Significance
Current cancer treatments ultimately fail owing to metastasis and relapse. The discovery of therapeutic approaches that counteract relapse and metastasis is, therefore, extremely important for advancing cancer medicine. Hypermalignant cancer cells, termed cancer stem cells or stemness-high cancer cells, have been isolated from patients with a variety of tumor types and found to be highly malignant, tumorigenic, and resistant to chemotherapies. Our data that BBI608, a cancer stemness inhibitor in clinical development, effectively blocks cancer relapse and metastasis in xenografted human cancers, suggest targeting cancer stemness as a novel approach to develop the next generation of cancer therapeutics to suppress cancer relapse and metastasis.
Keywords: cancer stemness, relapse, BBI608
Abstract
Partial or even complete cancer regression can be achieved in some patients with current cancer treatments. However, such initial responses are almost always followed by relapse, with the recurrent cancer being resistant to further treatments. The discovery of therapeutic approaches that counteract relapse is, therefore, essential for advancing cancer medicine. Cancer cells are extremely heterogeneous, even in each individual patient, in terms of their malignant potential, drug sensitivity, and their potential to metastasize and cause relapse. Indeed, hypermalignant cancer cells, termed cancer stem cells or stemness-high cancer cells, that are highly tumorigenic and metastatic have been isolated from cancer patients with a variety of tumor types. Moreover, such stemness-high cancer cells are resistant to conventional chemotherapy and radiation. Here we show that BBI608, a small molecule identified by its ability to inhibit gene transcription driven by Stat3 and cancer stemness properties, can inhibit stemness gene expression and block spherogenesis of or kill stemness-high cancer cells isolated from a variety of cancer types. Moreover, cancer relapse and metastasis were effectively blocked by BBI608 in mice. These data demonstrate targeting cancer stemness as a novel approach to develop the next generation of cancer therapeutics to suppress cancer relapse and metastasis.
Current cancer treatments ultimately fail owing to metastasis and relapse. Although chemotherapy can induce partial or even complete cancer regression in some patients, such initial responses are invariably followed by relapse, with the recurrent cancer being highly resistant to further chemotherapy, resulting in very limited survival benefits (1–3). Modern therapeutics designed to specifically target activating mutations are quite effective in inducing cancer regression in patients driven by such activating mutations; however, these treatments are also invariably followed by relapse with tumors that no longer respond to the targeted agent (2, 3). The discovery of novel therapeutic approaches that counteract cancer relapse is, therefore, urgently required for advancing cancer treatment.
The idea that cancer is composed of a group of near-homogenous, ectopically growing cells has been replaced with a more complex model in which cancer cells are extremely heterogeneous, even in each individual patient, in terms of their malignant potential to metastasize and cause relapse. The increased understanding of the genomic and proteomic complexity of tumor heterogeneity further highlights the extreme heterogeneity of cancer cells (4).
Subpopulations of cancer cells with extremely high tumorigenic potential, termed cancer stem cells or stem-like cancer cells, have been isolated from cancer patients with a variety of tumor types (5–13) and found to have high stemness properties (5–15). Stemness, initially defined by the expression of stem cells genes, is a property shared by embryonic stem cells and adult stem cells (16). In addition to distinct gene expression profiles, stemness can be measured by a cell’s ability to form spheres when cultured in stem cell media (17). Although it is still uncertain whether cancer stem cells isolated from cancer patients truly qualify as bona fide stem cells and how frequent these cells are, it has been demonstrated that these stemness-high malignant cells are extremely tumorigenic and are resistant to conventional chemotherapies and radiation. Moreover, chemotherapy and radiation have been found to induce stemness genes in cancer cells, converting stemness-low cancer cells to stemness-high cancer cells (18, 19). Such highly tumorigenic and drug-resistant stemness-high cancer stem cells are, therefore, likely to be “left over” after chemotherapy or radiotherapy and ultimately responsible for relapse (13, 14, 16, 20, 21). However, these stemness-high cancer stem cells are difficult to target owing to activation of prosurvival and antiapoptotic pathways, overexpression of drug efflux pumps, and increased DNA repair capacity (13, 14, 16, 20, 21). Therapeutic approaches based on the cancer stem cell hypothesis have centered on identifying specific cancer stem cell surface markers and the design of agents to selectively kill these marker-bearing cancer stem cells (22–24).
We hypothesized that cancer stemness inhibition can effectively suppress metastasis and relapse. To selectively target cancer stemness, it is critical to identify molecular targets that are required for cancer stemness, but not (or less so) by normal tissue stem cells. The feasibility of this approach has been demonstrated by gene expression profiling, whereby cancer stemness has been shown to more closely resemble embryonic stem cells than adult stem cells (25). Through gene-silencing approaches, we have identified signal transducer and activator of transcription 3 (Stat3) as critically important for maintaining cancer stemness, yet largely dispensable for hematopoietic stem cells. Here we show that BBI608, a small molecule identified by its ability to inhibit gene transcription driven by Stat3 and cancer stemness properties, can block spherogenesis of and kill stemness-high cancer cells isolated from a variety of cancer types and inhibit stemness gene expression. Moreover, cancer relapse and metastasis were effectively blocked by BBI608 in xenografted human cancers in mice. These data demonstrate targeting cancer stemness as a novel approach to develop the next generation of cancer therapeutics to suppress cancer relapse and metastasis.
Results
Inhibition of Cancer Relapse and Metastasis by BBI608.
Relapse after chemotherapy treatment is a common phenomenon (26). We hypothesized that stemness-high cancer cells are responsible for cancer relapse and that stemness inhibition can suppress cancer relapse. To effectively block cancer relapse it is, therefore, essential to target cancer stemness. Through a gene silencing-based approach, we identified Stat3 as a key driver for cancer stemness. We next set out to discover a druggable inhibitor of Stat3 to target cancer stemness, using in silico screening and computational biology to search for Stat3 binders, phenotype-driven testing to determine stemness inhibition, and Stat3-driven gene transcription to test for Stat3 inhibition. Through such an activity-oriented, quality-driven iterative research process, we identified BBI608.
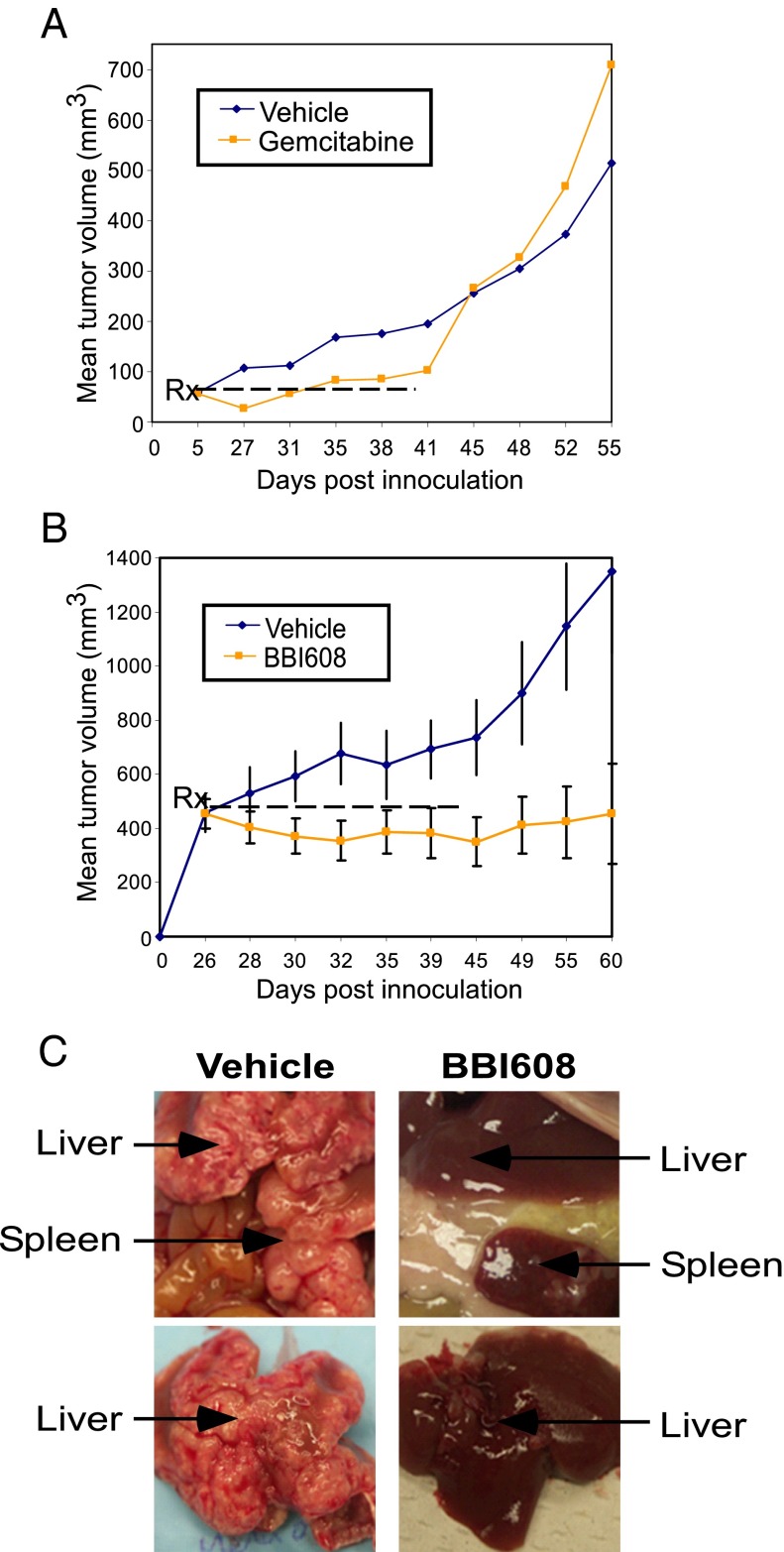
To evaluate the phenomenon of tumor relapse, we used a pancreatic cancer xenograft model and the chemotherapy agent gemcitabine. Treatment with gemcitabine inhibited PaCa-2 xenograft tumor growth, with tumor growth inhibition of 47.5% on day 41 (Fig. 1A). However, after cessation of treatment on day 41, the tumors in the gemcitabine-treated animals soon relapsed and even outgrew the tumors in the vehicle control group (Fig. 1A). Treatment with BBI608 also significantly inhibited PaCa-2 xenograft tumor growth on day 41 (Fig. 1B). However, unlike the gemcitabine-treated animals, no tumor regrowth was observed in the animals administered BBI608 during the 22-d posttreatment observation period (Fig. 1B). Furthermore, no signs of toxicity as evidenced by body weight measurement were observed (Fig. S1 A and B). These data suggest that BBI608, unlike chemotherapy, can inhibit the cells within the tumor mass that are responsible for tumor relapse.
Fig. 1.
Inhibition of cancer relapse by BBI608. (A) Immunosuppressed mice with established s.c. Paca-2 human pancreatic cancer were given gemcitabine (120 mg/kg) q3d, or vehicle control i.p. The animals received a total of 14 doses and were maintained 22 d for posttreatment observation. Tumor size was evaluated periodically during treatment and posttreatment observation. Each point represents the mean ± SEM of seven tumors. (B) Immunosuppressed mice with established s.c. PaCa-2 human pancreatic cancer were given BBI608 (20 mg/kg) daily or vehicle control i.p. and monitored as in A. (C) Immunosuppressed mice bearing intrasplenically inoculated HT29 human colon cancer cells were given daily BBI608 (20 mg/kg) or vehicle control i.p. Primary tumors at spleen and spontaneous liver metastases were examined macroscopically. A representative photograph is shown.
As another model for cancer relapse, we used the intrasplenic-nude mouse model system (ISMS) model to evaluate cancer relapse in a metastatic setting. This model involves the injection of colon cancer cells (HT29) into the spleen capsule of nude mice, and these colon cancer cells can form liver metastases spontaneously in a few weeks. To test the potential therapeutic role of BBI608 against metastasis, we used the ISMS model. BBI608 was found to effectively block spleen and liver metastases in the ISMS model (Fig. 1C). These data demonstrate that BBI608 is effective at blocking metastasis in vivo.
Depletion of Stemness-High Cancer Cells by BBI608.
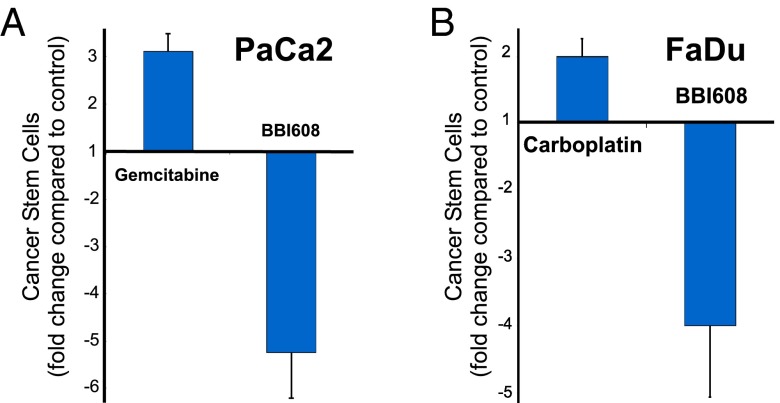
We hypothesized that the antirelapse activity of BBI608 may be attributed to its effect on stemness-high cancer cells. To test this hypothesis, we used two human xenograft tumor models. In the pancreatic tumor (PaCa2) model animals were treated with vehicle, gemcitabine, or BBI608. Tumors were collected after 7 d of treatment, single-cell suspensions obtained from the tumors, and frequency of stemness-high cancer cells was determined by their self-renewal capacity as measured by their ability to grow as spheres when cultured under serum-free, attachment-free stem cell culture conditions. Compared with control-treated animals, BBI608 treatment decreased the stemness-high subpopulation by fivefold (Fig. 2A). By contrast, gemcitabine treatment caused a threefold increase in stemness-high cell population. Similar data were also observed in a head and neck tumor (FaDu) model treated with vehicle, carboplatin, or BBI608 (Fig. 2B). These data demonstrate that BBI608 is effective at targeting the stemness-high cancer cells in vivo, whereas standard chemotherapy caused an enrichment of the stemness-high cancer cell subpopulation.
Fig. 2.
Depletion of stemness-high cancer cells by BBI608 in vivo. Mice were administered either vehicle, (A) gemcitabine (120 mg/kg [PaCa2]), (B) carboplatin (30 mg/kg [FaDu]), or 20 mg/kg of BBI608 i.p. After killing, tumors were collected after 7 or 14 d of treatment, for Paca-2 and FaDu cells, respectively. Single-cell suspensions were obtained after animal killing and sterile removal of tumors. Live cells were then counted and used to measure their ability to form spheres when cultured in cancer stem cell media.
BBI608 Can Block Survival and Self-Renewal of Stemness-High Cancer Cells.
To determine the effect of BBI608 on stemness-high cancer cells, we examined self-renewal of stemness-high cancer cells isolated or enriched by stem-cell culture selection (Fig. 3A), side population-based enrichment (Fig. 3B), or cancer stem cell surface markers (Fig. 3C). As shown in Fig. 3A, stemness-high colon cancer cells grown under stem cell culture conditions form spheres, and treatment with BBI608 blocked spherogenesis, suggesting that BBI608 inhibits self-renewal of stemness-high cancer cells
Fig. 3.
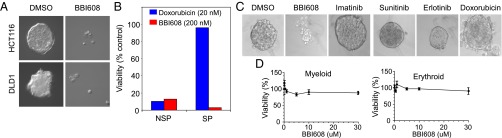
Inhibition of self-renewal of stemness-high cancer cells by BBI608. (A) Spheres derived from DLD1 and HCT116 cell lines were dissociated to single cells and allowed to form spheres in suspension with cancer stem cell medium for 48 h before treatment with BBI608 (2 μM). After 24 h, the drugs were removed and the cells were cultured in fresh cancer stem cell medium for another 24 h. Representative images are shown. (B) SW480 colon cancer cells were isolated by FACS based on Hoechst dye exclusion and were cultured for 72 h in cancer stem cell conditions before the addition of the indicated concentrations of therapeutic agents. Sphere growth was scored by counting the number of spheres possessing >50 cells. SP, side population; NSP, nonside population. (C) CD44high FaDu cells were isolated by FACS and were cultured for 72 h in cancer stem cell conditions before the addition of the indicated therapeutic agents (400 nM BBI608, 2 μM imatinib, 10 μM sunitinib, 10 μM erlotinib, 100 nM doxorubicin). Sphere growth was scored by counting the number of spheres possessing >50 cells. Representative images are shown. (D) CD34+ bone marrow mononuclear cells were treated with either DMSO or BBI608 for 6 h at 37 °C. Cells were then washed and plated in complete Methocult H4434 medium. Colonies of both erythroid and myeloid lineages containing >50 cells per colony were counted. Each treatment was performed in triplicate.
We determined whether the stemness-high population was resistant to chemotherapeutics and targeted kinase inhibitors. Stemness-high cancer cells were isolated by sorting for exclusion of Hoechst dye, followed by culture in the serum-free stem cell media. Sorting based on Hoechst dye exclusion provides two cell populations: a stemness-high side population and a stemness-low nonside population. Both the stemness-high side population and nonside population were killed by BBI608, with the side population being more sensitive to BBI608 (Fig. 3B). By contrast, whereas treatment of the nonside population with doxorubicin resulted in inhibition of viability, treatment of the side population with doxorubicin had little effect on viability (Fig. 3B). Stemness-high cancer cells were also isolated by sorting for high CD44 expression followed by culture in the serum-free stem cell media. Treatment of these cells with BBI608 resulted in inhibition of spherogenesis (Fig. 3C). By contrast, treatment with imatinib, sunitinib, erlotinib, or doxorubicin had little effect on the spherogenesis (Fig. 3C).
We next determined whether BBI608 affects normal stem cells. To address this issue we obtained CD34+ hematopoietic stem cells, treated them with BBI608, and then assessed their ability to form both erythroid and myeloid colonies. No inhibition of colony formation of CD34+ hematopoietic stem cells was observed by BBI608 treatment at up to 30 μM (the highest concentration tested) (Fig. 3D).
We next compared the potency of BBI608 and clinically used targeted therapeutics against stemness-high cancer cells and heterogeneous cancer cells under the same conditions. As shown in Table 1, stemness-high cancer cells displayed between threefold and 10-fold resistance to all targeted therapeutics tested, whereas the IC50 for BBI608 was lower in the stemness-high cancer cell population than in the bulk cancer cells. We next determined the IC50 of the inhibitory activity of BBI608 in stemness-high cancer cells isolated from a variety of human cancer cell lines, including head and neck, lung, brain, colon, liver, ovarian, pancreatic, and kidney cancer cell lines. As shown in Table 2, BBI608 was highly effective at targeting stemness-high cancer cells. These data suggest that stemness-high cancer cells are resistant to conventional chemotherapeutic and targeted agents tested but are sensitive to cancer stemness inhibitor BBI608.
Table 1.
Comparison of BBI608 with indicated compounds in regular cancer cells and stemness-high cancer cells
| Compound | IC50 (uM) | |
| Bulk cells | Cancer stem cells | |
| BBI608 | 0.395 | 0.142 |
| Sunitinib | 2.907 | 9.011 |
| Gefitinib | 1.950 | 22.283 |
| Regorafenib | 4.705 | 15.821 |
| Erlotinib | 1.807 | 12.172 |
FaDu cells cultured under normal growth conditions or cancer stem cell growth conditions. IC50s were performed in triplicate.
Table 2.
Broad spectrum activity of BBI608 against stemness-high cancer cells
| Cell line | IC50 (uM) |
| U87-MG (glioblastoma; astrocytoma) | 0.729 |
| U118 (glioblastoma; astrocytoma) | 0.930 |
| COLO205 (colorectal adenocarcinoma) | 0.870 |
| DLD1 (colorectal adenocarcinoma) | 0.996 |
| SW480 (colorectal adenocarcinoma) | 1.231 |
| HCT116 (colorectal carcinoma) | 1.249 |
| FaDu (head and neck squamous cell carcinoma) | 0.616 |
| ACHN (renal cell adenocarcinoma) | 1.190 |
| SNU-475 (hepatocellular carcinoma) | 0.479 |
| Huh7 (hepatocellular carcinoma) | 0.926 |
| HepG2 (hepatocellular carcinoma) | 1.057 |
| H1975 (non-small cell lung cancer; adenocarcinoma) | 0.549 |
| A549 (non-small cell lung cancer; adenocarcinoma) | 1.130 |
| H460 (large cell lung cancer; carcinoma) | 1.185 |
| CAOV-3 (ovarian adenocarcinoma) | 0.291 |
| SW-626 (ovarian adenocarcinoma) | 0.432 |
| PaCa2 (pancreatic carcinoma) | 0.624 |
Beginning with single-cell suspensions from dissociated sphere cultures, cancer stem cells were grown for 3 d to allow for sphere formation and then treated with BBI608, and viability was assessed after 24 h. Data (IC50, uM) represent averages of three separate experiments.
BBI608 Down-Regulates Stemness Gene Expression.
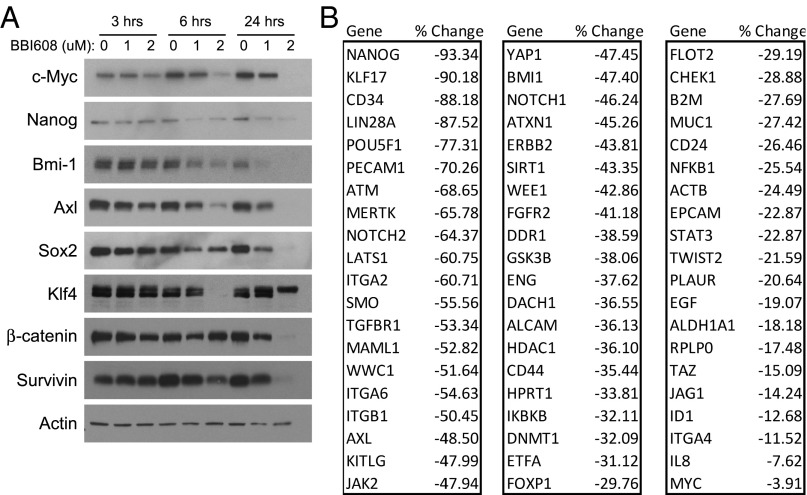
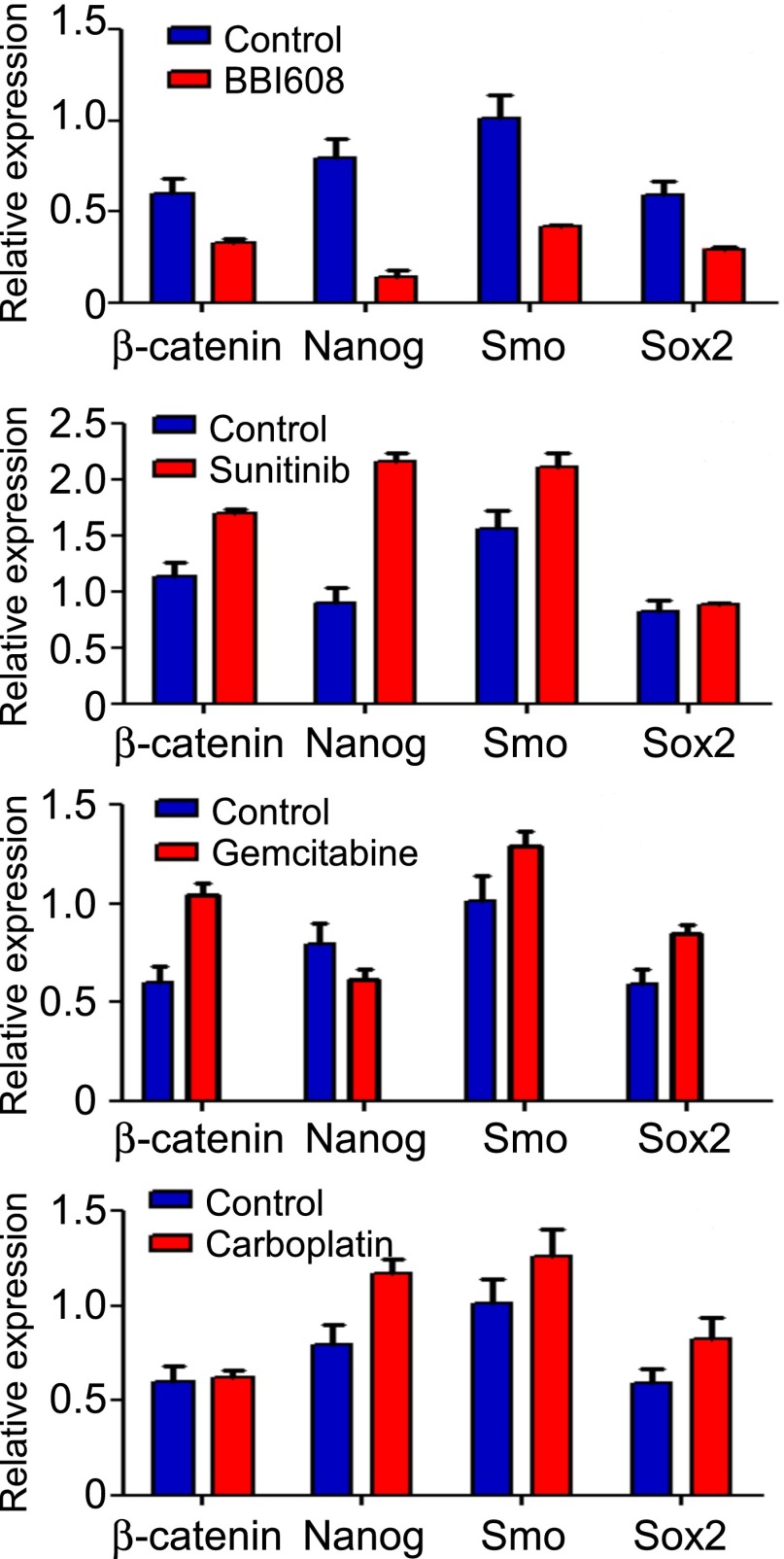
Multiple pathways regulating the self-renewal of stem cells have been identified (16). Stat3, the target of BBI608, regulates many of the genes implicated in cancer stem cell self-renewal, including c-Myc and β-catenin (27–32). We investigated inhibition of these signaling pathways after treatment with BBI608. We found that BBI608 treatment resulted in a dose-dependent decrease in Nanog, Axl, Sox-2, Klf4, survivin, c-Myc, Bmi-1, and β-catenin protein levels (Fig. 4A). Levels of some proteins, such as c-Myc and Axl, were decreased as early as 3 h after treatment, whereas most were lower at 6 h, with the majority of proteins still reduced after 24 h (Fig. 4A). To expand on this observation, we analyzed the changes in gene expression after treatment with BBI608 using a cancer stem cell PCR array. Numerous key molecular markers and genes responsible for cancer stem cell proliferation and self-renewal were found to be down-regulated by BBI608 treatment (Fig. 4B), among them Nanog, Smo, Axl, Atm, and Bmi-1. Given that BBI608 treatment resulted in inhibition of multiple self-renewal pathways, we next compared the effect of BBI608 with chemo- and targeted therapeutics on stemness gene expression. Treatment of stemness-high cancer cells with BBI608 resulted in decreased expression of the self-renewal genes β-catenin, Nanog, Smo, and Sox2 (Fig. 5). By contrast, treatment of stemness-high cancer cells with the chemotherapeutic agents gemcitabine or carboplatin either had no effect or resulted in increased cancer stem cell gene expression (Fig. 5). Likewise, treatment with the kinase inhibitor sunitinib also resulted in increased cancer stem cell gene expression (Fig. 5). Taken together, these results demonstrate that treatment with BBI608 resulted in decreased expression of multiple cancer stem cell genes, whereas treatment with chemo- or targeted therapeutics resulted in increased cancer stem cell gene expression.
Fig. 4.
BBI608 inhibits stemness gene expression. (A) FaDu cancer stem cells were treated for 3, 6, or 24 h with BBI608 at either 1 or 2 μM or with DMSO (0). Cell lysates from these treated cells were then analyzed by Western blotting. BBI608 down-regulates a number of stemness related proteins involved in the growth and maintenance of cancer stem cells. Actin is shown as a loading control. (B) FaDu sphere cultures were treated for 6 h with DMSO (control) or BBI608 at 2 μM. RNA was isolated and reverse transcribed and then the resulting cDNA analyzed using a quantitative PCR cancer stem cell array. GAPDH was used as a housekeeping gene to which the data were normalized. Data shows the top genes down-regulated after treatment with BBI608 normalized to the control treated sample.
Fig. 5.
Comparison between BBI608 and current cancer therapeutics. FaDu cancer stem cells were treated for 24 h with DMSO (control), BBI608 (2 μM), sunitinib (20 μM), gemcitabine (2 μM), or carboplatin (32 μM). RNA was isolated, reverse transcribed, and then analyzed by quantitative PCR for stem cell genes: β-catenin, Nanog, SMO, and SOX2. GAPDH was used as a housekeeping gene to which the data were normalized.
Discussion
In this study we show that BBI608, a small molecule identified on the basis of its inhibition of spherogenesis and Stat3-driven transcription, suppresses metastasis and cancer relapse. The chemotherapeutics and targeted agents tested showed little activity against spherogenesis of stemness-high cancer cells and had either no effect or a stimulatory effect on stemness gene expression. By contrast, BBI608 potently blocked spherogenesis of stemness-high cancer cells, killed stemness-high cancer cells isolated or enriched by surface-marker or side population flow cytometry, and down-regulated stemness gene expression. Moreover, BBI608 showed potent activity against metastasis in a spontaneous liver metastasis model of colorectal cancer and suppressed cancer relapse in a pancreatic cancer model. These data suggest cancer stemness inhibition as a novel approach for the development of cancer therapeutics against cancer relapse and metastasis.
Highly tumorigenic cancer cells have been enriched and isolated from heterogeneous cancer cell populations in patients of various tumor types (5–15). Such hypermalignant cells, termed cancer stem cells, are characterized by extremely high tumorgenic potential, spherogenesis in stem cell media, and expression of stemness genes (5–15). More recently, it has been demonstrated that nonstem cancer cells can acquire stemness phenotypes under certain conditions (33, 34), including clinically relevant chemotherapy or radiotherapy (18, 19). To compare BBI608 with chemotherapeutics agents, we used gemcitabine and carboplatin, which are two commonly used agents for the treatment of a variety of human cancers. We reproduced in vivo the situation that is often found in patients, in which standard therapies target the bulk of the tumor but fail to eradicate the cancer stem cell population, allowing the tumor to relapse (35). We found that treatment with BBI608 could prevent cancer relapse and metastasis in vivo. Moreover, BBI608 significantly reduced the expression of various stemness genes, including Nanog, Sox2, and Oct4 (POU5F1). These genes encode key stemness transcription factors that are important for the maintenance of pluripotency (36, 37). These data demonstrate the feasibility of inhibiting cancer stemness by modulating stemness gene expression.
Through a gene silencing-based approach we identified Stat3 as a critically important factor for maintaining cancer stemness (US patent 8,877,803), which has also been shown by other publications (38, 39). We have observed that Stat3 is constitutively activated in cancer stem cells independent of upstream signaling regulators (US patent 8,877,803). Therefore, cancer stem cells or stemness-high cancer cells are highly sensitive to direct Stat3 inhibition and are not sensitive to inhibition of upstream kinases, including Janus kinases. In this study we demonstrate that BBI608, a potent small-molecule inhibitor of Stat3 (US patent 8,877,803), has a broad spectrum of activity against stemness-high cancer cells, further supporting a significant role for Stat3 in cancer stemness. Targeting Stat3 is expected to trigger a cascade of down-regulation of stemness genes, via direct or indirect mechanisms (32). Our data show that BBI608 completely spares hematopoietic stem cells, a finding that is consistent with an observation made in Stat3 conditional knockout mice in which Stat3 was found to be dispensable for hematopoietic stem cells owing to compensation by other Stat family members (40). Thus, it seems that cancer stem cells hijacked the essential role of Stat3 in embryonic stem cells and become highly sensitive to Stat3 inhibition. In keeping with these in vitro findings, BBI608 is well tolerated and associated with no signs of adverse effects on hematopoietic or other normal adult stem cells in preclinical toxicology studies, as well as in clinical trials (41).
Cancer relapse and metastasis are the ultimate reasons of the failure of current cancer therapies (42–44). The initial responses to chemotherapy and targeted agents are almost always followed by recurrence of resistant cancer (45). The hypothesis that cancer stemness is at least partially responsible for cancer relapse and metastasis opens a novel avenue of research. Identifying and targeting the molecular mechanisms that regulate cancer stemness should help design the next generation of cancer therapeutics to block metastasis and suppress relapse.
Materials and Methods
Cell Culture and Reagents.
Colo205, Dld1, Hct116, RKO, SW480, AGS, MKN-28, MKN-45, 786-0, ACHN, Huh7, Panc1, Du145, HeLa, PC3, and A431 cells were obtained from ATCC. All cells were maintained in DMEM supplemented with 10% (vol/vol) FBS (Gemini) and 1% penicillin/streptomycin (Life Technologies). Cancer stem cells were maintained on noncell adhesive plates in DMEM nutrient mixture F-12 (DMEM/F-12) containing B-27 supplement (Life Technologies), 0.6% BSA, 20 ng/mL EGF, 10 ng/mL FGF (R&D Systems), and 1% penicillin/streptomycin. Hoechst dye exclusion cell sorting of the side population was performed as previously described (46). CD44high/CD24low were isolated by FACS and sorted cells maintained as described above. Human CD34+ bone marrow cells (AllCells) were cultured in MethoCult media to form colonies as described by the manufacturer (Stemcell Technologies). BBI608 (2-Acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione) was synthesized at Boston Biomedical. Sunitinib, regorafenib, gefitinib, erlotinib, carboplatin, gemcitabine, and doxorubicin were purchased from Selleckchem.
Western Blot Analysis.
Cells were washed twice with ice-cold PBS and lysed in lysis buffer [50 mM Hepes (pH 7.5), 1% Nonidet P-40, 150 mM NaCl, 1 mM EDTA, and 1× protease inhibitor mixture (EMD Millipore)]. Soluble protein (20 μg) was separated by SDS/PAGE and transferred to nitrocellulose membranes. Primary antibodies against c-Myc, Klf4, Nanog, Survivin, Sox2, Bmi-1, Axl, and β-catenin (Cell Signaling) were used in this study. The antigen–antibody complexes were visualized by enhanced chemiluminescence (BioRad).
IC50 Analysis.
For cancer stem cells, spheres were dissociated and plated under cancer stem cell culture conditions on coated 96-well plates. After 72 h of culture, wells were dosed with the indicated compounds. Seventy-two hours (Table 1) or 24 h (Table 2) after dosing, CellTiter-Glo 2.0 was added to each well, and the luminescence was measured as described by the manufacturer (Promega). IC50 values were calculated by fitting a four parameter dose–response curve to normalized data using GraphPad Prism software. For bulk cells, cells were plated at 5,000 cells per well on 96-well plates. Twenty-four hours after plating, cells were treated with the indicated compounds. Viability was determined at 72 h as described above.
Quantitative PCR.
FaDu cancer stem cells were seeded in attachment-free six-well plates with cancer stem cell media. Media was changed on day 3. On day 4 of culture, desired concentrations of BBI608, chemotherapy or targeted therapeutic, or DMSO were added to wells in quadruplicate. At the appropriate time point cells were harvested and RNA extracted using the SimplyRNA kit according to directions on the Promega Maxwell system. Reverse transcription was performed on 1 μg of RNA from each sample using the GoScript reverse transcription kit (Promega). Real-time PCR was carried out using RT2 qPCR Primer Assays and RT2 SYBR Green qPCR Mastermix (SABiosciences). Replicate wells were prepared for each sample. Expression of genes of interest was normalized to GAPDH.
CD34+ Bone Marrow Cells.
Frozen CD34+ bone marrow mononuclear cells (AllCells LLC) were thawed into RPMI 1640 medium (Gibco BRL) containing 10% (vol/vol) FBS and DNase I (Stem Cell Technologies). After two washes with medium, cells were incubated in medium containing DMSO or BBI608 for 6 h at 37 °C. Cells were then washed twice in medium, counted to determine viability, and plated in replicate plates at 4,000 cells per 35-mm dish in complete Methocult H4434 medium (Stem Cell Technologies). Cultures were maintained at 37 °C for 14 d to allow colony formation. Colonies of both erythroid and myeloid lineages containing more than 50 cells per colony were counted.
Mouse Models.
For xenograft studies, PaCa2 pancreatic cancer cells were inoculated s.c. into female athymic nude mice (6 × 106 cells per mouse) and allowed to form palpable tumors. Once the tumors reached ∼100 mm3, the animals were treated i.p. with BBI608 at 20 mg/kg, gemcitabine (120 mg/kg) every three days (q3d), or vehicle control daily (5 consecutive days, followed by a 2-d dosing holiday). The animals received a total of 14 doses of BBI608 or vehicle control. Tumors were measured throughout treatment and the posttreatment observation period. To determine whether BBI608 depletes cancer stem cells in vivo, female athymic nude mice were administered with either vehicle, gemcitabine (120 mg/kg), carboplatin (30 mg/kg), or 20 mg/kg of BBI608 i.p. Single-cell suspensions were obtained after animal killing after 14 d of treatment, and sterile removal of tumors. Live cells were then counted and used to measure their ability to form spheres when cultured in cancer stem cell media. Fresh media was added every 3 d, and sphere formation was determined after 10–14 d in culture. Spheres with >50 cells were scored. For the ISMS model, 2 × 106 HT29 cells in 0.1 mL PBS were injected under the spleen capsule of the nude mice. The spleen was replaced in the peritoneal cavity, and the incision was closed. Mice were killed when moribund or 5 wk after treatment. The spleen, liver, and lungs were removed and examined, and the number of tumor lesions was recorded. Mice were divided into two groups, a control group given vehicle (n = 4) and the other group receiving 20 mg/kg BBI608 (n = 4). Drug was administered via i.p. injection for 5 d/wk and for 4 wk from the fourth day after intrasplenic injection. The primary tumors at the spleen and spontaneous liver metastases were examined macroscopically and confirmed histologically.
Statistical Analysis.
All experiments were performed in triplicate. The data for the cell proliferation assays assay are expressed as the mean ± SD. The SDs for all of the measured biological parameters are displayed in the appropriate figures. A Student t test was used for single-variable comparisons, and a P value of <0.05 was considered statistically significant.
Animal Permits.
The protocol was approved by Institutional Animal Care and Use Committee of Boston Biomedical.
Supplementary Material
Acknowledgments
We thank our colleagues at Boston Biomedical for their advice, Katherine Geromini for excellent technical help, and Dr. Andrew Keates for critical reading of the manuscript.
Footnotes
Conflict of interest statement: All authors are employees or advisors of Boston Biomedical, Inc., Sumitomo Dainippon Global Oncology, and declare no equity ownership.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424171112/-/DCSupplemental.
References
- 1.Shekhar MP. Drug resistance: Challenges to effective therapy. Curr Cancer Drug Targets. 2011;11(5):613–623. doi: 10.2174/156800911795655921. [DOI] [PubMed] [Google Scholar]
- 2.Nakata A, Gotoh N. Recent understanding of the molecular mechanisms for the efficacy and resistance of EGF receptor-specific tyrosine kinase inhibitors in non-small cell lung cancer. Expert Opin Ther Targets. 2012;16(8):771–781. doi: 10.1517/14728222.2012.697155. [DOI] [PubMed] [Google Scholar]
- 3.Bucheit AD, Davies MA. Emerging insights into resistance to BRAF inhibitors in melanoma. Biochem Pharmacol. 2014;87(3):381–389. doi: 10.1016/j.bcp.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eramo A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15(3):504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 8.Kim MP, et al. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS ONE. 2011;6(6):e20636. doi: 10.1371/journal.pone.0020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 11.Prince ME, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 13.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 14.Blagosklonny MV. Cancer stem cell and cancer stemloids: From biology to therapy. Cancer Biol Ther. 2007;6(11):1684–1690. doi: 10.4161/cbt.6.11.5167. [DOI] [PubMed] [Google Scholar]
- 15.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 16.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 17.Chen SF, et al. Nonadhesive culture system as a model of rapid sphere formation with cancer stem cell properties. PLoS ONE. 2012;7(2):e31864. doi: 10.1371/journal.pone.0031864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghisolfi L, Keates AC, Hu X, Lee DK, Li CJ. Ionizing radiation induces stemness in cancer cells. PLoS ONE. 2012;7(8):e43628. doi: 10.1371/journal.pone.0043628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, et al. Induction of cancer cell stemness by chemotherapy. Cell Cycle. 2012;11(14):2691–2698. doi: 10.4161/cc.21021. [DOI] [PubMed] [Google Scholar]
- 20.Milas L, Hittelman WN. Cancer stem cells and tumor response to therapy: Current problems and future prospects. Semin Radiat Oncol. 2009;19(2):96–105. doi: 10.1016/j.semradonc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Vermeulen L, de Sousa e Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: Clinical challenges and opportunities. Lancet Oncol. 2012;13(2):e83–e89. doi: 10.1016/S1470-2045(11)70257-1. [DOI] [PubMed] [Google Scholar]
- 22.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12(10):1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 23.Jin L, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5(1):31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Kikushige Y, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7(6):708–717. doi: 10.1016/j.stem.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Wong DJ, et al. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2(4):333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colak S, Medema JP. Cancer stem cells—important players in tumor therapy resistance. FEBS J. 2014;281(21):4779–4791. doi: 10.1111/febs.13023. [DOI] [PubMed] [Google Scholar]
- 27.Garner JM, et al. Constitutive activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor κB signaling in glioblastoma cancer stem cells regulates the Notch pathway. J Biol Chem. 2013;288(36):26167–26176. doi: 10.1074/jbc.M113.477950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee TK, et al. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9(1):50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 30.Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19(21):2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 31.Bromberg JF, et al. Stat3 as an oncogene. Cell. 1999;98(3):295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 32.Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2(10):740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 33.Gupta PB, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146(4):633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Vermeulen L, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12(5):468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 35.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 36.Rizzino A. Concise review: The Sox2-Oct4 connection: Critical players in a much larger interdependent network integrated at multiple levels. Stem Cells. 2013;31(6):1033–1039. doi: 10.1002/stem.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saunders A, Faiola F, Wang J. Concise review: Pursuing self-renewal and pluripotency with the stem cell factor Nanog. Stem Cells. 2013;31(7):1227–1236. doi: 10.1002/stem.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gujral TS, et al. A noncanonical frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell. 2014;159(4):844–856. doi: 10.1016/j.cell.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marotta LL, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44⁺CD24⁻ stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121(7):2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato Y, et al. Selective activation of STAT5 unveils its role in stem cell self-renewal in normal and leukemic hematopoiesis. J Exp Med. 2005;202(1):169–179. doi: 10.1084/jem.20042541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langleben A, et al. A dose-escalation phase I study of a first-in-class cancer stemness inhibitor in patients with advanced malignancies. J Clin Oncol. 2013;31(15 Suppl):2542. [Google Scholar]
- 42.Dave B, Chang J. Treatment resistance in stem cells and breast cancer. J Mammary Gland Biol Neoplasia. 2009;14(1):79–82. doi: 10.1007/s10911-009-9117-9. [DOI] [PubMed] [Google Scholar]
- 43.Li S, et al. Model of tumor dormancy/recurrence after short-term chemotherapy. PLoS ONE. 2014;9(5):e98021. doi: 10.1371/journal.pone.0098021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 45.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13(10):714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 46.Wulf GG, et al. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98(4):1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.