Significance
Tuberculosis (TB) is the second most lethal pathogen worldwide. Pulmonary granulomas are a hallmark of this disease. By discovering similarities between granulomas and solid cancerous tumors, we identified a novel therapeutic target for TB, the abnormal granuloma-associated vasculature that contributes to the abnormal granuloma microenvironment. We then asked if we could “normalize” granuloma vasculature by blocking VEGF signaling, an approach originally shown to enhance cancer treatment. Our results demonstrate that bevacizumab, a widely prescribed anti-VEGF antibody for cancer and eye diseases, is able to create more structurally and functionally normal granuloma vasculature and improve the delivery of a low-molecular-weight tracer. This effect suggests that vascular normalization in combination with anti-TB drugs has the potential to enhance treatment in patients with TB.
Keywords: antiangiogenesis, hypoxia, host-directed therapy, Mycobacterium tuberculosis, rabbit model
Abstract
Tuberculosis (TB) causes almost 2 million deaths annually, and an increasing number of patients are resistant to existing therapies. Patients who have TB require lengthy chemotherapy, possibly because of poor penetration of antibiotics into granulomas where the bacilli reside. Granulomas are morphologically similar to solid cancerous tumors in that they contain hypoxic microenvironments and can be highly fibrotic. Here, we show that TB-infected rabbits have impaired small molecule distribution into these disease sites due to a functionally abnormal vasculature, with a low-molecular-weight tracer accumulating only in peripheral regions of granulomatous lesions. Granuloma-associated vessels are morphologically and spatially heterogeneous, with poor vessel pericyte coverage in both human and experimental rabbit TB granulomas. Moreover, we found enhanced VEGF expression in both species. In tumors, antiangiogenic, specifically anti-VEGF, treatments can “normalize” their vasculature, reducing hypoxia and creating a window of opportunity for concurrent chemotherapy; thus, we investigated vessel normalization in rabbit TB granulomas. Treatment of TB-infected rabbits with the anti-VEGF antibody bevacizumab significantly decreased the total number of vessels while normalizing those vessels that remained. As a result, hypoxic fractions of these granulomas were reduced and small molecule tracer delivery was increased. These findings demonstrate that bevacizumab treatment promotes vascular normalization, improves small molecule delivery, and decreases hypoxia in TB granulomas, thereby providing a potential avenue to improve delivery and efficacy of current treatment regimens.
As one of the most prevalent infectious diseases in the world today, Mycobacterium tuberculosis (MTB) infects roughly one-third of the global population, resulting in 2 million deaths annually (1). Although current treatment regimens are largely successful in curing the disease (2), they require 6–8 mo of treatment with up to four agents (3), and multidrug-resistant bacterial strains have emerged and proliferated (4). Resistance to front-line therapies necessitates treatment with up to five or six second-line agents that are poorly tolerated, and treatment success is only achieved in 40–70% of patients (5). Failure to cure drug-resistant disease leads to acquisition of further resistance with a progressively poorer prognosis for these patients, thus fueling an emerging epidemic of drug-resistant disease that threatens to overwhelm fragile health care systems in developing countries (6).
When infected with the tuberculosis (TB) bacilli, the body triggers an immune response that walls off the bacteria in dense cellular masses known as granulomas, or tubercular lesions (7). These abnormal tissue structures, which can vary in size within the same host, are surrounded by fibrous cuffs that serve to contain the MTB bacilli (7, 8). Recent studies have demonstrated a wide variation in the spatial distribution of drugs within TB granulomas, with very few agents able to penetrate the central regions (9). This differential ability of drugs to penetrate TB granulomas has been incorporated into modern TB drug development programs as a criterion for optimizing lead molecules and selecting efficacious combinations (10). However, the mechanisms that contribute to this differential penetration of drugs are not fully understood, and novel strategies to improve TB drug delivery and efficacy are urgently needed.
Following infection with MTB, pulmonary granulomas form in humans and develop heterogeneous microenvironments, often featuring hypoxia (i.e., low levels of oxygen) and central necrosis, which are recapitulated in nonhuman primate and rabbit models of the disease (11). Large lesions appear to develop their own vasculature, presumably allowing them to continue to grow (7). However, the morphological and functional characteristics of granuloma-associated vessels are largely unknown. In solid tumors, cancer cells can form similar dense tissue masses with abnormal associated vasculature. The physiological abnormalities that characterize tumor vessels have been investigated extensively (12, 13). For example, hypoxia, a common feature in solid tumors, stimulates the overproduction of proangiogenic factors, such as VEGF. Proangiogenic factors enhance the formation of new immature, tortuous, and hyperpermeable vessels (12, 14), often with excess endothelial cells, a lack of associated pericytes (i.e., perivascular cells), and uneven basement membranes (15–17). These atypical features result in an impaired blood flow that further compromises delivery of drugs and oxygen (13). Hypoxia also causes immunosuppression, inflammation, and fibrosis, and it can also confer resistance to many drugs (18). Here, we propose that TB granulomas share many characteristics with solid tumors, namely, that they are associated with abnormal and dysfunctional vasculature that can impair the delivery of small molecules, such as oxygen and antibiotics.
Because VEGF is a critical growth factor required for new blood vessel formation (16), anti-VEGF agents were originally developed to block tumor growth by inhibiting blood vessel formation (19). However, bevacizumab, a humanized monoclonal antibody developed to neutralize human VEGF, failed to improve survival benefit as a monotherapy but conferred survival benefit only in combination with chemotherapy or immunotherapy (18). A potential explanation for the success of combined therapies is that bevacizumab “normalizes” the abnormal vasculature of tumors, resulting in improved delivery of concurrently administered anticancer drugs, as well as alleviation of hypoxia (13, 15, 18, 20, 21). However, this strategy has not been tested in a TB disease model. In this study we show, for the first time to our knowledge, in a rabbit model of TB that treatment with bevacizumab normalizes granuloma vasculature, reduces hypoxia, and enhances small molecule delivery during a “window of normalization,” a transient effect observed in tumors (15, 20). Because anti-VEGF drugs have been approved for both malignant and nonmalignant diseases (18), our findings could be rapidly tested in the clinic to enhance TB treatment, shorten treatment duration, and avert the development of treatment resistance.
Results
Human and Rabbit TB Granulomas Express VEGF.
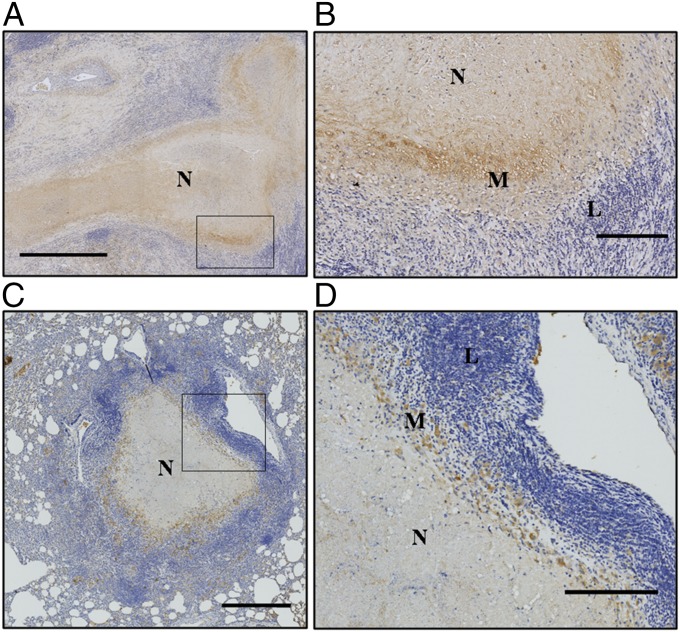
First, we examined VEGF expression levels in histological sections from patients with TB who had received elective lung resection surgery and from experimentally infected rabbits (Fig. 1). Granulomas are characterized by concentric layers of differing cellular composition, often organized around a central necrotic core (8). The predominant cells found in the outer layer are lymphocytes, whereas those cells nearer the necrotic core are primarily epithelioid macrophages (Fig. 1); these two layers are frequently surrounded by a fibrotic rim (8). We observed that VEGF was detectable in the granulomatous regions of the lung (Fig. 1 A and C) and that it was most highly expressed in the inner macrophage layer (Fig. 1 B and D) of both human and rabbit granulomas. We examined similarly sized lymphoid aggregates in human lungs (Fig. S1 A and B) and rabbit lungs (Fig. S1 C and D), and found that VEGF expression was minimal in these structures, where lymphocytes are the dominant cell type.
Fig. 1.
Human and rabbit granulomas express VEGF. Representative line-scanned histological sections from human patients after lung tissue resection surgery (A and B) and from necropsied rabbits (C and D) show VEGF expression (brown) in the cellular layers surrounding the central necrosis of necrotizing (N) granulomas in humans (A) and rabbits (C), with magnified regions shown in B and D. Macrophages (M) express higher levels of VEGF than lymphocytes (L) in granulomas. (Scale bars: A, 1 mm; B and D, 200 μm; C, 500 μm.)
Microvessels in TB Granulomas Are Morphologically and Spatially Heterogeneous.
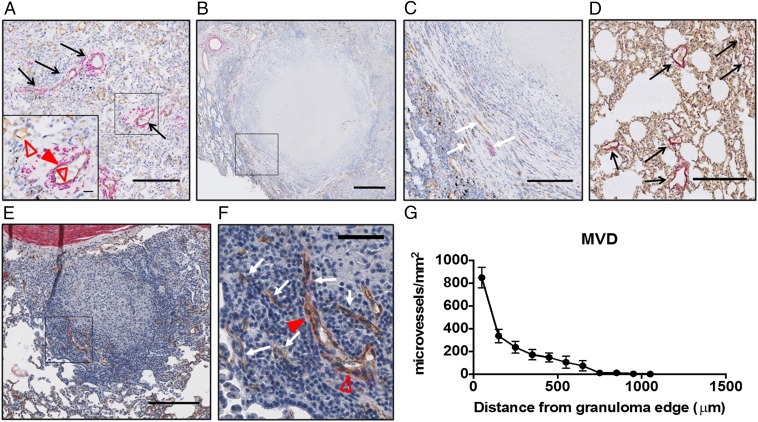
To assess blood vessel morphology and spatial distribution in granulomas, we double-stained tissue sections with antibodies detecting CD31 for endothelial cells and α smooth muscle actin (αSMA) for pericytes (Fig. 2). We found that TB granulomas have a lower overall density of microvessels compared with normal lung tissue in both humans (Fig. 2 A–C) and rabbits (Fig. 2 D–F). Analysis of the spatial distribution of the vessels within granulomas showed a higher microvessel density (MVD; quantified as the number of microvessels per square millimeter of viable tissue) in the granuloma periphery (i.e., a rim of CD31-positive vessels) that dramatically decreases to an avascular central region, as quantified in rabbit tissues (Fig. 2G). Furthermore, we observed that many granuloma-associated vessels were compressed or collapsed in the interior region of granulomas, as indicated by a lack of open lumen in some CD31-positive vessels. Pericytes, which support the endothelial layer of blood vessels, play a key role in vessel maturation and function (16, 22). Therefore, we also analyzed the fraction of CD31-positive vessels surrounded by αSMA-positive cells. In the normal lung, αSMA-positive cells were seen mostly surrounding larger vessels and were sparse around microvessels (Fig. 2 A and D). Furthermore, TB granuloma-associated vessels were found to have spatially heterogeneous αSMA-positive coverage, which was even more sparse than the αSMA-positive coverage of the normal lung (Fig. 2 C and F). These results suggest that the granuloma vasculature is abnormal both morphologically and structurally.
Fig. 2.
TB granuloma blood vessels are morphologically abnormal and have heterogeneous spatial densities. Human resection tissues (A–C) and rabbit tissues from an experimental MTB infection (D–F) labeled with CD31 (brown, open red triangles) and αSMA (pink, closed red triangles) are shown. The MVD is higher in human (A and Inset) and rabbit (D) normal lung tissue adjacent to lesions than in human (B and C) and rabbit (E and F) granulomas. Staining for endothelial cells revealed that in both human (B and C) and rabbit (E and F) granulomas, blood vessels are restricted to the granuloma periphery and tend to be absent from the central region, as demonstrated by quantification of MVD (the number of CD31-positive microvessels per square millimeter of viable tissue) in rabbit granulomas (G). Granuloma vessels are often compressed along the lesion periphery and collapsed within the interior (C and F, white arrows), as indicated by a positive CD31 stain and lack of a visible open vessel lumen, unlike the open, often αSMA-covered vessels in the normal lung (A and D, black arrows). αSMA costaining with CD31 shows that granuloma-associated blood vessels lack significant pericyte coverage in both human and rabbit tissues. (Scale bars: A, C, D, and E, 200 μm; A, Inset, 20 μm; B, 500 μm; F, 50 μm.)
TB Lesion-Associated Blood Vessels Are Functionally Abnormal.
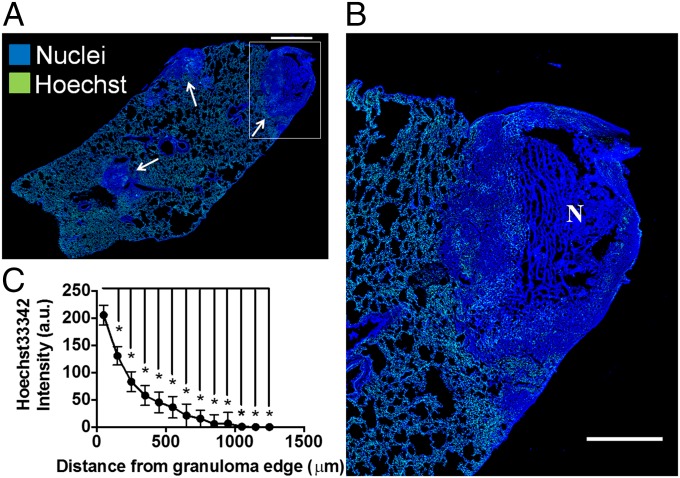
To evaluate the function of TB granuloma blood vessels, we analyzed the delivery and distribution of the fluorescent probe Hoechst33342 (Hoechst), a low-molecular-weight dye that serves as a model for small molecule first-line anti-TB agents, in MTB-infected rabbits that had been sacrificed 7 min after i.v. administration of this agent (Fig. 3). Although this small molecule was well distributed and gave an intense fluorescent signal throughout normal lung parenchyma, its intensity was found to be very low in granulomas and it was heterogeneously distributed (Fig. 3A). Analysis of the spatial distribution of this small molecule showed a statistically significant higher concentration in the periphery of the lesion, falling off rapidly toward the central necrotic region (Fig. 3B), correlating with high MVD in this region (Fig. 2G).
Fig. 3.
Delivery of a low-molecular-weight dye into rabbit granulomas is poor in contrast to lung. Small molecule delivery was assessed by analyzing the distribution of the low-molecular-weight fluorescent probe Hoechst (green) into rabbit granulomas. (A) Representative immunofluorescent confocal microscope image of a rabbit lung shows that Hoechst delivery is reduced in granulomas (white arrows) compared with the surrounding lung tissue, as indicated by low, heterogeneous distribution of the dye in these areas of dense nuclei (blue). As seen in a magnified necrotizing granuloma image (B) and via quantitative analysis (C), there is significantly higher delivery of the dye in the periphery of granulomas than in the central regions of the granulomas. a.u., arbitrary units; N, necrosis. *P < 0.05, Student’s t test. (Scale bars: A, 1 mm; B, 500 μm.)
Bevacizumab Treatment Reduces Vessel Number and Increases Vessel Pericyte Coverage and Lumen Area.
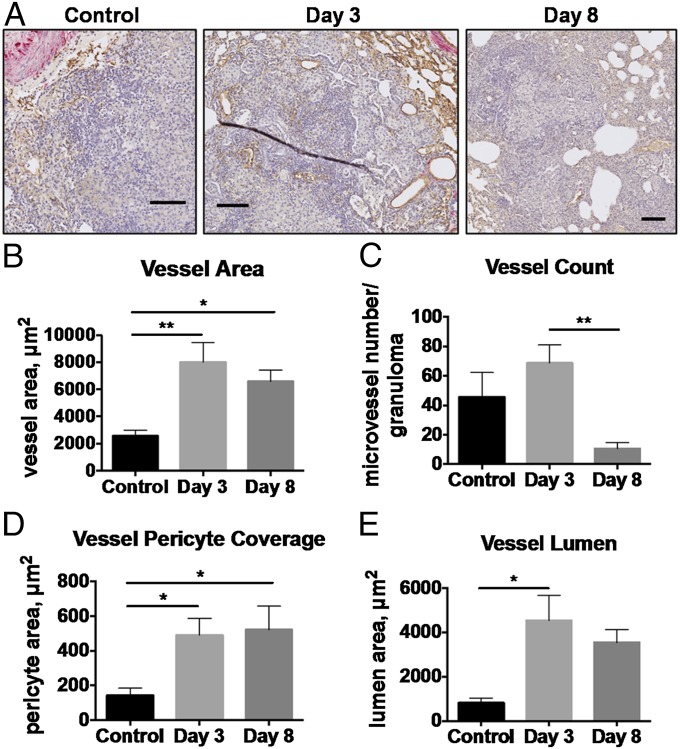
Because the vasculature in TB granulomas was found to be structurally and functionally abnormal, with impaired small molecule delivery, we next treated TB-infected rabbits with bevacizumab (Avastin; Genentech, Inc.) administered at a dose of 5 mg/kg i.v. once weekly (23). Granulomas were analyzed 3 and 8 d posttreatment from necropsied rabbit tissues. Granuloma-associated vasculature was assessed using a custom-built semiautomatic vessel segmentation algorithm (Visiopharm). This program was used to select for endothelial cells, pericytes, and vessel lumen in CD31/αSMA double-stained rabbit lung tissues. Representative images of vessels in the control and treated groups show the differences seen in vessel morphology with bevacizumab treatment (Fig. 4A). We found that the vessel area (i.e., average area of the granuloma-associated vessels) was significantly increased 3 and 8 d after bevacizumab treatment compared with controls (Fig. 4B). We also found that the number of vessels per granuloma was reduced 8 d posttreatment compared with 3 d posttreatment (Fig. 4C). The MVD featured a decreasing trend at both 3 and 8 d posttreatment, although this difference was not statistically significant (Fig. S2). Furthermore, we found that the pericyte coverage of granuloma-associated vessels, quantified as the average area of pericytes per vessel, was significantly increased at both 3 and 8 d posttreatment, and that the vessel lumen, quantified as average lumen area per vessel, was also significantly increased 3 d posttreatment compared with the control group (Fig. 4 D and E).
Fig. 4.
Bevacizumab treatment normalizes granuloma-associated vessel structure. (A) Vessel endothelial cells (CD31, brown), associated pericytes (αSMA, pink), and the lumen were quantified in granulomas in tissue sections from rabbits treated with bevacizumab using the semiautomatic Visiopharm software. (Scale bars: 100 μm.). The mean vessel area per granuloma was significantly higher in the treated groups compared with controls (B), and the mean vessel number per granuloma was significantly reduced between days 3 and 8 posttreatment (C). The average pericyte area per vessel was increased on both 3 and 8 d posttreatment (D), and the lumen area per vessel was increased 3 d after treatment with bevacizumab (E). The lumen area was decreased 8 d posttreatment compared with controls, although not significantly (P = 0.06). *P < 0.05; **P < 0.01.
Bevacizumab Treatment Improves Hoechst Delivery and Oxygenation in TB Granulomas.
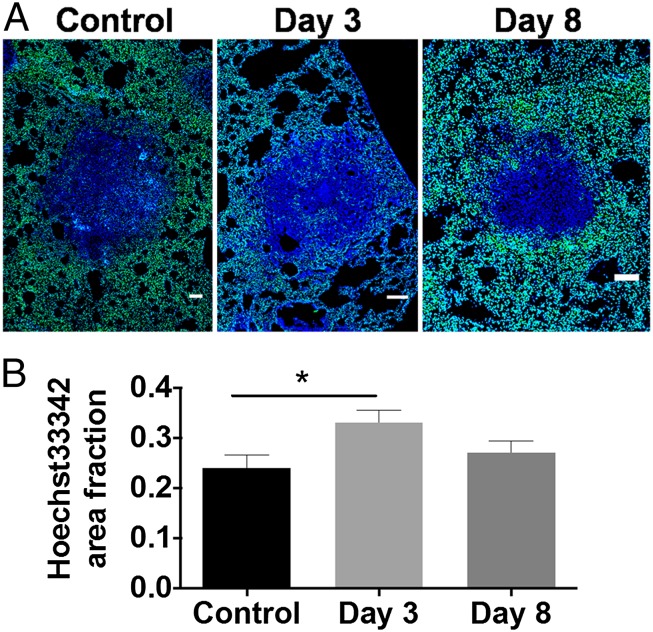
In cancer, normalization of vessels and improvements in vascular perfusion can enhance the intratumoral distribution of both low- and high-macromolecular-weight drugs in tumors (18, 20, 21). Because bevacizumab treatment normalized the structure and morphology of granuloma vasculature, we investigated if small molecule delivery had been improved via normalization of vessel function. Hoechst delivery to granulomas was quantified as an area fraction (Fig. 5). There was a significant increase in dye delivery to TB lesions 3 d after bevacizumab treatment compared with control animals (Fig. 5B). Although the level of dye penetration was increased 8 d posttreatment, the area fraction was not significantly higher compared with the controls. Of note, bevacizumab treatment did not affect TB granuloma volume, density, or activity, as assessed via [18F]fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) (Fig. S3 A–C).
Fig. 5.
Bevacizumab treatment enhances low-molecular-weight dye delivery in rabbit granulomas. (A) Amount of Hoechst delivered into the granuloma was assessed in immunofluorescent images by quantifying the area fraction of Hoechst (green) of granulomas (dense blue regions), that is, the Hoechst dye area per viable granuloma area, as shown in the representative images. (Scale bars: 100 μm.) (B) Amount of Hoechst delivered to TB lesions was found to be significantly higher 3 d after bevacizumab treatment compared with controls (*P = 0.04); by 8 d posttreatment, however, the area fraction was no longer significantly higher than the area fraction in untreated animals.
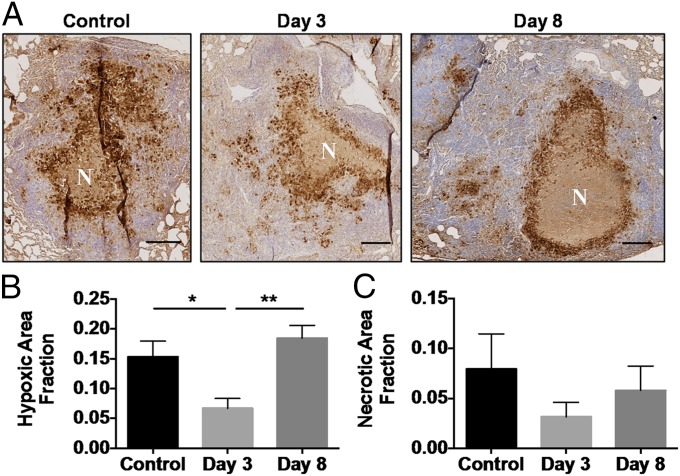
Large TB granulomas are characterized by the presence of central necrosis surrounded by a rim of hypoxic cells in necrotizing granulomas (11), as seen in representative images from the rabbit model (Fig. 6A). The hypoxic area fraction of the viable (i.e., nonnecrotic) granuloma tissue from treated rabbits was significantly reduced 3 d posttreatment with bevacizumab (Fig. 6B), but the necrotic fraction (Fig. 6C), overall volume (Fig. S3A), and density (Fig. S3C), as assessed by CT, were not different, suggesting a true reduction in the hypoxic fraction. Treatment with bevacizumab did not change the bacterial burden of lesions or the adjacent lung tissue (Fig. S3D).
Fig. 6.
Bevacizumab treatment reduces hypoxia in rabbit granulomas. (A) Immunohistochemical sections of rabbit granulomas were quantified for necrosis (N) and hypoxia (brown) via pimonidazole staining, as shown in representative images. (Scale bars: 500 μm.) (B) Hypoxic fraction of the viable (nonnecrotic) granuloma tissue area was found to be reduced 3 d posttreatment with bevacizumab (*P = 0.03), but this reduction did not persist. At 8 d posttreatment, the hypoxic fraction is significantly higher than in granulomas from day 3 (**P = 0.002). (C) Necrotic fraction is also reduced on day 3, but this reduction is not significant.
Discussion
TB treatment in drug-susceptible disease fails due to resistance in as many as 1–5% of patients despite simultaneous treatment with four active agents (24). Theoretically, the emergence of resistance should be very difficult in the face of four agents (25). Recent results using scanning mass spectrometric imaging of drug penetration in TB lesions suggest that different drugs have differential abilities to diffuse into TB lesions, resulting in discrete spatial localization of different agents (9, 10), which suggests that drug delivery is limited in regions of TB lesions and that bacterial populations in specific locations may be subjected to, effectively, monotherapy. Understanding of the mechanisms that limit TB drug delivery is urgently needed for the development of effective treatments. Similarly, cancer treatment can be hindered by limited drug delivery (26, 27). Characterization of the abnormal tumor vasculature has had a profound influence on understanding tumor progression and the limitations of treatment. The concept of vascular normalization using judicious doses of antiangiogenic drugs (e.g., bevacizumab) has offered a clinically translatable approach to overcome some of these limitations (13, 18). Therefore, we sought to exploit the similarities between blood vessels formed in tumors and blood vessels formed in TB granulomas to understand the impact of these structures on TB disease progression and examine the potential normalizing effect of antiangiogenic therapy on the structure and function of granuloma-associated vessels.
Granulomas in the rabbit TB model used in this study are known to harbor regions of hypoxia and necrosis (11). Hypoxia is known to increase production of proangiogenic cytokines, including VEGF, in solid tumors (19). Indeed, we found that VEGF is highly expressed in granulomas compared with normal lung tissue in both the rabbit model and samples of human patients with TB. TB granulomas, similar to solid tumors, contain spatially heterogeneous vessels that are compressed or collapsed. These vessels are structurally abnormal, with a lack of pericyte coverage, and dysfunctional, as demonstrated by poor delivery of Hoechst dye. In solid tumors, the abnormal vasculature creates an abnormal tumor microenvironment (i.e., interstitial hypertension, hypoxia, acidosis) that fuels tumor progression and treatment resistance. Hypoxic tumor cells often show a more aggressive phenotype with enhanced metastatic potential (28). Furthermore, the hostile microenvironment impairs the function of antitumor immune cells (29). Importantly, tumor response to therapy is also affected because hypoxic tumor cells often demonstrate reduced sensitivity to radiation and chemotherapy (18), and the delivery of systemically administered chemotherapeutics into tumors is impeded (18). Previous studies showed that in MTB, hypoxia triggers a set of adaptive transcriptional programs by the bacteria that result phenotypically in dramatic reductions in drug susceptibility (30).
Given these physiological similarities between vasculatures in TB granulomas and solid cancerous tumors, we next assessed the ability of bevacizumab to normalize granuloma-associated vasculatures. In solid tumors, to reach cancer cells, blood-borne therapeutic agents must cross vessel walls and diffuse through the intervening interstitium (31). The structural and functional abnormalities of tumor vasculature hinder drug delivery, and the delivery and efficacy of blood-borne antitumor agents are impaired as a result. A number of anti-VEGF agents have been shown to normalize tumor vessel structure and function in animal models and patients (18). In the hope of applying this concept to TB to improve drug delivery, we treated TB-infected rabbits with bevacizumab, a VEGF-neutralizing antibody. Moreover, a recent complimentary study has demonstrated that inhibiting the VEGF receptor early in infection in a zebrafish model of TB reduced pathogenicity and improved treatment efficacy (32).
In the rabbit TB model, bevacizumab treatment did not result in aggravation of the disease state, evidenced by no significant change in lesion volume, density, or inflammation, as measured by FDG uptake, and bacterial load. However, we found that immature vessels are pruned by bevacizumab treatment and that the remaining vessels are structurally normalized, as indicated by an increase in vessel pericyte coverage. The improved vessel structure is associated with an increase in average vessel lumen area. Because the nonpruned vessels are either already associated with or have actively recruited pericytes, the vessel walls become more structurally normal and are better able to resist compression or collapse. This pruning effect explains why vessel area is increased 8 d posttreatment despite the reduction in vessel number.
We found that hypoxic areas in granulomas were significantly reduced by bevacizumab 3 d posttreatment and that delivery of the Hoechst dye was significantly increased at that time point. By 8 d posttreatment, however, hypoxia rebounded and the Hoechst dye area was no longer significantly increased. These data suggest that by day 8, although the granuloma-associated vessels are more structurally and functionally normal than vessels in control granulomas, the reduction in the number of vessels due to vascular pruning cannot sustain the maximal level of small molecule delivery that was observed 3 d posttreatment. Thus, our data show that bevacizumab treatment leads to a transient “window of opportunity” between the start of treatment and 8 d posttreatment, during which the granuloma-associated vasculature is structurally typical and features improved functionality in terms of oxygen and small molecule delivery, as has been observed in tumor studies. Although the Hoechst dye used is an imperfect model for TB drugs, our results suggest that permeation of some drugs will be enhanced in granulomas. The effective concentration of drug in the compartments bearing bacteria will be based on perfusion into and diffusion out of these lesions, and will need to be assessed on a drug-by-drug basis to optimize killing of the bacteria. These results suggest that delivery of some drugs into granulomas would be improved during this window, which would be the optimal time to administer either higher dose therapies or boost drugs given intermittently.
In summary, our study suggests that TB granulomas feature abnormal vasculature and that bevacizumab is transiently able to normalize granuloma-associated vessel structure and function. Although human disease may have a longer duration of chronicity, the lesions are strikingly dynamic (33) and their overall structure with respect to vasculature is remarkably similar to the lesions in rabbits; therefore, we expect a similar response with antiangiogenic agents in humans as we have seen in this animal model. Nonetheless, there may be lesions with differential structure resulting from irreversible pathology, such as cavities that were not assessed in this analysis, that would behave differently in response to antiangiogenic treatment. If administered in conjunction with anti-TB drugs, antiangiogenic agents, such as bevacizumab, have the potential to improve the delivery of antimicrobial drugs into granulomas and stimulate sensitivity to drug treatment through improved oxygen delivery into the lesions during the window of normalization. Furthermore, this type of “host-directed therapy” that targets the abnormal granuloma vasculature and reduces hypoxia may lead to a more robust immune response again the bacteria. Through this mechanism, vessel normalization has the potential to reduce the overall duration of TB chemotherapies and possibly avoid localized exposure of the bacterium to monotherapy, thereby avoiding the development of drug resistance, which is one of the main issues in the global control of TB.
Materials and Methods
Human granuloma tissues were obtained from subjects undergoing lung resection surgery for multidrug-resistant TB, who gave consent for the clinical protocol NCT00816426. Female New Zealand White rabbits were infected by aerosol with MTB strain HN878, and were allowed 10–11 wk to develop TB disease pathology, as described previously (11). Rabbits were treated with 5 mg/kg of bevacizumab (Avastin; Genentech, Inc.) by slow intravascular infusion (23) or with a sham infusion of saline. [18F]FDG-PET/CT was used to monitor granuloma volume, density, and FDG uptake as previously described (11, 34). Rabbits were killed at the described time points, after pimonidazole (11) and Hoechst (Sigma–Aldrich) injections, and the lung tissues were collected for histology. The tissues were assessed for hypoxia (pimonidazole), small molecule dye delivery (Hoechst), endothelial cells (CD31), and pericytes (αSMA). Experimental procedures are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Carolyn Smith for outstanding technical support of the immunohistochemistry studies, and Drs. Matija Snuderl and Rehka Samuel for their scientific input. We thank all of the participants who enrolled in the study, as well as the staff of the International Tuberculosis Research Center, Asan Medical Center, Pusan National University Hospital, and the National Medical Center for their assistance with this work. We acknowledge Seokyong Eum for his service as a pathologist for this study. This study was supported, in part, by Grant P01CA080214 (to R.K.J.) and by grants from the Bill and Melinda Gates Foundation (to R.K.J.), from the American Cancer Society (to L.X.), from the Children’s Tumor Foundation (to L.X.), and from the Susan G. Komen Foundation [Grant PDF14301739 (to G.S.)]; through the Grand Challenges in Global Health Program to Douglas Young, Imperial College (to C.E.B.); by the Intramural Research Program of the NIH/National Institute of Allergy and Infectious Diseases (C.E.B.); and by the Korean Ministry of Health and Welfare.
Footnotes
Conflict of interest statement: R.K.J. received consultant fees from Enlight, Ophthotech, and SynDevRx. R.K.J. owns equity in Enlight, Ophthotech, SynDevRx, and XTuit and serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, and the Tekla Healthcare Opportunities Fund. No reagents or funding from these companies was used in these studies.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424563112/-/DCSupplemental.
References
- 1.Dye C, et al. Measuring tuberculosis burden, trends, and the impact of control programmes. Lancet Infect Dis. 2008;8(4):233–243. doi: 10.1016/S1473-3099(07)70291-8. [DOI] [PubMed] [Google Scholar]
- 2.Zumla AI, et al. New antituberculosis drugs, regimens, and adjunct therapies: Needs, advances, and future prospects. Lancet Infect Dis. 2014;14(4):327–340. doi: 10.1016/S1473-3099(13)70328-1. [DOI] [PubMed] [Google Scholar]
- 3.Zumla A, Nahid P, Cole ST. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov. 2013;12(5):388–404. doi: 10.1038/nrd4001. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto K. The pathology of Mycobacterium tuberculosis infection. Vet Pathol. 2012;49(3):423–439. doi: 10.1177/0300985811429313. [DOI] [PubMed] [Google Scholar]
- 5.Falzon D, et al. Multidrug-resistant tuberculosis around the world: What progress has been made? Eur Respir J. 2015;45(1):150–160. doi: 10.1183/09031936.00101814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang S, et al. Risk factors for poor treatment outcomes in patients with MDR-TB and XDR-TB in China: Retrospective multi-center investigation. PLoS ONE. 2013;8(12):e82943. doi: 10.1371/journal.pone.0082943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell DG, Barry CE, 3rd, Flynn JL. Tuberculosis: What we don’t know can, and does, hurt us. Science. 2010;328(5980):852–856. doi: 10.1126/science.1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell DG. Who puts the tubercle in tuberculosis? Nat Rev Microbiol. 2007;5(1):39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 9.Dartois V. The path of anti-tuberculosis drugs: From blood to lesions to mycobacterial cells. Nat Rev Microbiol. 2014;12(3):159–167. doi: 10.1038/nrmicro3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dartois V, Barry CE., 3rd A medicinal chemists’ guide to the unique difficulties of lead optimization for tuberculosis. Bioorg Med Chem Lett. 2013;23(17):4741–4750. doi: 10.1016/j.bmcl.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Via LE, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76(6):2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 13.Jain RK. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146(5):1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 15.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 16.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15(1):102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Tong RT, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 18.Jain RK. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 20.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 21.Goel S, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg JI, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456(7223):809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez ES, Rizzo MM, Croxatto JO, Mazzolini G, Gallo JE. Suramab, a novel antiangiogenic agent, reduces tumor growth and corneal neovascularization. Cancer Chemother Pharmacol. 2011;67(3):723–728. doi: 10.1007/s00280-010-1457-z. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad S, Mokaddas E. Current status and future trends in the diagnosis and treatment of drug-susceptible and multidrug-resistant tuberculosis. J Infect Public Health. 2014;7(2):75–91. doi: 10.1016/j.jiph.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Munck C, Gumpert HK, Wallin AI, Wang HH, Sommer MO. Prediction of resistance development against drug combinations by collateral responses to component drugs. Sci Transl Med. 2014;6(262):262ra156. doi: 10.1126/scitranslmed.3009940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46(1-3):149–168. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12(11):958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeClerck K, Elble RC. The role of hypoxia and acidosis in promoting metastasis and resistance to chemotherapy. Front Biosci (Landmark Ed) 2010;15:213–225. doi: 10.2741/3616. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73(10):2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rustad TR, Harrell MI, Liao R, Sherman DR. The enduring hypoxic response of Mycobacterium tuberculosis. PloS One. 2008;3(1):e1502. doi: 10.1371/journal.pone.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Clin Oncol. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oehlers SH, et al. Interception of host angiogenic signalling limits mycobacterial growth. Nature. 2014 doi: 10.1038/nature13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman MT, et al. PET/CT imaging reveals a therapeutic response to oxazolidinones in macaques and humans with tuberculosis. Sci Transl Med. 2014;6(265):265ra167. doi: 10.1126/scitranslmed.3009500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Via LE, et al. Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [¹⁸F]2-fluoro-deoxy-D-glucose positron emission tomography and computed tomography. Antimicrob Agents Chemother. 2012;56(8):4391–4402. doi: 10.1128/AAC.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.