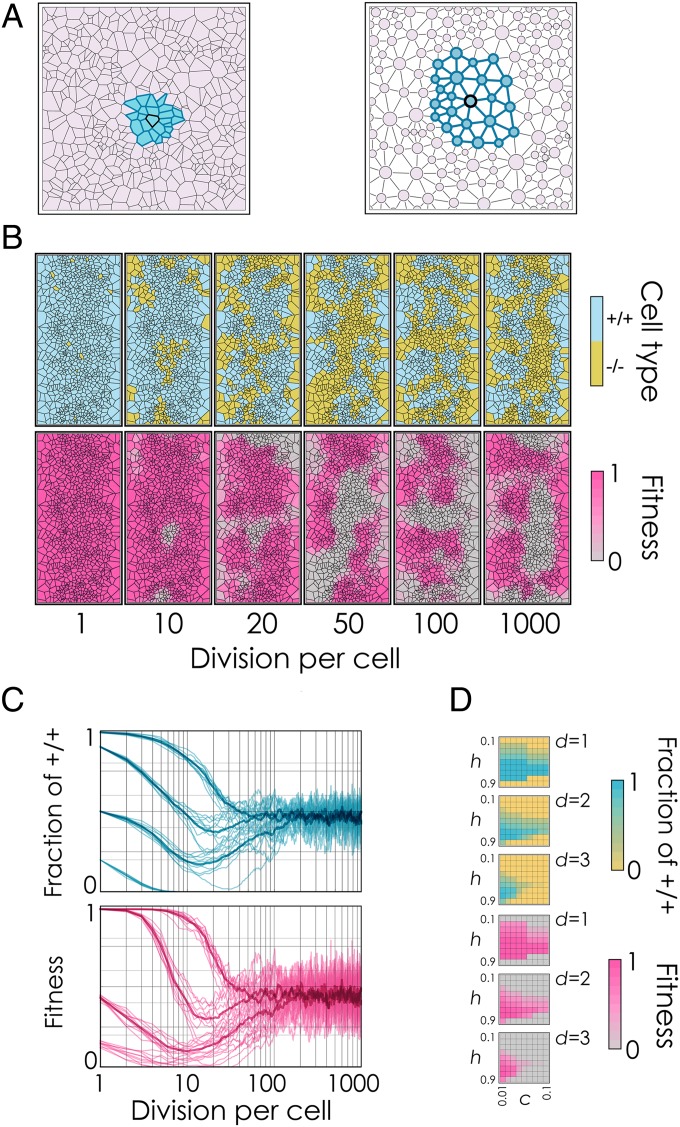
Although we have assumed a well-mixed population of cells in the above arguments about conflict and cooperation, many solid tumors, including the insulinomas produced by our cells, are populations with a defined spatial structure, a detail that is known to be important in studies on the evolution of cooperation (25, 26). To verify the importance of spatial structure on the dynamics, we ran simulations of evolution in spatial planar networks, in line with previous models of cooperation in spatially structured populations, with the difference that monolayers of cells, being heterogeneous planar networks, were modeled as Voronoi graphs (Fig. 5A and SI Materials and Methods). Voronoi graphs resemble the distributions of polygons in cell tissues (27) rather than regular lattices (in which all nodes have the same number of neighbors) or a scale-free network (which is not planar), which are the two topologies generally used in the study of cooperation in social networks (25, 26).
Fig. 5.
Growth factor production as a public goods game on a network. (A) Cells in a monolayer occupy the nodes of a planar heterogeneous graph. The number of edges within the diffusion range d of the growth factor defines the interaction group [here, d = 2 (blue cells)]. (B) Snapshots of simulations in which −/− cells invade a population of +/+ cells (d = 3, c = 0.02, h = 0.5, s = 20). (C) Changes in the fraction of producer cells (+/+) and in tumor fitness over time in simulations (thick lines are the average of 10 simulations). (D) Fraction of +/+ cells and fitness at equilibrium as a function of the inflection point h and of the cost of production c for different values of the diffusion range d.
Simulations of the evolution of growth factor production on Voronoi networks showed that the +/+ and −/− cells can coexist in stable equilibrium (Fig. 5 B–D). Similar to what happens in well-mixed populations, increasing the diffusion range of the growth factors reduces the amount of producer cells and the overall fitness of the populations. Cooperation is more efficient when the cost/benefit ratio of producing the growth factor is lower and when intermediate levels of producers are required (i.e., when the benefit function has an inflection at intermediate frequencies of producers) (Fig. 5D). Increasing the diffusion range d of the growth factor reduces the fraction of +/+ cells, and therefore the growth rate of the tumor (Fig. 5D), because it increases the size of the group that benefits from a cell’s production. Whereas the overall fraction of +/+ and −/− cells remains relatively stable after a period of adjustment (Fig. 5C), the position of the +/+ and −/− clusters continues to change over time (Fig. 5B and Fig. S3). Our simulations show that the dynamics are not significantly affected by cell density (Fig. S4) or by the frequency of cell passaging during culture (Fig. S5).
Discussion
Overall, our experimental results are in line with the predictions of models of nonlinear public goods in the framework of evolutionary game theory (23, 25, 26): Stable coexistence of producers and nonproducers (and therefore stable heterogeneity) can be maintained if the effect of the growth factor is a nonlinear function of the frequency of producers. Although cooperation requires positive assortment in linear public goods games, and the dynamics in spatially structured populations are therefore significantly different from the dynamics in well-mixed populations (25, 26), in the case of nonlinear benefits, cooperation is maintained in both well-mixed and spatial populations by frequency-dependent selection. Strict interdependence between subclones (10, 11) is not the case in our system, and, as we have shown, it is not necessary to maintain heterogeneity if the effect of the growth factor is nonlinear.
Because the fraction of producers is directly proportional to the concentration of the growth factor, our results imply that stable heterogeneity can be maintained if the benefit of a growth factor is a nonlinear function of its concentrations. Although we have focused on IGF-II, cooperation for the production of diffusible factors is probably common in cancer cell proliferation and in other processes that require diffusible molecules, such as sustained angiogenesis, immune system evasion, and metastasis (10). Collective effects have been previously reported for acidic FGF-1 in bladder carcinoma cells (28). Nonlinear effects are likely to be the rule for most biological molecules; the nonlinearity is generally a sigmoid shape described by the Hill equation (29), and examples of sigmoid effects have been reported for IGF-II and other growth factors (24, 30, 31).
Our results are related to studies of cooperation and competition in microbial cell populations where examples of cooperation and defection for the production of public goods have been observed (13–15). Similar to what happens for antibiotic resistance in microbial populations (32), which is promoted by the production of diffusible public goods (14), cooperation between tumor cells is an obstacle for therapies that target growth factors. Reducing the amount of circulating growth factors may lead to a reduction in tumor growth in the short term, but it will also increase the inflection point of the benefit function (the number of producer cells necessary to achieve a benefit), thus simply shifting the equilibrium to a higher fraction of +/+ cells, which may potentially explain the relapse observed in patients treated with therapies targeting growth factors (33). On the other hand, modifying the dynamics of the production of growth factors, by increasing their diffusion range for instance, might lead to a stable reduction of tumor proliferation.
Although the view that cancer is an evolutionary process (1, 2) is now widely accepted (3, 4), and the importance of understanding its dynamics has been recognized (32, 34), evolutionary methods are still largely neglected in the study of resistance to anticancer therapies (35). Our results suggest that further work on the dynamics of “social” interactions among cancer cells may reveal further insight into the dynamics of cancer, and hopefully guide research toward evolutionarily stable therapies (36).
Materials and Methods
Cell Lines.
β-tumor cell lines were derived from WT Rip1Tag2 mice (+/+) (16) and from the same transgenic mice carrying a homozygous deletion of the IGF-II gene (−/−) (17). The cell lines were maintained in culture in DMEM supplied with 10% (vol/vol) FBS, 1% glutamine, and 1% antibiotics. Conditioned medium was obtained from subconfluent cultures kept for 48 h in DMEM supplied with 5% (vol/vol) FBS, 1% glutamine, and 1% antibiotics.
Proliferation Assay.
A total of 30,000 cells were plated per well in 24-multiwell plates. After treatment, cells were fixed with 2.5% glutaraldehyde dissolved in PBS for 30 min at room temperature (RT). After washing twice with deionized water, crystal violet 0.1% solution in 20% methanol was added in each well for 15 min. Afterward, the solution was removed and each well was washed with water and allowed to dry at RT. The color was dissolved in 50 μL of 10% acetic acid solution and transferred in 96-multiwell plates, and intensity was measured by a plate reader at 595 nm. The growth rate is defined as the relative change in density during the log phase (after 10 d).
Measuring Frequencies by Flow Cytometry.
The producer (+/+) type was stably transduced by lentiviral infection with a pLenti-EGFP plasmid and selected for EGFP expression by puromycin (2 ng/mL) treatment for 48 h. The producer (+/+) type therefore expresses EGFP constitutively. We measured the fraction of the two types in mixed populations using an Accuri C6 flow cytometer (Becton Dickinson) (488-nm excitation, 533-nm emission, 300-nm emission filter) after gating out cellular debris and selecting only single cells for analysis. We typically counted 50,000 cells.
Public Goods Game.
A cell can be a producer (+/+) or a nonproducer (−/−) of IGF-II. Producers pay a fixed cost c that nonproducers do not pay. A cell benefits from the IGF-II produced by all of the cells in its group of size n. We assume that the benefit function has a sigmoid shape. The benefits for +/+ and −/− cells are therefore, respectively, the normalized versions of V(j + 1) and V(j), where V(j) = β/[1+e−s(j/n−h)]. We assume that β = 1 and 0 < c < 1, j is the number of +/+ cells among the other n − 1 cells, h defines the position of the inflection point (h→1 gives strictly increasing returns, and h→0 gives strictly diminishing returns), and s defines the steepness of the function at the inflection point (s→∞ models a threshold public goods game, s→0 models an N-person prisoner’s dilemma) (23).
Evolutionary Dynamics on Networks.
In well-mixed populations, groups of size n are updated at every generation. In spatially structured populations, group size n is a function of the diffusion range d of the growth factor, measured as the lowest number of edges (the shortest distance) between nodes (Fig. 5A). The spatial network is a Voronoi graph obtained by a Delaunay triangulation of random points on a sphere (to avoid distortions due to edge effects) (SI Materials and Methods). The average connectivity is six, with a unimodal distribution; nodes with fewer than four or more than eight connections are rare (27). Individual cells occupy the nodes of a network of size 1,000. The process starts with a number of nonproducers placed at random on the graph. At each game round, strategies are updated according to a standard death–birth process in which the probability that a node reproduces is proportional to its fitness (25, 26) (SI Materials and Methods). Results are obtained by averaging the final 20% of 1 million generations, averaged over 10 runs.
Supplementary Material
Acknowledgments
We thank Dieter Ebert, István Scheuring, Dylan Edwards, and Stephen Robinson for comments on the manuscript; Stephen Robinson, Aleksander Gontarczyk, and Linh Le for help with cell culture; and Ernesta Fagiani and Darren Sexton for help with flow cytometry. This work was supported by Natural Environment Research Council Grant NE/H015701/1, the Swiss National Science Foundation, 7th Framework Programme of the European Union TuMIC HEALTH-F2-2008-201662, and the SystemsX.ch Research, Technology and Development project Cellplasticity.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414653112/-/DCSupplemental.
References
- 1.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255(5505):197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 2.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 3.Merlo LMF, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6(12):924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 4.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenman C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446(7132):153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navin N, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472(7341):90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dexter DL, Leith JT. Tumor heterogeneity and drug resistance. J Clin Oncol. 1986;4(2):244–257. doi: 10.1200/JCO.1986.4.2.244. [DOI] [PubMed] [Google Scholar]
- 8.Maley CC, et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat Genet. 2006;38(4):468–473. doi: 10.1038/ng1768. [DOI] [PubMed] [Google Scholar]
- 9.Almendro V, Marusyk A, Polyak K. Cellular heterogeneity and molecular evolution in cancer. Annu Rev Pathol. 2013;8:277–302. doi: 10.1146/annurev-pathol-020712-163923. [DOI] [PubMed] [Google Scholar]
- 10.Axelrod R, Axelrod DE, Pienta KJ. Evolution of cooperation among tumor cells. Proc Natl Acad Sci USA. 2006;103(36):13474–13479. doi: 10.1073/pnas.0606053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleary AS, Leonard TL, Gestl SA, Gunther EJ. Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature. 2014;508(7494):113–117. doi: 10.1038/nature13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S, Goel S, Alexander CM. Differentiation generates paracrine cell pairs that maintain basaloid mouse mammary tumors: Proof of concept. PLoS ONE. 2011;6(4):e19310. doi: 10.1371/journal.pone.0019310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West SA, et al. The social lives of microbes. Annu Rev Ecol Evol Syst. 2007;38:53–77. [Google Scholar]
- 14.Lee HH, Molla MN, Cantor CR, Collins JJ. Bacterial charity work leads to population-wide resistance. Nature. 2010;467(7311):82–85. doi: 10.1038/nature09354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore J, Youk H, van Oudenaarden A. Snowdrift game dynamics and facultative cheating in yeast. Nature. 2009;459(7244):253–256. doi: 10.1038/nature07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christofori G, Naik P, Hanahan D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature. 1994;369(6479):414–418. doi: 10.1038/369414a0. [DOI] [PubMed] [Google Scholar]
- 17.Lamm GM, Christofori G. Impairment of survival factor function potentiates chemotherapy-induced apoptosis in tumor cells. Cancer Res. 1998;58(4):801–807. [PubMed] [Google Scholar]
- 18.Burtscher I, Christofori G. The IGF/IGF-1 receptor signaling pathway as a potential target for cancer therapy. Drug Resist Updat. 1999;2(1):3–8. doi: 10.1054/drup.1998.0061. [DOI] [PubMed] [Google Scholar]
- 19.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 21.Maynard Smith J. Evolution and the Theory of Games. Cambridge Univ Press; Cambridge, UK: 1982. [Google Scholar]
- 22.Hardin G. The tragedy of the commons. The population problem has no technical solution; it requires a fundamental extension in morality. Science. 1968;162(3859):1243–1248. [PubMed] [Google Scholar]
- 23.Archetti M, Scheuring I. Review: Game theory of public goods in one-shot social dilemmas without assortment. J Theor Biol. 2012;299:9–20. doi: 10.1016/j.jtbi.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Valenzano KJ, Heath-Monnig E, Tollefsen SE, Lake M, Lobel P. Biophysical and biological properties of naturally occurring high molecular weight insulin-like growth factor II variants. J Biol Chem. 1997;272(8):4804–4813. doi: 10.1074/jbc.272.8.4804. [DOI] [PubMed] [Google Scholar]
- 25.Nowak MA, Tarnita CE, Antal T. Evolutionary dynamics in structured populations. Phil Trans R Soc Lond B Biol Sci. 2010;365(1537):19–30. doi: 10.1098/rstb.2009.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perc M, Gómez-Gardeñes J, Szolnoki A, Floría LM, Moreno Y. Evolutionary dynamics of group interactions on structured populations: A review. J R Soc Interface. 2013;10(80):20120997. doi: 10.1098/rsif.2012.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson WT, Gibson MC. Cell topology, geometry, and morphogenesis in proliferating epithelia. Curr Top Dev Biol. 2009;89:87–114. doi: 10.1016/S0070-2153(09)89004-2. [DOI] [PubMed] [Google Scholar]
- 28.Jouanneau J, Moens G, Bourgeois Y, Poupon MF, Thiery JP. A minority of carcinoma cells producing acidic fibroblast growth factor induces a community effect for tumor progression. Proc Natl Acad Sci USA. 1994;91(1):286–290. doi: 10.1073/pnas.91.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornish-Bowden A. Fundamentals of Enzyme Kinetics. 4th Ed Wiley Blackwell; Hoboken, NJ: 2012. [Google Scholar]
- 30.Karey KP, Sirbasku DA. Differential responsiveness of human breast cancer cell lines MCF-7 and T47D to growth factors and 17 beta-estradiol. Cancer Res. 1988;48(14):4083–4092. [PubMed] [Google Scholar]
- 31.Jourdan M, et al. Delineation of the roles of paracrine and autocrine interleukin-6 (IL-6) in myeloma cell lines in survival versus cell cycle. A possible model for the cooperation of myeloma cell growth factors. Eur Cytokine Netw. 2005;16(1):57–64. [PubMed] [Google Scholar]
- 32.Lambert G, et al. An analogy between the evolution of drug resistance in bacterial communities and malignant tissues. Nat Rev Cancer. 2011;11(5):375–382. doi: 10.1038/nrc3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bozic I, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. eLife. 2013;2:e00747. doi: 10.7554/eLife.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aktipis CA, Kwan VS, Johnson KA, Neuberg SL, Maley CC. Overlooking evolution: A systematic analysis of cancer relapse and therapeutic resistance research. PLoS ONE. 2011;6(11):e26100. doi: 10.1371/journal.pone.0026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Archetti M. Evolutionarily stable anti-cancer therapies by autologous cell defection. Evol Med Public Health. 2013;2013(1):161–172. doi: 10.1093/emph/eot014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.