Significance
Conjugation of large polypeptides such as Neural precursor cell expressed, developmentally down-regulated 8 (Nedd8) and ubiquitin to proteins is a critical regulatory process for normal cell function. There are more than 1,000 E3 ligases that are involved in the ubiquitin pathway. The predominant activity of one E3, murine double minute-2 protein (Mdm2), has been characterized to mediate ubiquitination of the tumor suppressor p53 in response to genotoxic stress. We show that under growth conditions, active Src kinase binds to and phosphorylates Mdm2 at Y281 and Y302, which recruits the Nedd8 E2 enzyme, Ubc12. This recruitment converts Mdm2 to an E3 that neddylates p53. We provide the first evidence, to our knowledge, showing how phosphorylation may redirect ligase activity. This mechanism may be applicable to numerous E3 ligases and provides a basis for therapeutic intervention.
Keywords: Mdm2, Src, Nedd8
Abstract
Murine double minute-2 protein (Mdm2) is a multifaceted phosphorylated protein that plays a role in regulating numerous proteins including the tumor suppressor protein p53. Mdm2 binds to and is involved in conjugating either ubiquitin or Nedd8 (Neural precursor cell expressed, developmentally down-regulated 8) to p53. Although regulation of the E3 ubiquitin activity of Mdm2 has been investigated, regulation of the neddylating activity of Mdm2 remains to be defined. Here we show that activated c-Src kinase phosphorylates Y281 and Y302 of Mdm2, resulting in an increase in Mdm2 stability and its association with Ubc12, the E2 enzyme of the neddylating complex. Mdm2-dependent Nedd8 conjugation of p53 results in transcriptionally inactive p53, a process that is reversed with a small molecule inhibitor to either Src or Ubc12. Thus, our studies reveal how Mdm2 may neutralize and elevate p53 in actively proliferating cells and also provides a rationale for using therapies that target the Nedd8 pathway in wild-type p53 tumors.
Although c-Src is rarely mutated in human cancers, aberrant activation of this nonreceptor tyrosine kinase is correlated with clinical progression of cancer in numerous tumors derived from different organ systems. Specifically, c-Src is involved in a multitude of tumor progression properties including survival, angiogenesis, migration, and adhesion (1). However, c-Src alone is not a strong transforming agent, which is not surprising considering that transformation is a multifaceted process requiring activation of oncogenes and hyperactivation of signaling pathways (2, 3). One of the downstream effectors of c-Src is the survival factor Akt. Akt has been shown to phosphorylate many substrates to promote cell survival, including the murine double minute-2 protein (Mdm2) (4, 5). Mdm2 undergoes nuclear translocation in response to Akt phosphorylation at serine 166 and 186 (5). Once in the nucleus, Mdm2 binds to tumor suppressor p53 and inhibits its activity. However, in response to genotoxic stress, Mdm2 is phosphorylated by numerous serine, threonine, and tyrosine kinases that result in increased abundance and activity of p53 (6–10). Conversely, under growth conditions, p53 is elevated, yet largely inactive, suggesting there are distinct pathways involved in regulating p53.
One intriguing mechanism that leads to the inactivation of p53 is Mdm2-mediated conjugation of Nedd8 (Neural precursor cell expressed, developmentally down-regulated 8) (11). In a similar fashion to the ubiquitin system, neddylation involves the activation and transfer of Nedd8 from E1 (APP-BP1 in human) to E2 (Ubc12) and E3 really interesting new gene (RING) ligases. In addition, there are de-neddylating enzymes, such as constitutive photomorphogenic (COP9), COP9 signalisome (CSN), and Nedd8 specific protease1 (NEDP1), that act on p53 in response to genotoxic stress (12–14). Physiologically, the Nedd8 pathway is crucial for viability in mice, Caenorhabditis elegans, and Schizosaccharomyces pombe (15), and pathologically, Nedd8-conjugated proteins have been shown to be elevated in certain types of cancer (16).
Thus, dependent on the cellular signaling, the Mdm2–p53 interplay may be differentially regulated. Here we examine the possibility that Mdm2 activity is altered and becomes a neddylating enzyme in response to growth factors. We show a novel Mdm2/c-Src interaction that results in the phosphorylation of Mdm2 at Y281 and Y302. Further analysis revealed that under growth conditions, neither Mdm2 nor p53 is able to be ubiquitinated. The neddylation activity of Src-phosphorylated Mdm2, in contrast, was evident, as both Mdm2 and p53 were conjugated with Nedd8. This switch of Mdm2 activity results in stagnant but elevated levels of p53. Src-mediated activation of the neddylating function of Mdm2 provides a mechanism for inactive but stable p53.
Results
Mdm2 Interacts with the Src Homology 3 Domain of c-Src in Vitro and in Vivo.
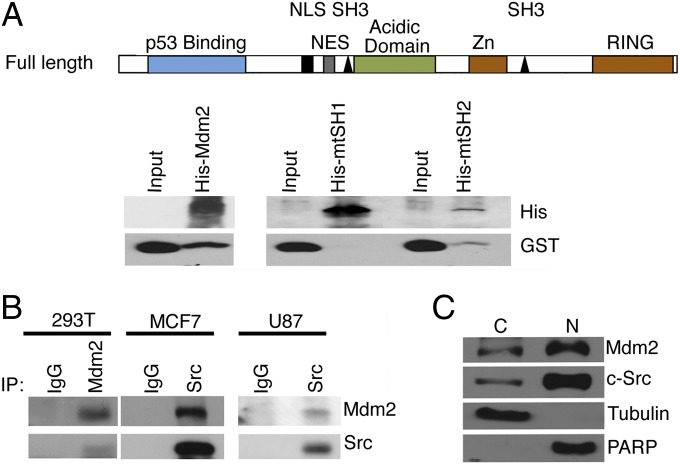
Mdm2 binds to and is a substrate for the nonreceptor kinase c-Abl under genotoxic stress conditions (7). c-Abl contains a Src homology 3 (SH3) domain, which binds to partner proteins also containing an SH3 binding domain (PxxP). Human Mdm2 contains two SH3 binding domains, so we tested whether Mdm2 could bind to other SH3 domain-containing tyrosine kinases by screening an SH3 domain array from Panomics. Using recombinant human Mdm2, we found that Mdm2 binds to c-Abl, Abl2, c-Src, and Hck (Table S1). Because of the prominent role of c-Src in oncogenesis, we pursued the interaction between c-Src and Mdm2 further. A series of Mdm2 truncations were generated, and a GST-pull-down assay showed that they all bound to the SH3-Src domain, but not to GST alone (Fig. S1A), revealing that the portion of Mdm2 needed for the Src interaction is between residues 102 and 268, where the SH3-1 domain was located. Mutation (prolines-alanines) of the amino terminal SH3 binding domain (mtSH3-1) of Mdm2 did not bind GST-SH3Src in a nickel pull-down assay. The carboxy terminal SH3 domain mutant (mtSH3-2) and wild-type Mdm2 maintained the ability to bind GST-SH3Src (Fig. 1A). To further validate this interaction in vivo, an immunoprecipitation of endogenous c-Src or endogenous Mdm2 (MCF7, U87, or 293T cells) revealed that Mdm2 and c-Src form a complex (Fig. 1B). Furthermore, c-Src and Mdm2 were predominantly found in the nuclear fraction (Fig. 1C). Thus, in vitro and in vivo data provide evidence for an Mdm2/c-Src complex.
Fig. 1.
Mdm2 and c-Src form a complex in vitro and in vivo. (A) Schematic of Mdm2 is shown (Top). His-tagged Mdm2 and GST-tagged SH3Src from a nickel pull-down of His-Mdm2, His-Mdm2 SH3 domain mutant 1, and His-Mdm2 SH3 domain mutant 2 were immunoblotted for GST and His antibodies. Input is GST-SH3Src. (B) Immunoprecipitations of endogenous Mdm2 from 293T (Left) or c-Src from MCF7 and U87 cell extracts (Center and Right) were probed with Src and Mdm2 antibodies. (C) Mdm2 and c-Src were detected by immunoblot of cytoplasmic and nuclear extracts from MCF7 cells. Detection of tubulin and PARP indicate extract purity.
c-Src Phosphorylates Mdm2 at Y281 and Y302.
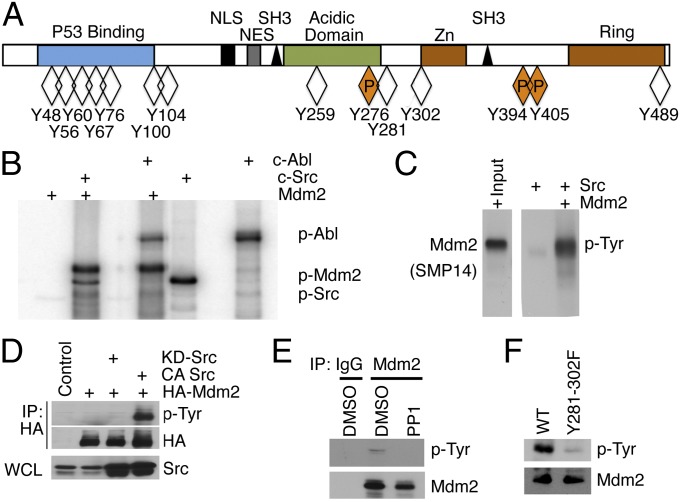
Mdm2 is a highly phosphorylated protein, and yet only two tyrosine kinases (Abl and EGFR) have been identified as phosphorylating Mdm2 (7, 17, 18). There are 14 conserved tyrosines in Mdm2 (human and mouse), three of which are already identified as c-Abl phosphorylation sites (Fig. 2A). To determine whether c-Src could phosphorylate Mdm2, an in vitro phosphorylation reaction was performed using 32P γ-labeled ATP. As expected, Mdm2 was phosphorylated by c-Src and c-Abl (positive control) (Fig. 2B). Using the phospho-tyrosine antibody 4G10, we show that Mdm2 (recombinant and transiently expressed) was tyrosine phosphorylated by Src (Fig. 2 C and D). Endogenous analysis of Mdm2 showed that treatment of MCF7 cells for 16 h with PP1 (a selective Src inhibitor) resulted in a loss of tyrosine phosphorylation of Mdm2 (Fig. 2E). Thus, our in vitro and in vivo data show that Mdm2 is a substrate for Src.
Fig. 2.
c-Src phosphorylates Mdm2 at tyrosines 281 and 302. (A) The total and the defined tyrosine phosphorylation sites (P) in Mdm2 are illustrated. (B) An in vitro phosphorylation reaction of Mdm2 (p-Mdm2) by c-Src or c-Abl using 32P was detected by autoradiography. Autophosphorylation of c-Src and c-Abl, along with c-Abl phosphorylation of Mdm2, served as internal controls. (C) Immunoblot of in vitro phosphorylation reactions with Src and Mdm2 was probed with the 4G10 antibody to detect phospho-tyrosines (pTyr) (Right). (Left) Immunoblot of the input probed with SMP14. (D) HA-Mdm2 was immunopurified from H1299 cells that have been transiently transfected with HA-Mdm2 and KD-Src or CA-Src. Immunoblots were probed with anti-HA antibodies or the p-Tyr antibody, 4G10, to show tyrosine phosphorylation of Mdm2. Immunoblot (Bottom) was probed with Src to show expression in lysates. (E) Mdm2 was immunoprecipitated from MCF7 extracts after treatment with 10 μM PP1 or DMSO for 16 h and immunoblotted for p-Tyr and Mdm2. (F) In vitro phosphorylation reactions were performed with Src on His-tagged 90–383 Mdm2 and the Y281-302F double mutant.
To identify the c-Src phosphorylation sites, we performed mass spectrometry on Mdm2 isolated from actively proliferating T47D and MDA 231 cells. The data show, with high confidence, that b and y ions were recovered for peptides that contained Y302 of Mdm2 (Fig. S1B). We also noted that the peptide containing Y281 was recovered but was in low abundance. The difficulty in recovering this peptide was most likely a result of issues involving enzymatic digestion and fragment sizes amenable for analysis. With high confidence, peptides that contained Y276, Y394, and Y405 (Abl phosphorylation sites) were recovered, but these sites were not phosphorylated. To verify the mass spectrometry data, we performed in vitro phosphorylation reactions and found that the double mutant (Y281-302F) was also not phosphorylated (Fig. 2F). These data show, through diverse approaches, that Y281 and Y302 are bona fide phosphorylation sites for Src.
c-Src Elevates Mdm2 Protein Levels.
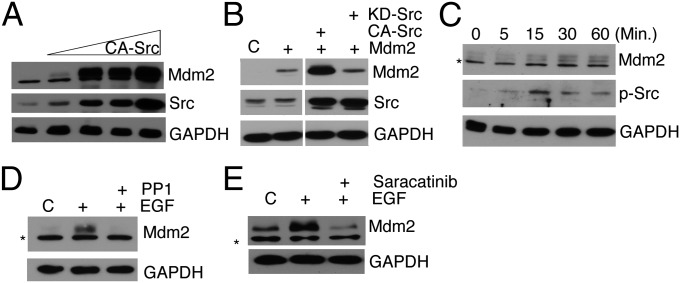
We examined the possibility that Src phosphorylation would decrease the levels of Mdm2, as we have previously shown that Abl phosphorylation induces destabilization (6). Contrary to Abl phosphorylation of Mdm2, we unexpectedly observed that Mdm2 protein levels increased concomitantly as constitutively active (CA)-Src levels increased (Fig. 3A), and as Fig. 3B shows, the increase in Mdm2 levels was dependent on the kinase activity of Src. It is noteworthy that the observed increases in Mdm2 protein levels are not dependent on p53 (H1299 cells are devoid of p53) or Src signaling-mediated mdm2 gene expression (Fig. S2 A and B).
Fig. 3.
c-Src phosphorylation results in an enhancement of Mdm2 protein levels. (A) Immunoblot analysis was performed with H1229 cells overexpressing Mdm2 that had been treated with increasing concentrations (0.5, 1, 5, 10 μg) of CA-Src. (B) Cellular extracts of H1299 cells ectopically expressing Mdm2 alone or with CA-Src or KD-Src were immunoblotted for Mdm2, c-Src, and GAPDH. (C) MCF7 cells were serum starved for 48 h and then treated with 200 ng/mL EGF for the time indicated. Endogenous Mdm2, p-Src, and GAPDH (for loading) were analyzed by immunoblot. The asterisk indicates a nonspecific band. (D) MCF7 cells were serum starved for 48 h and then pretreated with either DMSO or PP1 (10 μM) for 1 h before the addition of EGF (200 ng/mL). EGF treatment of 1 h was followed by immunoblot analysis of Mdm2 and GAPDH. The asterisk indicates a nonspecific band. (E) Mdm2 levels (and GAPDH as control) in MCF7 cells pretreated with saracatanib or DMSO overnight followed by EGF for 1 h were detected by immunoblotting. The asterisk indicates a nonspecific band.
To determine whether growth factors could alter Mdm2 levels through the activation of c-Src, epidermal growth factor (EGF) was incubated with MCF7 cells that were serum-starved for 48 h. Mdm2 levels increased with EGF stimulation until a plateau was achieved at 30 min (Fig. 3C). Pretreatment with c-Src inhibitors PP1 (Fig. 3D) or saracatanib (Fig. 3E) prevented the increase in Mdm2. Thus, the increase in endogenous Mdm2 protein is a result of activated c-Src.
Src Phosphorylation Alters the Enzymatic Activity and Half-Life of Mdm2.
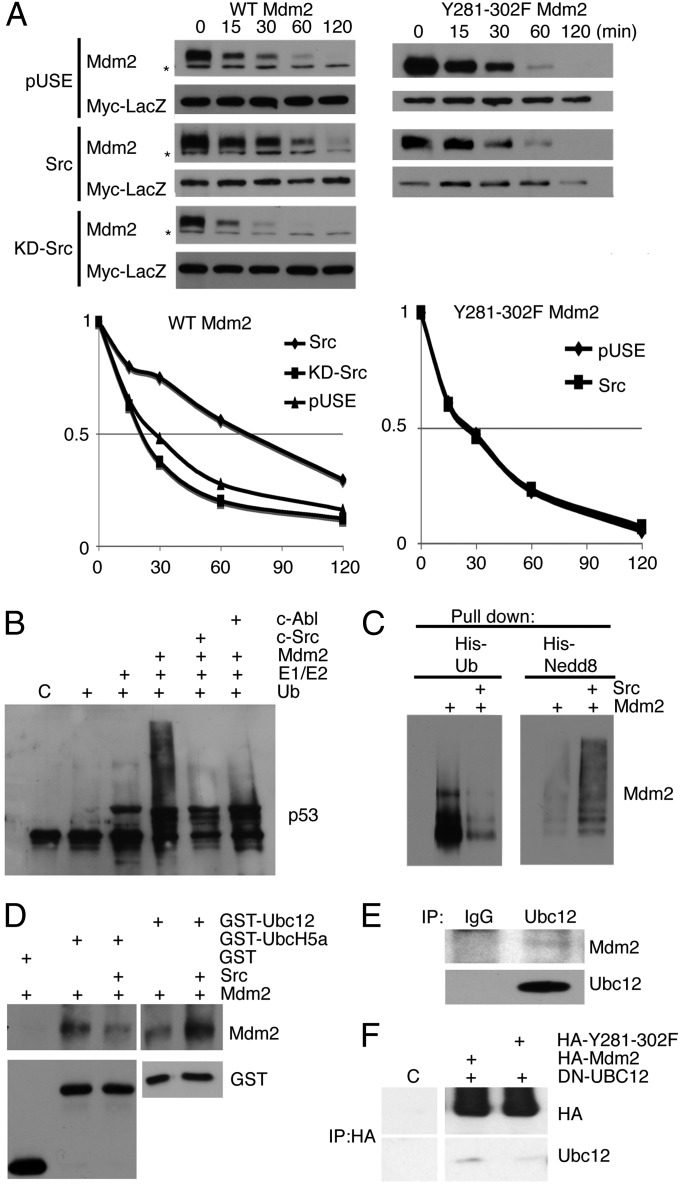
We next examined whether the increased Mdm2 levels in response to Src activation were a result of an increase in half-life by treating transfected H1299 cells with cycloheximide. In the presence or absence of kinase dead Src (KD)-Src, the half-life of Mdm2 was 25 min (Fig. 4A, Left). In the presence of CA-Src, the half-life of Mdm2 more than doubled, increasing to 70 min (Fig. 4A, Left). The half-life of the mutant Mdm2 remained at 25 min, the same as wild-type Mdm2 without Src or in the presence of KD-Src (Fig. 4A, Right). Therefore, Src-dependent phosphorylation of Mdm2 increased the half-life of Mdm2, suggesting the ubiquitin ligase activity of Mdm2 is not operative.
Fig. 4.
c-Src expression increases the half-life of Mdm2 through inhibition of its E3 ubiquitination activity. (A) H1299 cells were transfected with Mdm2 (Left) or Y281-302F Mdm2 (Right) and CA-Src, KD-Src, or empty vector (pUSE) in the presence of Myc-LacZ vector. Twenty-four hours posttransfection, the cells were treated with 50 μg/mL cycloheximide (CHX) and harvested at different points, as indicated. The cell lysates were immunoblotted with anti-Mdm2 and anti-Myc antibodies, as indicated (Top). The density of Mdm2 in each lane was quantified against the level of Myc-LacZ and plotted in a graph (Bottom). The asterisk indicates a nonspecific band. (B) An in vitro ubiquitination assay was performed with recombinant p53, using Mdm2 or Mdm2 phosphorylated by c-Src/c-Abl. Immunoblot was probed for p53 (D01). (C) A His pull-down assay was used to determine whether Mdm2 was either His-ubiquitinated (Left) or His-neddylated (Right) in the presence of CA-Src. Immunoblots were used to detect Mdm2. (D) A GST-pull-down of GST, GST-Ubc5, or GST-Ubc12 was used to determine whether Mdm2 or c-Src-phosphorylated Mdm2 would preferentially complex to an E2. Mdm2 and GST were detected by immunoblot. (E) Cellular extracts from MCF7 cells were used to immunoprecipitate Ubc12 or isotype control. Mdm2 and Ubc12 were detected by immunoblot. (F) Immunoblots detected Ubc12 and HA, from immunoprecipitations of HA from 239T cells transiently transfected with combinations of HA-Mdm2, HA-Y281-302F Mdm2, and DN-Ubc12.
When the autoregulatory loop is engaged, Mdm2 is associated with decreased p53 levels; this decrease is a result of the Mdm2-dependent ubiquitination of p53. In fact, ectopic expression that leads to high levels of Mdm2 has been reported to facilitate poly-ubiquitination of p53 (19). Because c-Src phosphorylation results in increased Mdm2 levels, we examined whether ubiquitination of p53 would be evident. An in vitro ubiquitination assay of p53 was performed using unphosphorylated, Src, or Abl-phosphorylated Mdm2. c-Abl is known to inhibit Mdm2 ubiquitination of p53, and thus served as a control (6, 7). As shown in Fig. 4B, phosphorylation with c-Src inhibited the ubiquitination of p53 to the same extent as c-Abl. Furthermore, we examined whether the loading of ubiquitin to Mdm2 was altered by phosphorylation in transient transfection assays. In the presence of CA-Src, ubiquitinated Mdm2 was dramatically diminished compared with Mdm2 alone (Fig. 4C, Left). These data are consistent with the in vitro assays in Fig. 4B. Because tyrosine phosphorylation of Mdm2 was implicated with loss of activity (Fig. 4B), we also determined whether the neddylating activity associated with Mdm2 was also decreased with Src phosphorylation. To our surprise, Mdm2 was neddylated in the presence of c-Src (Fig. 4C, Right).
Because Mdm2 activity is specified by which E2 it binds, we determined whether Src phosphorylation of Mdm2 increased the association with Ubc12 (the E2 ligase for Nedd8). Immunoblot analysis of a GST pull-down assay shows that unphosphorylated Mdm2 bound to Ubc5a (the E2 ligase for ubiquitin) preferentially over Ubc12, which was diminished with Src-phosphorylated Mdm2. Conversely, an increase in the Ubc12/Mdm2 interaction was observed with Src-phosphorylated Mdm2 (Fig. 4D). This experiment provides evidence that Src phosphorylation of Mdm2 changes the recruitment of E2 enzymes. Indeed, endogenous Ubc12 copurified endogenous Mdm2 (Fig. 4E) and endogenous Mdm2 copurified endogenous Ubc12 (Fig. S2C). We also examined whether transiently expressed HA-Mdm2 or HA-Mdm2 (Y281-302F) would purify Ubc12. Dominant negative Ubc12 was used to eliminate the potential for a transient interaction between Mdm2 and an active Ubc12. The data in Fig. 4F show that immunoprecipitation of Mdm2 purified Ubc12, but not the Y281-302F mutant of Mdm2. These data support a novel mechanism by which Src-mediated tyrosine phosphorylation of Mdm2 increases binding to Ubc12 and the formation of a neddylating complex.
Regulation of p53 by c-Src-Phosphorylated Mdm2.
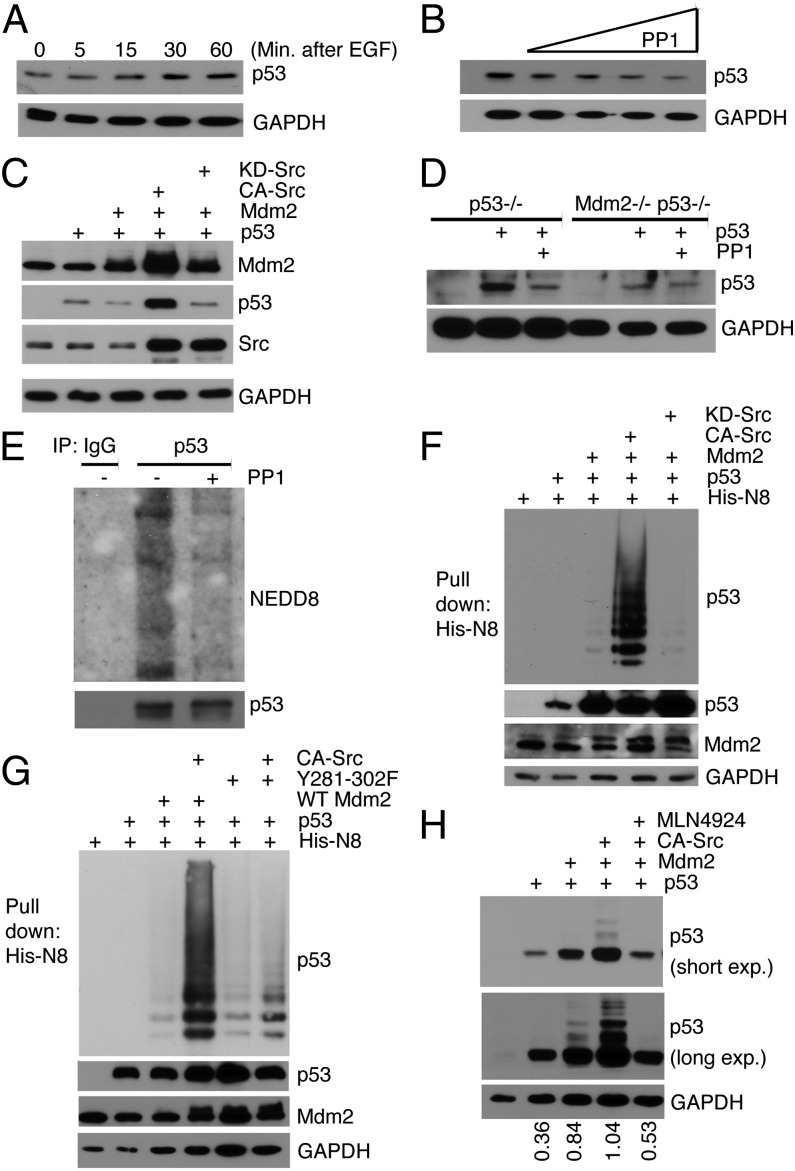
Because we have established that Src-phosphorylated Mdm2 complexes with Ubc12, we examined whether p53 would be a substrate. Initially, we incubated MCF7 cells with EGF and examined endogenous p53 levels over a time course. Immunoblot analysis shows that p53 increased (Fig. 5A), which is consistent with c-Src activation, increased Mdm2 protein levels (Fig. 3C), and decreased ubiquitination (Fig. 4B). To examine whether blockade of c-Src would affect p53 levels, actively growing MCF7 cells were treated with PP1. We observed decreasing levels of p53 as PP1 concentrations increased (Fig. 5B). This supports a switch in Mdm2 from a Src-dependent neddylating enzyme to a ubiquitinating enzyme. In addition, transfection of Mdm2 alone and with KD-Src resulted in decreased p53, whereas p53 levels were elevated in the presence of Mdm2 and CA-Src (Fig. 5C). To confirm that p53 stabilization in response to active Src requires Mdm2, we transiently transfected p53 into p53−/− or p53−/− Mdm2−/− murine embryo fibroblasts (MEFs), and treated with PP1 (Fig. 5D). In the absence of Mdm2 (p53−/−Mdm2−/−), p53 levels remained unchanged with PP1 treatment. Conversely, in the presence of Mdm2 (p53−/−), PP1 treatment resulted in a decrease in ectopically expressed p53. Collectively, these results show that c-Src phosphorylation of Mdm2 results in the stabilization of p53 levels. Furthermore, Fig. 5E shows that in the presence of PP1 treatment, fewer Nedd8 conjugated proteins copurified with p53 compared with actively growing cells. Although these data show that a p53 complex is neddylated in response to c-Src activation, this does not provide direct evidence that p53 was neddylated. To address this issue, we transfected H1299 cells with a His-Nedd8 construct, along with p53, Mdm2, and CA-Src or KD-Src, and conducted a nickel pull-down assay to isolate His- tagged, neddylated proteins. Fig. 5F illustrates that in the presence of CA-Src, neddylated p53 dramatically increases. As expected, Nedd8 conjugated to p53 was absent when KD-Src was present (Fig. 5F), which indicates that the neddylating activity of Mdm2 is dependent on active Src. To provide further evidence that the switch of Mdm2 to neddylating activity was dependent on Src-phosphorylation, a His-Nedd8 pull-down assay using the Mdm2 phosphorylation-deficient mutant Y281-302F was performed. The neddylation of p53 increases dramatically in the presence of WT Mdm2 and CA-Src, but not in the presence of Y281-302F and CA-Src (Fig. 5G). Furthermore, we found that the Y281-302 mutant of Mdm2 generated more ubiquitinated p53 than wild-type Mdm2 (Fig. S2D). Considering that Mdm2 is functioning as part of a neddylating complex under growth conditions, we sought to determine whether this activity could be disrupted pharmacologically. Recently, a potent inhibitor, MLN4924, that targets the Nedd8-activating enzyme was developed (20). This compound blocked the Mdm2-mediated p53 neddylation (Fig. 5H). Taken together, increased p53 levels result from c-Src activation of the neddylating function of Mdm2.
Fig. 5.
p53 protein levels are regulated by c-Src-phosphorylated Mdm2. (A) MCF7 cells were serum starved for 48 h and incubated with EGF over a time course of 1 h. Extracts were immunoblotted for p53 and GAPDH. (B) MCF7 cells were treated with increasing concentrations of PP1 (1, 5, 10, 15, 20 μM) for 16 h. Cellular extracts were immunoblotted for p53 and GAPDH. (C) Lysates from H1299 cells overexpressing combinations of p53, Mdm2, CA-Src, or KD-Src were immunoblotted for Mdm2, p53, Src, and GAPDH. (D) Cellular extracts from p53−/− or p53−/− Mdm2−/− MEFs that had been transiently transfected with p53 and treated with PP1 (10 μM) or DMSO were immunoblotted for p53 and GAPDH. (E) Immunoprecipitations of p53 and IgG control from MCF7 cellular extracts were used to detect endogenous Nedd8 and p53 in immunoblots. Cells were either treated with PP1 (10 μM) or DMSO for 16 h. (F) p53 was detected by immunoblot from a His-Nedd8 pull-down assay conducted in H1299 cells expressing His-Nedd8, p53, WT-Mdm2, CA-Src, or KD-Src. To verify expression, lysates were subjected to immunoblot analysis, as indicated. (G) His-Nedd8 pull-down was performed as in F, but with the use of Mdm2-Y281-302F, as indicated. (H) H1299 cells were transfected with p53, Mdm2, and CA-Src 24 h before treatment with either DMSO or 0.3 μM of the Nedd8-activating enzyme inhibitor MLN4924 for 16 h. Immunoblot detected p53 and GAPDH and relative amounts of p53 were determined.
p53 Levels Are Divorced from Transcriptional Activity.
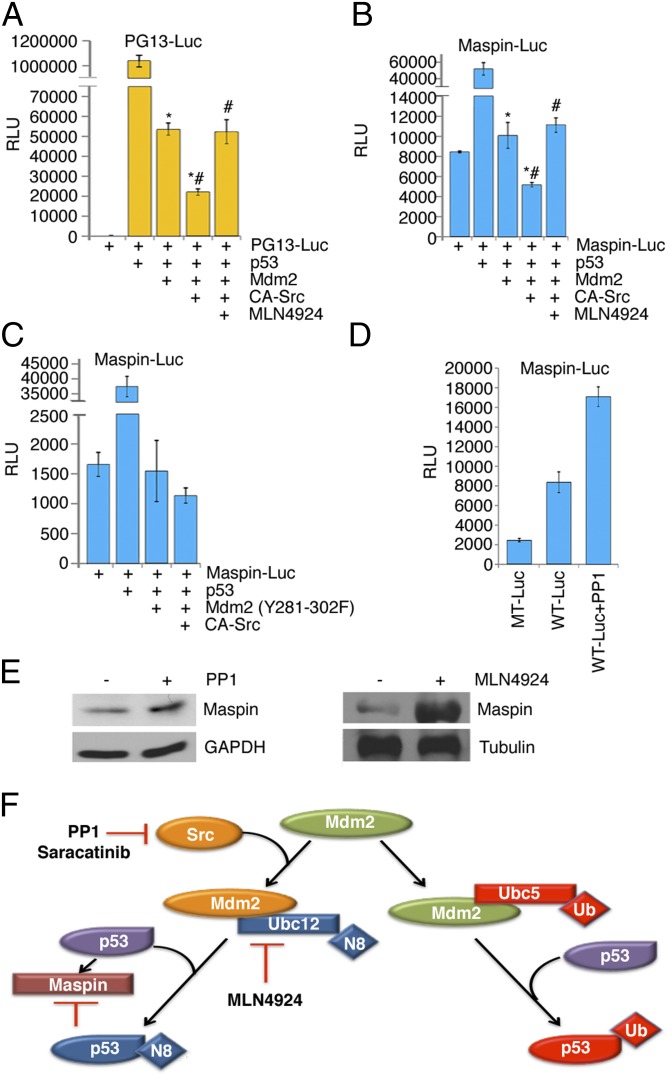
We have established that Src-phosphorylation leads to increased neddylated-p53, and considering that increased levels of p53 are not necessarily an indication of transcriptional activity, we tested whether p53 was transcriptionally active under conditions of neddylation. We initiated our study using PG13-Luc, which is an artificial p53 responsive promoter linked upstream of luciferase, along with combinations of p53, Mdm2, CA-Src, and beta-galactosidase (internal control for transfection efficiency) and/or treatment with MLN4924. As predicted, in the presence of Mdm2, p53 activity was diminished, and a further decrease was evident when CA-Src was present. This decrease in p53 activity was reversed to a level similar to that in Mdm2 alone with MLN4924 treatment (Fig. 6A).
Fig. 6.
Increased p53 levels do not translate to increased transcriptional activity. (A) PG13-Luc activity was assayed using H1299 cells transfected with Myc-LacZ, p53, Mdm2, and CA-Src. Twenty-four hours posttransfection, cells were treated with DMSO or 0.3 μM of the Nedd8-activating enzyme inhibitor MLN4924 for 16 h. (B) H1299 cells were treated the same as in A, except luciferase was assayed using Maspin-Luc and Myc-LacZ. (C) maspin-luciferase activity was examined in H1299 cells transfected with p53, Mdm2 Y281-302F, and CA-Src. (D) Luciferase assay of MCF7 cells transfected with either Maspin-Luc or Mutant Maspin-Luc (MT) and Myc-LacZ was conducted. Twenty-four hours after transfection, cells were treated with DMSO or 10 μM PP1 for 16 h. (A–D) Y axis measurements are relative luciferase units (RLU). The RLU were calculated from the ratio of luciferase/β-gal activity. Error bars represent SD of three separate experiments. *,#P < 0.05. (E) MCF7 cells were treated with DMSO or 10 μM PP1 and subjected to immunoblotting for Maspin and GAPDH. (F) In response to activated Src, Mdm2 is phosphorylated and selectively binds to Ubc12 to function as a neddylating enzyme. The respective inhibitors to the pathway are denoted.
We next determined whether the Src-Mdm2-Nedd8-p53 axis regulates the tumor suppressor Maspin, which plays an important role in regulating tumor cell invasion and metastasis (21). We have previously shown that p53 is required for the up-regulation of maspin under hypoxic conditions in glioblastoma cell lines (22). The data in Fig. 6B show that p53 drives the expression of luciferase from the maspin promoter and that a decrease is seen with Mdm2 overexpression. Just like with the PG13-Luc, expression of CA-Src further inhibits p53 activity on the maspin promoter, and this inhibition is dependent on neddylation. The Src phosphorylation sites in Mdm2 were necessary for the additional decrease in p53 activity, as maspin-luc activity was equally inhibited with or without CA-Src when the Y281-302F Mdm2 was present (Fig. 6C). This experiment shows that Mdm2, when phosphorylated at Y281 and Y302, is more effective at repressing p53-mediated maspin gene expression. When MCF7 cells were transfected with the maspin-luc construct or a mutant maspin-luc (MT1) that is unresponsive to p53, activity from the MT1 promoter was significantly lower than that from the maspin-luc promoter, demonstrating that luciferase activity was dependent on endogenous p53 (Fig. 6D). Treatment of cells with PP1 resulted in an increase in endogenous p53 activity (Fig. 6D). We next examined whether the activity observed in the transient reporter assays was reflected in endogenous Maspin protein levels. Similar to the reporter assays, when the neddylating activity of Mdm2 was impeded by treatment with PP1 or MLN4924, Maspin protein levels increased as a result of the alleviated repression of p53 activity (Fig. 6E). Together, these findings show that neddylation of p53 is responsible for a reduction in p53 activity.
Discussion
Mdm2 is interesting clinically, as the gene is amplified in 10% of all human cancers, yet the protein is detectable in 40–90% of cases (23). This increase in detectable Mdm2 in cancer raises questions as to how Mdm2 is elevated. Mdm2 is undetectable in normal tissue, and external stimuli might be involved in elevating levels in cancer. Indeed, the current paradigm is that the mdm2 gene is a transcriptional target of the p53 tumor suppressor, in response to stress signals such as low genotoxic stress. This pathway then forms an autoregulatory feedback loop to down-regulate p53 through the proteasome. However, there may be other mechanisms that can elevate Mdm2. Because the tumor microenvironment has high concentrations of numerous growth factors and cytokines, these factors may play a role in regulating Mdm2. In fact, recent work shows that sustained TGFβ1 exposure induces the Smad3/Smad4 complex to bind the promoter of mdm2 and increase gene expression, an event independent of p53 activity (24).
Several growth factors including EGF can activate c-Src (25), and considering that c-Src is downstream of many growth factors, it is not surprising that c-Src functions poorly as an oncogenic factor on its own. c-Src is integrated into a number of normal and pathologic processes, including proliferation, survival, motility, and angiogenesis (1, 26). In support of Src mediating oncogenic signaling, we show that the oncogene Mdm2 interacts with, and is a downstream target of, c-Src (Figs. 1 A–C and 2 B–F). The phosphorylation of Mdm2 by c-Src occurs on tyrosines 281 and 302 (Figs. 2F and S1B), which are within or close proximity to the zinc finger domain of Mdm2. Recent work has shown that the zinc finger domain is important for the half-life of Mdm2, which is supported by a C305F mutation that results in loss of ubiquitin ligase activity (27). Through the use of ectopically expressed constitutively active Src or activation by EGF, we show that Src phosphorylation of Mdm2 results in an increase in Mdm2 protein stability (Figs. 3 and 4). Therefore, our study and published work on the zinc finger support the notion that this domain of Mdm2 is highly regulated and is pivotal in controlling Mdm2 activity.
There is a complex interplay between p53 and Mdm2, where Mdm2 may block p53 transcriptional activity without destabilization by masking p53’s N-terminal transactivation domain (28). This phenomenon is evident as C464S, the RING finger mutant of Mdm2, is embryonically lethal, akin to the mdm2−/− mouse, despite still being efficient at inhibiting p53 transcriptional activity (29). This finding provides evidence that the ligase activity of Mdm2 is necessary to regulate p53 during specific windows of development. In parallel with these studies, our data illustrate that the C464S mutant can inhibit p53, yet the RING domain of Mdm2 is required for a robust reduction in p53 activity in the presence of CA-Src (Fig. S3A).
Although much is known about the role of Mdm2 during genotoxic stress, very little information has been generated on how Mdm2 is functioning under conditions of growth. We show that signaling pathways emanating from cell surface receptors result in c-Src phosphorylation at Y281 and Y302, which “flips the switch” for Mdm2 to become a neddylating enzyme (Figs. 4C and 5 E–H). Phosphorylation at these sites is required for recruitment of Ubc12, the conjugation of Nedd8 to p53, and thus, inactivation of p53 (Figs. 5 and 6). Initial work showing that Mdm2 has neddylating activity on p53 did not provide a mechanism of how this activity was engaged (11).
The conjugation of nedd8 to p53 could be reversed in response to genotoxic stress, as the deneddylating enzyme NEDP1 is activated (30) and can then reverse neddylation of p53. This finding shows that there are distinct pathways that regulate p53, which are dependent on specific stimuli. It could be envisioned that if growth signals are dominant, then the genotoxic signaling would be tempered, and p53 activity minimalized. Thus, understanding how signaling pathways promote growth and cell death will be key for the development of therapeutic approaches to activate a robust p53 response.
Inhibition of global neddylation with MLN4924 leads to apoptotic death in human cancer cells and suppresses the growth of human tumors in mouse xenografts (23). In fact, we observed that loss of Src/Nedd8 activity leads to decreased cell survival (Fig. S3B). This result corresponded with inhibition of the neddylation activity of Mdm2 with PP1 and MLN4924 (Fig. 6), unneddylated p53, and the induction of the downstream target maspin gene. p53 can target many genes including maspin, which can be induced in the absence of extrinsic genotoxic stress (22). Maspin is normally highly expressed in normal breast epithelial cells, but is down-regulated in invasive and metastatic breast carcinoma cells (31). Clinically, its expression correlated with decreased microvessel staining in human breast cancer samples (32), and our work shows induction when we inhibit the neddylation activity of Mdm2 with PP1 and MLN4924 (Fig. 6). Thus, we have identified Src, Mdm2, Ubc12, and NEDD8 as components of a pathway that impedes the ability of p53 to induce maspin (Fig. 6F). This pathway can be reversed pharmacologically to increase protein levels, which may negatively affect tumor progression in cancers that maintain p53 genetically.
Methods
Cell Culture and Treatments.
Cells were cultured are described in SI Methods.
Protein Analysis and Immunoprecipitation.
Whole-cell extracts (lysates) were prepared as described and used for Western blot analysis or immunoprecipitation (6). Additional information regarding reagents and buffers and in vitro reactions is located in SI Methods.
Statistical Analysis.
All statistical analysis was done using a two-tailed t test with unequal variance.
Supplementary Material
Acknowledgments
We thank Dr. Lawrence Quilliam for the SH3 domain proteins, Dr. Brenda Schulman for the Ubc12 constructs, and Dr. Stephen Jones for the MEFs. We also thank Dr. Mu Wang at the Proteomic Core facility at Indiana University School of Medicine, and Phil Wubbolding for generation of reagents. This work was supported by National Cancer Institute R01 (CA172256), Research Support Funds Grant from Indiana University Purdue University at Indianapolis, and Riley Children’s Foundation grants (to L.D.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416656112/-/DCSupplemental.
References
- 1.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4(6):470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 2.Shalloway D, Coussens PM, Yaciuk P. Overexpression of the c-src protein does not induce transformation of NIH 3T3 cells. Proc Natl Acad Sci USA. 1984;81(22):7071–7075. doi: 10.1073/pnas.81.22.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: Epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2(3):203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA. 2001;98(20):11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waning DL, Lehman JA, Batuello CN, Mayo LD. c-Abl phosphorylation of Mdm2 facilitates Mdm2-Mdmx complex formation. J Biol Chem. 2011;286(1):216–222. doi: 10.1074/jbc.M110.183012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg Z, et al. Tyrosine phosphorylation of Mdm2 by c-Abl: Implications for p53 regulation. EMBO J. 2002;21(14):3715–3727. doi: 10.1093/emboj/cdf384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hjerrild M, et al. Phosphorylation of murine double minute clone 2 (MDM2) protein at serine-267 by protein kinase CK2 in vitro and in cultured cells. Biochem J. 2001;355(Pt 2):347–356. doi: 10.1042/0264-6021:3550347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khosravi R, et al. Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci USA. 1999;96(26):14973–14977. doi: 10.1073/pnas.96.26.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayo LD, Turchi JJ, Berberich SJ. Mdm-2 phosphorylation by DNA-dependent protein kinase prevents interaction with p53. Cancer Res. 1997;57(22):5013–5016. [PubMed] [Google Scholar]
- 11.Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell. 2004;118(1):83–97. doi: 10.1016/j.cell.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Liakopoulos D, Doenges G, Matuschewski K, Jentsch S. A novel protein modification pathway related to the ubiquitin system. EMBO J. 1998;17(8):2208–2214. doi: 10.1093/emboj/17.8.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyapina S, et al. Promotion of NEDD-CUL1 conjugate cleavage by COP9 signalosome. Science. 2001;292(5520):1382–1385. doi: 10.1126/science.1059780. [DOI] [PubMed] [Google Scholar]
- 14.Xirodimas DP. Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochem Soc Trans. 2008;36(Pt 5):802–806. doi: 10.1042/BST0360802. [DOI] [PubMed] [Google Scholar]
- 15.Pan ZQ, Kentsis A, Dias DC, Yamoah K, Wu K. Nedd8 on cullin: Building an expressway to protein destruction. Oncogene. 2004;23(11):1985–1997. doi: 10.1038/sj.onc.1207414. [DOI] [PubMed] [Google Scholar]
- 16.Chairatvit K, Ngamkitidechakul C. Control of cell proliferation via elevated NEDD8 conjugation in oral squamous cell carcinoma. Mol Cell Biochem. 2007;306(1-2):163–169. doi: 10.1007/s11010-007-9566-7. [DOI] [PubMed] [Google Scholar]
- 17.Dias SS, Milne DM, Meek DW. c-Abl phosphorylates Hdm2 at tyrosine 276 in response to DNA damage and regulates interaction with ARF. Oncogene. 2006;25(50):6666–6671. doi: 10.1038/sj.onc.1209671. [DOI] [PubMed] [Google Scholar]
- 18.Arasada RR, Carpenter G. Secretase-dependent tyrosine phosphorylation of Mdm2 by the ErbB-4 intracellular domain fragment. J Biol Chem. 2005;280(35):30783–30787. doi: 10.1074/jbc.M506057200. [DOI] [PubMed] [Google Scholar]
- 19.Li M, et al. Mono- versus polyubiquitination: Differential control of p53 fate by Mdm2. Science. 2003;302(5652):1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- 20.Soucy TA, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458(7239):732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 21.Zou Z, et al. p53 regulates the expression of the tumor suppressor gene maspin. J Biol Chem. 2000;275(9):6051–6054. doi: 10.1074/jbc.275.9.6051. [DOI] [PubMed] [Google Scholar]
- 22.Eitel JA, et al. PTEN and p53 are required for hypoxia induced expression of maspin in glioblastoma cells. Cell Cycle. 2009;8(6):896–901. doi: 10.4161/cc.8.6.7899. [DOI] [PubMed] [Google Scholar]
- 23.Rayburn E, Zhang R, He J, Wang H. MDM2 and human malignancies: Expression, clinical pathology, prognostic markers, and implications for chemotherapy. Curr Cancer Drug Targets. 2005;5(1):27–41. doi: 10.2174/1568009053332636. [DOI] [PubMed] [Google Scholar]
- 24.Araki S, et al. TGF-beta1-induced expression of human Mdm2 correlates with late-stage metastatic breast cancer. J Clin Invest. 2010;120(1):290–302. doi: 10.1172/JCI39194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osherov N, Levitzki A. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur J Biochem. 1994;225(3):1047–1053. doi: 10.1111/j.1432-1033.1994.1047b.x. [DOI] [PubMed] [Google Scholar]
- 26.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23(48):7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 27.Lindström MS, Jin A, Deisenroth C, White Wolf G, Zhang Y. Cancer-associated mutations in the MDM2 zinc finger domain disrupt ribosomal protein interaction and attenuate MDM2-induced p53 degradation. Mol Cell Biol. 2007;27(3):1056–1068. doi: 10.1128/MCB.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 29.Itahana K, et al. Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell. 2007;12(4):355–366. doi: 10.1016/j.ccr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Watson IR, et al. Chemotherapy induces NEDP1-mediated destabilization of MDM2. Oncogene. 2010;29(2):297–304. doi: 10.1038/onc.2009.314. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6(2):196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 32.Hojo T, et al. Association of maspin expression with the malignancy grade and tumor vascularization in breast cancer tissues. Cancer Lett. 2001;171(1):103–110. doi: 10.1016/s0304-3835(01)00569-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.