Significance
The control of organ growth involves cell–cell communication that is mediated by signal transduction pathways. The Hippo signaling pathway has emerged as an essential regulator of organ size in Drosophila and mammals, and defects in Hippo signaling drive cancer progression. An important unresolved question in the growth control field is, How is the Hippo pathway regulated? Recent reports show that adherens junctions and cell polarity complexes regulate the Hippo pathway, but controversy exists about the mechanisms involved. Here we report that in Drosophila and in mammalian cells, adherens junctions and basolateral polarity complexes regulate the Hippo pathway independently of each other. These results thus deepen our knowledge of this important growth control and tumor suppressor pathway.
Keywords: apical–basal cell polarity, Hippo pathway, adherens junction, basolateral protein, Drosophila imaginal discs
Abstract
Adherens junctions (AJs) and cell polarity complexes are key players in the establishment and maintenance of apical–basal cell polarity. Loss of AJs or basolateral polarity components promotes tumor formation and metastasis. Recent studies in vertebrate models show that loss of AJs or loss of the basolateral component Scribble (Scrib) cause deregulation of the Hippo tumor suppressor pathway and hyperactivation of its downstream effectors Yes-associated protein (YAP) and Transcriptional coactivator with PDZ-binding motif (TAZ). However, whether AJs and Scrib act through the same or independent mechanisms to regulate Hippo pathway activity is not known. Here, we dissect how disruption of AJs or loss of basolateral components affect the activity of the Drosophila YAP homolog Yorkie (Yki) during imaginal disc development. Surprisingly, disruption of AJs and loss of basolateral proteins produced very different effects on Yki activity. Yki activity was cell-autonomously decreased but non–cell-autonomously elevated in tissues where the AJ components E-cadherin (E-cad) or α-catenin (α-cat) were knocked down. In contrast, scrib knockdown caused a predominantly cell-autonomous activation of Yki. Moreover, disruption of AJs or basolateral proteins had different effects on cell polarity and tissue size. Simultaneous knockdown of α-cat and scrib induced both cell-autonomous and non–cell-autonomous Yki activity. In mammalian cells, knockdown of E-cad or α-cat caused nuclear accumulation and activation of YAP without overt effects on Scrib localization and vice versa. Therefore, our results indicate the existence of multiple, genetically separable inputs from AJs and cell polarity complexes into Yki/YAP regulation.
Epithelial tissues are barriers that separate body structures from their environment. A key characteristic of epithelial cells is their highly organized apical–basal polarity (1). Apical–basal cell polarity must be tightly controlled for proper development and function of organs, and loss of cell polarity is involved in tumor development (1). Apical–basal cell polarity is controlled by the concerted action of protein modules that localize to specific positions along the apical–basal axis: the apically localized Crumbs (Crb) and Par/atypical protein kinase C (aPKC) modules, the laterally localized Scribble (Scrib) module, and the adjacent adherens junction (AJ) complex (2). All three modules of polarity proteins are highly conserved from Drosophila to humans (3). In Drosophila, the Crb module contains the transmembrane domain protein Crb and the adaptor proteins Stardust and PatJ (2); the Par/aPKC module includes the serine/threonine kinase aPKC and the PDZ domain containing proteins Par6 and Bazooka (2). Both apical modules antagonize the function of the basolateral module, which comprises the proteins Scrib, Discs large (Dlg), and Lethal giant larvae (Lgl) (2). AJs are physically located between the apical and the basolateral membrane and serve as a boundary between apical and basal domains (4). The main components of AJs are E-cadherin (E-cad), α-Catenin (α-Cat), and β-Catenin (β-Cat) (4). A complex network of repressive and cooperative interactions between apical determinants, basolateral determinants, and AJ proteins establishes and maintains cell polarity to properly integrate cells into epithelial tissues (2, 4, 5). Loss of apical–basal polarity or AJ function is frequently observed in epithelial defects, many of which are closely associated with tumor formation and metastasis (6). Apical–basal polarity components and AJ proteins have thus been implicated as essential regulators of growth, and in particular as regulators of the Hippo growth control pathway, although the mechanisms of this regulation are poorly understood (7, 8).
Originally characterized in Drosophila, the Hippo pathway has been studied extensively in recent years in both Drosophila and mammals (7–11). Upstream components of the Hippo pathway signal to a core kinase cascade, which in Drosophila comprises the Hippo (Hpo) and Warts (Wts) kinases that regulate the phosphorylation of the transcriptional coactivator Yorkie (Yki), leading to retention of phosphorylated Yki in the cytoplasm. Nonphosphorylated Yki enters the nucleus and forms complexes with transcription factors such as Scalloped (Sd) that then drive the expression of downstream target genes. All of the core components of the Hippo pathway have mammalian homologs that function in an analogous fashion. The Yki homologs Yes-associated protein (YAP) and Transcriptional coactivator with PDZ-binding motif (TAZ) are phosphorylated by the core kinases MST1/2 (Hpo homologs) and LATS1/2 (Wts homologs), which phosphorylate and prevent YAP/TAZ from entering the nucleus to induce target gene transcription (9–11).
Recent studies suggest that several apical–basal cell polarity components regulate the activity of the Hippo pathway (7, 8). In Drosophila, Crb directly interacts with the upstream Hippo pathway component Expanded (Ex) and recruits it to the apical membrane (12–15). Basolateral proteins also regulate Hippo signaling. Drosophila larvae that are homozygous mutant for scrib, dlg, or lgl show highly elevated Yki activity and massive overgrowth of their imaginal discs (16–18). Similar observations have also been reported in mammalian cells. The Crb complex is required to recruit upstream components, such as Angiomotin to the apical membrane (19), and Scrib forms a protein complex with MST1/2, LATS1/2, and TAZ (20). Loss of Crb or loss of Scrib deregulates the Hippo pathway and allows YAP and/or TAZ to enter the nucleus and drive target gene expression (20, 21). Together, these reports indicate that the apical–basal cell polarity modules are required for the proper functioning of the Hippo pathway.
Components of AJs have also been implicated as regulators of the Hippo pathway. In mammals, homophilic interaction of E-cad in cultured cells decreases cell proliferation and promotes nuclear export of YAP (22). Conditional deletion of α-Cat, which serves as a link between the actin cytoskeleton and AJs (4), caused nuclear accumulation of YAP in α-catenin (α-cat) mutant keratinocytes in vitro and in vivo (23, 24). However, although AJs and apical–basal cell polarity modules have individually been shown to regulate Hippo pathway activity, whether these regulatory pathways act via the same or parallel mechanisms is not known.
In this study, we report that disruption of AJs or knockdown of basolateral components in Drosophila epithelial imaginal discs causes distinct effects on Yki activity. Using the UAS-RNAi system, we were able to genetically separate and investigate the roles of AJs and basolateral complexes in Hippo signaling regulation. We also found that AJs and basolateral components can regulate YAP activity separately in mammalian cells. Our results indicate that AJs and apical–basal cell polarity complexes act through distinct molecular pathways to regulate Yki/YAP activity.
Results
Loss of AJs and Basolateral Components Has Different Effects on Yki Activity.
Several studies found that apical and basolateral cell polarity components regulate the Hippo pathway in vivo using Drosophila imaginal discs as a model system (12–15, 17, 25), but whether AJs regulate Hippo signaling in imaginal discs is not known. We thus sought to investigate the effects of disrupting the AJs on Hippo signaling in imaginal discs by removing the AJ proteins E-cad and α-Cat. However, animals homozygous mutant for DE-cad (the Drosophila E-cad homolog, referred hereafter simply as E-cad) or α-cat are embryonic lethal, and clones of cells mutant for E-cad or α-cat are cell lethal in imaginal discs (26, 27), thereby preventing their analysis. We thus turned to RNAi-mediated knockdown of E-cad and α-cat in imaginal discs using the UAS/Gal4 system, which allows tissue-specific knockdown of gene expression. We combined different Gal4 drivers with UAS-RNAi–expressing transgenes that target E-cad or α-cat and co-expressed GFP to mark the RNAi-expressing cells. Expression of GFP alone under the control of the patched-Gal4 (ptc-Gal4) driver, which drives expression of UAS-transgenes in a stripe in developing wing discs (Fig. 1 A and A′′), did not affect Yki activity as assayed by the expanded-lacZ (ex-lacZ) reporter, a commonly used and sensitive readout for Yki activity (Fig. 1A′) (28). However, co-expression of UAS-RNAi transgenes that target and down-regulate E-cad or α-cat (Fig. S1 A and B) caused a strong increase in ex-lacZ expression in a stripe in the center of wing imaginal discs to levels that reached even higher than endogenous expression (Fig. 1 B and C). To verify the specificity of the RNAi constructs, we repeated this experiment using UAS-RNAi lines targeting different regions of the E-cad and α-cat genes, and all of them showed similar phenotypes although with varying strengths (Fig. S1 C and D). Other Yki targets were similarly deregulated upon E-cad or α-cat knockdown (Fig. S1 F and J, compare to Fig. S1 E and I). Together, these results indicate that loss of the AJ components E-cad or α-cat causes deregulation of the Hippo pathway.
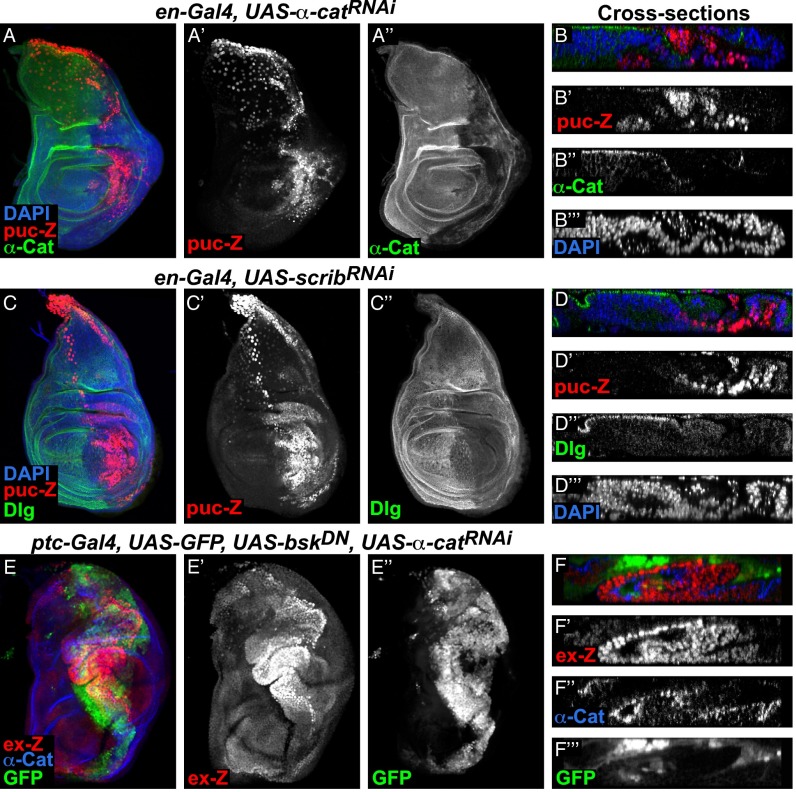
Fig. 1.
Disruption of AJs and basolateral components cause different effects on Yki activity. Confocal images of third instar imaginal discs of the indicated genotypes showing the effects of expression of different RNAi constructs under the control of the ptc-Gal4 driver. A–D show full stacks of the top view, and E–H show optical cross-sections. Yellow dashed lines indicate the position of the cross-sections. The ptc-Gal4 expression domains are marked by coexpression of GFP (green or gray in the ” panels). Discs were stained for β-galactosidase to reveal the expression of the Yki activity reporter ex-lacZ (ex-Z, red or gray in the ‘ panels). Knockdown of E-cad (B and F) or α-cat (C and G) caused cell-autonomous decrease and strong non-autonomous increase of ex-lacZ expression, whereas knockdown of scrib (D and H) caused mainly cell-autonomous up-regulation of ex-lacZ expression. Anterior is to the left for all discs.
Surprisingly, when we examined the cell autonomy of these effects, we found that ex-lacZ was induced non–cell-autonomously along the stripe of RNAi-expressing cells, whereas E-cad or α-cat knockdown cells had decreased levels of ex-lacZ expression (Fig. 1 F and G and Fig. S1 G and H). This effect was most pronounced in the presumptive dorsal hinge region and best visible by examining optical cross-sections through the imaginal discs (Fig. 1 F and G and Fig. S1 G and H). These data therefore show that disruption of AJs results in a cell-autonomous reduction and a non–cell-autonomous increase in Yki activity.
We then compared these effects to those of scrib knockdown, which is known to induce Hippo reporter activation (25). We observed strong cell-autonomous induction of ex-lacZ in scrib knockdown cells in agreement with previous reports (Fig. 1 D and H) (25) and weaker and limited non-autonomous effects (Fig. 1 D and H). Notably, however, most but not all scrib knockdown cells had elevated ex-lacZ. A recent study reported that knockdown of scrib induces spindle orientation defects and causes mutant cells to delaminate from the epithelium and to overproliferate (29). Indeed, many of the ex-lacZ–expressing nuclei lie below the disc epithelium, although many are also located in the normal nuclear region of the epithelium (Fig. 1H′′). Thus, in the dorsal hinge region, we often observed up-regulation of Yki activity in most of the scrib knockdown cells, which comprised cells in the epithelium and delaminated cells (Fig. 1H′). In any case, we saw strong up-regulation of Yki activity in scrib knockdown cells, which is in contrast to the effects of α-cat knockdown. Similar autonomous induction of ex-lacZ was observed in discs with knockdown of lgl or dlg (Fig. S2) (12, 18, 25). In summary, disruption of AJs and knockdown of basolateral polarity components cause different effects on Yki activity.
Knockdown of AJ and Basolateral Components Has Distinct Effects on Polarity Protein Localization.
To investigate how disruption of AJs and basolateral components causes such different effects on Hippo signaling, we first examined the effects of E-cad, α-cat, and scrib knockdown on AJs and polarity complexes. Interestingly, we again found that knockdown of AJ components and scrib caused different phenotypes. On the one hand, knockdown of α-cat caused mislocalization of E-cad and the Drosophila β-Cat homolog Armadillo (Arm) (Fig. 2A and Fig. S3A), therefore disrupting AJs as expected. Additionally, reduction of α-Cat also caused mislocalization of members of the apical complexes, including aPKC and Crb, as well as the apically localized adaptor protein and Hippo pathway component Merlin (Mer) (Fig. S3 A–C). However, the localization of the basolateral protein Dlg was largely retained in α-cat knockdown cells, suggesting that the basolateral polarity module was primarily intact (Fig. 2A′′). We saw similar mislocalization of α-Cat and properly localized Dlg in E-cad knockdown cells (Fig. 2B). On the other hand, knockdown of scrib disrupted the basolateral module, as evidenced by disruption of Dlg localization as expected (Fig. 2C), and also caused mislocalization of aPKC and Mer (Fig. S3D), indicating that the basolateral module is important for maintaining the apical domain. However, knockdown of scrib caused only minor effects on AJs in many cells, as E-cad (Fig. 2C′) remained largely properly localized. Altogether, these data show that under these knockdown conditions, disruption of AJs has a strong impact on apical domain maintenance, but not on the localization of the basolateral proteins, whereas loss of basolateral components can have a strong effect on apical proteins with only limited effects on AJ protein localization in imaginal discs. Although the RNAi knockdowns likely produce hypomorphic effects, their use uncoupled defects in AJs from defects in the basolateral module, thereby revealing the existence of multiple genetically separable inputs from AJs and the basolateral module into the regulation of Yki activity.
Fig. 2.
Disruption of AJs and basolateral components has distinct effects on polarity protein localization and tissue size. (A–C) Confocal cross-sections of wing discs that coexpressed GFP (green in all panels) with different RNAi constructs driven by ptc-Gal4. (A) Knockdown of α-cat caused loss of E-cad from the plasma membrane (A′) but not Dlg (A′′). (B) Knockdown of E-cad caused loss of α-Cat (B′) but not Dlg (B′′). (C) Knockdown of scrib caused loss of Dlg (C′′) but not E-cad (C′). (D–F) Full stack confocal images of wing discs that coexpressed GFP with different RNAi constructs in the posterior compartment driven by hh-Gal4. (E) Knockdown of α-cat reduced whereas (F) knockdown of scrib increased the size of the posterior compartment.
Disruption of AJs and Basolateral Components Has Opposite Effects on Tissue Size.
Our data show that disruption of AJs or basolateral components results in distinct effects on Hippo pathway activity. Because the Hippo pathway is an important regulator of tissue growth, we next investigated the effect of loss of AJs or basolateral components on tissue size. To knock down genes in a broad domain, we used hedgehog-Gal4 (hh-Gal4), which drives UAS-transgene expression in the entire posterior compartment of wing discs. Compared with control discs (Fig. 2D), knockdown of α-cat by hh-Gal4 strongly reduced ex-lacZ expression and the size of the posterior compartment (Fig. 2E), as shown by a drastically reduced GFP-expressing region (Fig. 2E′). In contrast, knockdown of scrib resulted in an increase in tissue size (Fig. 2F) and ex-lacZ expression. To test whether the reduced tissue size seen when AJ components are knocked down is caused by excessive apoptotic activity, we examined the levels of cleaved caspase 3. We observed widespread apoptosis in α-cat knockdown tissue, as evidenced by increased levels of cleaved caspase 3 staining in the GFP-expressing region (Fig. S4B) compared with controls (Fig. S4A). In contrast, scrib knockdown only caused a slight increase in cleaved caspase 3 staining (Fig. S4C). Together, these results show that in addition to having different effects on Yki activity, loss of AJs and basolateral components also have different effects on cell survival and overall tissue growth.
Suppression of Yki Activity in α-cat Knockdown Cells Does Not Require JNK.
The Jun N-terminal kinase (JNK) signaling pathway is activated in response to damage or cellular stress and can lead to elimination of damaged cells by apoptosis (30). We thus tested whether JNK is activated in α-cat knockdown cells and, if so, whether JNK signaling is required for the high levels of apoptosis and the suppression of Yki in knockdown cells. We found that the JNK reporter puckered-lacZ (puc-lacZ) was induced in α-cat (Fig. 3 A and C) as well as scrib knockdown regions (Fig. 3 B and D) as previously observed (31–33). In contrast to the distinct effects on Hippo pathway activity and cell polarity, knockdown of α-cat and scrib both cause autonomous up-regulation of JNK signaling. To determine whether the cell death and decrease in Yki activity in α-cat knockdown cells depends on JNK activity, we inhibited JNK by expressing a dominant negative version of the Drosophila JNK homolog basket (bskDN) and assayed growth, cell death, and Yki activity. We observed a reduction of cleaved caspase 3 staining (Fig. S4D, compare with Fig. S4B) and an expansion of the α-cat knockdown area (Fig. 3 E and F, compare with Fig. 1 A and E) when JNK activity was blocked, suggesting that the cell death in α-cat knockdown tissues is mediated by JNK signaling. However, ex-lacZ was still decreased in α-cat knockdown cells (Fig. 3F). Therefore, the suppression of Yki activity in α-cat knockdown cells does not require JNK signaling.
Fig. 3.
JNK is activated in α-cat and scrib knockdown cells. (A–F) Confocal images of wing discs stained for β-Gal to detect the expression of the JNK pathway reporter puc-lacZ (A–D) or the Yki reporter ex-lacZ (E and F). (A, C, and E) Full stack images and (B, D, and F) optical cross-sections. Knockdown of α-cat (A and B) or scrib (C and D) induced puc-lacZ expression. (E and F) Coexpression of a dominant negative JNK (bskDN) together with α-catRNAi did not activate ex-lacZ expression in knockdown cells.
Wts-Dependent Yki Regulation Is Functional in α-cat Knockdown Cells.
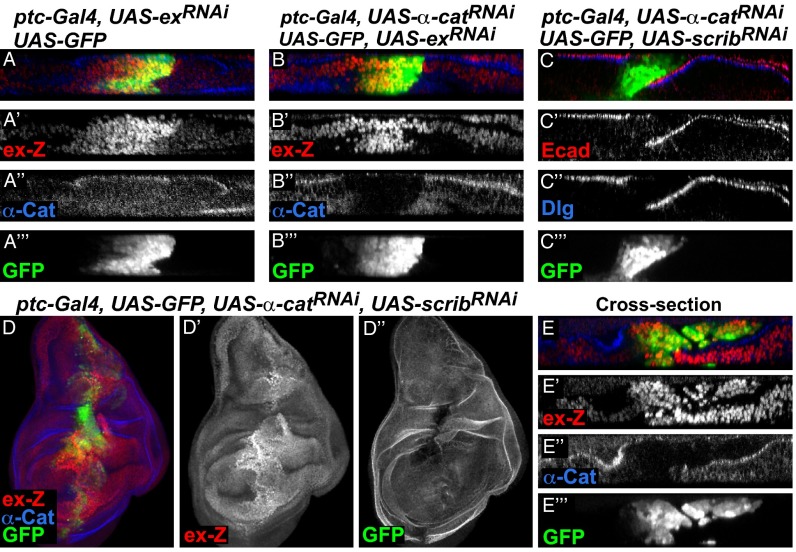
Several lines of evidence from mammalian tissue culture experiments indicate that the Hippo pathway effector YAP is regulated by F-actin–dependent mechanisms that do not require the function of the core kinases MST1/2 and LATS1/2, the mammalian homologs of Hpo and Wts (34). We thus tested whether the core components of the Hippo pathway are still functioning in α-cat knockdown wing disc cells. To test this, we expressed UAS-RNAi transgenes of core components and Yki alone or together with α-catRNAi and assayed Yki reporter activity. In a wild-type background, knockdown of ex or wts and overexpression of Yki strongly induced cell-autonomous expression of ex-lacZ as expected (Fig. 4A and Fig. S5 A and C). When coexpressed with α-catRNAi, these constructs rescued the suppression of ex-lacZ expression caused by α-catRNAi, resulting in cell-autonomous induction of ex-lacZ (Fig. 4B and Fig. S5 B and D). These components are thus epistatic to α-Cat in regulating Yki activity, indicating that they are active in α-cat knockdown cells and that α-Cat may affect the activity of the core of the Hippo pathway.
Fig. 4.
Additive effects of α-cat and scrib double knockdown on Hippo signaling. Confocal images of wing discs that expressed different constructs under the control of the ptc-Gal4 driver and coexpressing GFP. (A and B) Cross-sections showing that ex-lacZ expression was induced in cells expressing exRNAi (A) or exRNAi together with α-catRNAi (B). (C–E) Coexpression of α-catRNAi and scribRNAi disrupted E-cad (C′) and Dlg (C′′) localization. (D) Full stack image of a wing disc with α-catRNAi and scribRNAi double knockdown and an optical cross-section (E) revealing cell-autonomous and non-autonomous induction of ex-lacZ.
Basolateral Components and AJs Act in Parallel to Regulate Yki.
Our data show that disruption of AJs induces non–cell-autonomous activation of Yki activity, whereas loss of basolateral proteins results mainly in cell-autonomous activation of Yki, suggesting that AJs and basolateral components regulate Yki via different mechanisms. If AJs and basolateral proteins work through separate mechanisms to regulate the Hippo pathway, simultaneous disruption of AJs and basolateral components should produce additive effects on Hippo pathway reporter activity. To test this hypothesis, we performed a double knockdown experiment where we coexpressed UAS-α-catRNAi and UAS-scribRNAi. Immunofluorescent detection of α-Cat (Fig. 4D), E-cad (Fig. 4C′), and Dlg (Fig. 4C′′) confirmed that AJs and basolateral complexes were both disrupted in the RNAi-expressing cells. Supporting the hypothesis that AJs and basolateral complexes are independent regulators of Yki activity, we observed both cell-autonomous and non–cell-autonomous up-regulation of ex-lacZ in the α-cat and scrib double knockdown cells (Fig. 4 D and E). In addition, we wanted to know whether the non-autonomous effect of α-cat knockdown was due to loss of basolateral module function in neighboring cells. However, Dlg localization was not obviously disrupted throughout the wing region, including cells that were next to α-cat knockdown cells that had up-regulated ex-lacZ expression (Fig. S6 A and A′, arrows). Altogether, our data show that AJs and basolateral complexes regulate Hippo pathway activity via distinct mechanisms.
AJs Regulate YAP Independently of Scrib in Mammalian Cells.
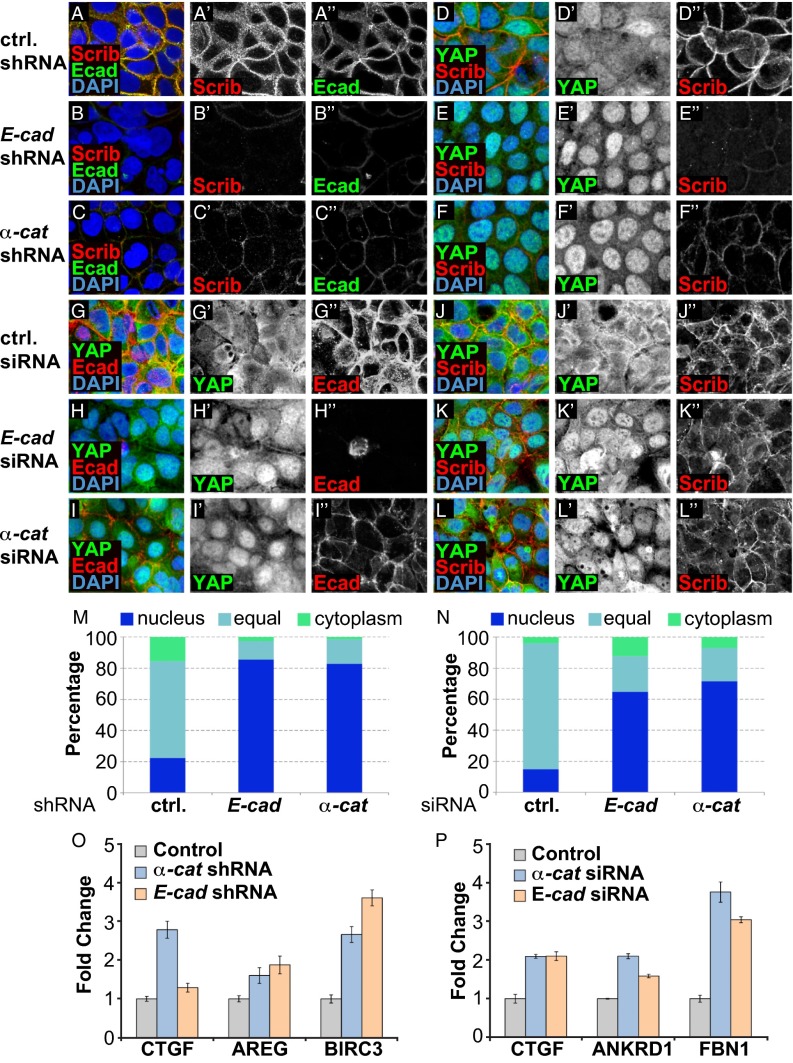
Our Drosophila data show that disruption of AJs causes activation of Yki only non–cell-autonomously. However, several studies in mammalian systems reported that YAP activity is induced cell-autonomously in cells with impaired AJ function (22–24). Interestingly, Scrib is mislocalized in cells with disrupted AJs (20, 35), and loss of Scrib activates YAP (20, 21). Similarly, we found that confluent wild-type Caco-2 cells had E-cad and Scrib localized at cell–cell junctions and YAP localized to both the cytoplasm and nucleus. Prolonged knockdown of E-cad or α-cat by shRNA-mediated expression caused nuclear translocation of YAP, elevated expression of YAP target genes, and disruption of Scrib localization (Fig. 5 A–F and M). Likewise, knockdown of E-cad or α-cat in confluent MDCK cells or disruption of AJs in confluent Caco-2 cells by calcium depletion caused mislocalization of E-cad and Scrib and nuclear translocation and activation of YAP (Figs. S7 and S8). These results indicate that the activation of YAP in AJ knockdown cells may be caused by the loss of Scrib localization.
Fig. 5.
AJs act independently of Scrib to regulate YAP activity in mammalian cells. (A–L) Confocal images of Caco-2 cells with shRNA- or siRNA-mediated knockdown of E-cad or α-cat and scrambled RNA as indicated. Cells were stained to detect Scrib, E-cad, and YAP as indicated, and nuclei were visualized by DAPI. (M and N) Quantification of YAP localization. Cells were binned into pools with predominantly nuclear YAP (blue), predominantly cytoplasmic YAP (green), or even distribution (light blue). (O and P) Quantitative RT-PCR of YAP target genes in Caco-2 cells with (O) shRNA- or (P) siRNA-mediated knockdown of control (gray), α-cat (blue), or E-cad (orange), normalized to levels in control cells.
To address this possibility, we sought to test the effects when AJs are lost without concomitant loss of Scrib localization. To do this, we induced acute and short-term down-regulation of AJs by siRNA transfection. Under these conditions, Scrib was still localized in E-cad or α-cat knockdown cells (Fig. 5 K and L). Interestingly, such siRNA-mediated short-term down-regulation of E-cad or α-Cat caused nuclear translocation of YAP and induction of its target genes even under conditions where cells maintained proper Scrib localization (Fig. 5 G–L and N). Conversely, Scrib knockdown by shRNA had minor effects on E-Cad localization but nevertheless caused YAP nuclear translocation (Fig. S7). These data therefore indicate that AJs and basolateral components regulate YAP through distinct pathways in mammalian cells, as well as in Drosophila.
Discussion
In this report, we addressed the effects of AJs and basolateral cell polarity determinants on the activity of the Hippo pathway in Drosophila imaginal discs. We found that knockdown of AJs and basolateral components both induced ectopic activation of Yki. However, knockdown of AJs and basolateral proteins had strikingly different effects on Yki. Disruption of the basolateral module induced mainly a cell-autonomous increase in Yki activity, whereas knockdown of AJs caused non-autonomous induction of Yki reporters. Therefore, our data identify and genetically uncouple multiple different molecular pathways from AJs and the basolateral module that regulate Yki activity.
Our studies further show that knockdown of AJs induces cell-autonomous reduction of Yki activity and causes cell death and decreased size of Drosophila imaginal discs (Figs. 1–3). Likewise, E-cad and α-cat mutant clones do not survive in imaginal discs (26, 27). This effect may be mediated by LIM domain proteins of the Zyxin and Ajuba subfamilies, which regulate Hippo signaling by directly inhibiting Wts/Lats kinases and by interacting with Salvador (Sav), an adaptor protein that binds to the Hpo/MST kinases (36, 37). A recent report shows that α-Cat recruits Ajuba and indirectly Wts to AJs (37) and loss of Ajuba leads to activation of Wts and hence phosphorylation and inhibition of Yki and diminished tissue size (36, 37). Thus, α-cat mutant cells may inactivate Yki because they lose Ajuba function.
In contrast, in mammalian systems, several in vivo and in vitro studies, including our own, showed the opposite effect on Hippo signaling upon AJ disruption; knockdown of E-cad or α-cat caused an increase in cell proliferation and nuclear accumulation of YAP (Fig. 5) (22), and conditional knockout of α-cat in mouse skin cells caused tumor formation and elevated nuclear YAP staining (23, 24). This suggests that AJ components have a tumor suppressor function in mammals. The observation that Scrib is mislocalized upon disruption of AJs in several different mammalian cell lines (Fig. 5) (20, 35) suggested that YAP activation could be due to the concomitant disruption of the basolateral module. However, our finding that acute disruption of AJs can cause YAP activation without disrupting Scrib localization and vice versa indicates that AJs and the basolateral module also act independently on the Hippo pathway in mammalian cells. In mammalian cells, α-Cat forms a complex with YAP and 14-3-3 proteins, thereby sequestering phosphorylated YAP at the plasma membrane (23, 24). However, α-Cat may function as a tumor suppressor only in epidermal stem cells, as conditional deletion of α-cat in differentiated cells only caused a mild phenotype with no overgrowth and tumor formation (38, 39). Therefore, it is possible that the negative regulation of YAP by α-Cat is cell type-specific, although further testing is required to fully address this issue.
The non–cell-autonomous effect of AJ knockdown on the Hippo pathway is an intriguing phenomenon. Several groups reported non-autonomous effects on the Hippo pathway in Drosophila in other mutant conditions. Disrupting the expression gradients of the atypical Cadherin Dachsous or that of its regulator Four-jointed (40, 41), clones of cells mutant for the tumor suppressor genes vps25 or hyperplastic discs (hyd) (16, 42), clones of cells overexpressing Src64 (43), or overexpression of the proapoptotic gene reaper or the JNK signaling ligand eiger (18) all cause non-autonomous activation of Yki. This non-autonomous activation of Yki may be part of a regenerative response that stimulates cell proliferation in cells neighboring tissue defects (16, 18). The signals that activate Yki in these situations are not known, nor is it known whether these mutant conditions activate the same or different signaling mechanisms. The non-autonomous activation of Yki around cells with AJ knockdown may be mediated by changes in mechanical forces. AJs are important for maintaining tension between cells across epithelia, and disruption of AJs leads to an imbalance of apical tension. Mechanical forces are known to regulate the Hippo pathway (8, 34, 37, 44, 45), and YAP/TAZ act as mediators of mechanical cues from the cellular microenvironment such as matrix stiffness (34). In particular, the Zyxin and Ajuba family LIM domain proteins can act as sensors of mechanical forces (36, 46) and may be involved in the non-autonomous activation of Yki. The effects on Hippo signaling of solely changing Zyxin and Ajuba may not be as strong as those described here, and these proteins may thus cooperate with other molecular conduits to regulate the activity of the Hippo pathway in response to changes in AJ strength. Unraveling these mechanisms will provide important new insights into understanding how cells interact with neighboring cells to regulate proliferation, apoptosis, and the Hippo pathway.
It is currently unknown whether AJs also exert non-autonomous effects on the Hippo pathway in mammalian tissues. Amphiregulin, an EGF ligand, is a downstream target of YAP and can induce non–cell-autonomous cell proliferation through EGFR signaling (47). However, it is not known whether YAP itself is activated non–cell-autonomously to contribute to the hyperproliferation phenotypes observed upon disruption of AJs in vivo and in vitro. It will be interesting to determine whether AJs and other cell–cell signaling mechanisms also have non–cell-autonomous effects on the activity of YAP in mammalian tissues, for example during regeneration.
Finally, the apical proteins aPKC and Crb modulate the activity of the Hippo pathway (13–15), and many Hippo pathway components are apically localized, which is important for their activity (7, 8). The data presented here add to these findings. Disruption of AJs causes reduced Yki activity, despite the fact that Crb and Mer are mislocalized. Thus, AJs and cell polarity components regulate Yki activity through multiple, genetically separable inputs. It will be interesting to decipher all of the different underlying molecular mechanisms of how AJs and basolateral proteins regulate the Hippo pathway and how these mechanisms evolved in Drosophila and in mammals.
Methods
Methods for Drosophila culture and imaginal disc immunostaining and mammalian cell culture methods were performed as described in refs. 5 and 28. Antibodies, shRNA, siRNA, and quantitative RT-PCR (qRT-PCR) information is given in SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. M. Shahmoradgoli and Dr. C. Ruteshouser for qRT-PCR consulting and Dr. M. Schroeder and Dr. W. Bossuyt for critical reading of the manuscript. We thank G. Mardon, D. Pan, J. Jiang, I. G. Macara, the Bloomington Drosophila stock center, the Vienna Drosophila RNAi Center, Transgenic RNAi Project at Harvard Medical School [National Institutes of Health (NIH)/National Institute of General Medical Science R01-GM084947], and the Developmental Studies Hybridoma Bank (University of Iowa) for fly stocks and reagents. C.-C.Y. was funded by the Center for Genetics and Genomics, University of Texas MD Anderson Cancer Center. A.B.G. was funded by the Department of Defense (W81XWH-14-1-0053). This work was supported by an NIH grant and an Odyssey grant (Fonds Wetenschappelijk Onderzoek Belgium) (to G.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420850112/-/DCSupplemental.
References
- 1.McCaffrey LM, Macara IG. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011;21(12):727–735. doi: 10.1016/j.tcb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Dow LE, Humbert PO. Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int Rev Cytol. 2007;262:253–302. doi: 10.1016/S0074-7696(07)62006-3. [DOI] [PubMed] [Google Scholar]
- 3.Tepass U. The apical polarity protein network in Drosophila epithelial cells: Regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol. 2012;28:655–685. doi: 10.1146/annurev-cellbio-092910-154033. [DOI] [PubMed] [Google Scholar]
- 4.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192(6):907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19(5):727–739. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Genevet A, Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem J. 2011;436(2):213–224. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder MC, Halder G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin Cell Dev Biol. 2012;23(7):803–811. doi: 10.1016/j.semcdb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13(8):877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halder G, Johnson RL. Hippo signaling: Growth control and beyond. Development. 2011;138(1):9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20(7):573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 13.Ling C, et al. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci USA. 2010;107(23):10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol. 2010;20(7):582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CL, et al. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci USA. 2010;107(36):15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grusche FA, Degoutin JL, Richardson HE, Harvey KF. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev Biol. 2011;350(2):255–266. doi: 10.1016/j.ydbio.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Chen CL, Schroeder MC, Kango-Singh M, Tao C, Halder G. Tumor suppression by cell competition through regulation of the Hippo pathway. Proc Natl Acad Sci USA. 2012;109(2):484–489. doi: 10.1073/pnas.1113882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev Biol. 2011;350(1):139–151. doi: 10.1016/j.ydbio.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varelas X, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell. 2010;19(6):831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 21.Mohseni M, et al. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat Cell Biol. 2014;16(1):108–117. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. 2011;108(29):11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlegelmilch K, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144(5):782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvis MR, et al. α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4(174):ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doggett K, Grusche FA, Richardson HE, Brumby AM. Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling. BMC Dev Biol. 2011;11:57. doi: 10.1186/1471-213X-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarpal R, et al. Mutational analysis supports a core role for Drosophila α-catenin in adherens junction function. J Cell Sci. 2012;125(Pt 1):233–245. doi: 10.1242/jcs.096644. [DOI] [PubMed] [Google Scholar]
- 27.Tepass U, et al. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 1996;10(6):672–685. doi: 10.1101/gad.10.6.672. [DOI] [PubMed] [Google Scholar]
- 28.Hamaratoglu F, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8(1):27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima Y, Meyer EJ, Kroesen A, McKinney SA, Gibson MC. Epithelial junctions maintain tissue architecture by directing planar spindle orientation. Nature. 2013;500(7462):359–362. doi: 10.1038/nature12335. [DOI] [PubMed] [Google Scholar]
- 30.Igaki T. Correcting developmental errors by apoptosis: Lessons from Drosophila JNK signaling. Apoptosis. 2009;14(8):1021–1028. doi: 10.1007/s10495-009-0361-7. [DOI] [PubMed] [Google Scholar]
- 31.Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22(21):5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlirova M, Jasper H, Bohmann D. Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc Natl Acad Sci USA. 2005;102(37):13123–13128. doi: 10.1073/pnas.0504170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Igaki T, Pagliarini RA, Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol. 2006;16(11):1139–1146. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 34.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 35.Navarro C, et al. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24(27):4330–4339. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- 36.Rauskolb C, Pan G, Reddy BV, Oh H, Irvine KD. Zyxin links fat signaling to the hippo pathway. PLoS Biol. 2011;9(6):e1000624. doi: 10.1371/journal.pbio.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rauskolb C, Sun S, Sun G, Pan Y, Irvine KD. Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell. 2014;158(1):143–156. doi: 10.1016/j.cell.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheikh F, et al. alpha-E-catenin inactivation disrupts the cardiomyocyte adherens junction, resulting in cardiomyopathy and susceptibility to wall rupture. Circulation. 2006;114(10):1046–1055. doi: 10.1161/CIRCULATIONAHA.106.634469. [DOI] [PubMed] [Google Scholar]
- 39.Nemade RV, et al. Biogenesis and function of mouse mammary epithelium depends on the presence of functional alpha-catenin. Mech Dev. 2004;121(1):91–99. doi: 10.1016/j.mod.2003.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15(2):309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci USA. 2008;105(39):14897–14902. doi: 10.1073/pnas.0805201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graves HK, Woodfield SE, Yang CC, Halder G, Bergmann A. Notch signaling activates Yorkie non-cell autonomously in Drosophila. PLoS ONE. 2012;7(6):e37615. doi: 10.1371/journal.pone.0037615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enomoto M, Igaki T. Src controls tumorigenesis via JNK-dependent regulation of the Hippo pathway in Drosophila. EMBO Rep. 2013;14(1):65–72. doi: 10.1038/embor.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sansores-Garcia L, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30(12):2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138(18):3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 46.Smith MA, et al. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev Cell. 2010;19(3):365–376. doi: 10.1016/j.devcel.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11(12):1444–1450. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.