Abstract
An epidemic of morbidity and mortality has swept across the United States related to the use of prescription opioids for chronic noncancer pain. More than 100 000 people have died from unintentional overdose, making this one of the worst manmade epidemics in history.
Much of health care delivery in the United States is regulated at the state level; therefore, both the cause and much of the cure for the opioid epidemic will come from state action.
We detail the strong collaborations across executive health care agencies, and between those public agencies and practicing leaders in the pain field that have led to a substantial reversal of the epidemic in Washington State.
Prescription opioid–related morbidity and mortality constitute a national public health crisis, requiring an urgent need for more effective policy responses.1,2 States play a central role in protecting public health and public safety; regulate health care and practice of health professions; are primary payers of health care through Medicaid, state employee benefits, corrections, and workers’ compensation; and manage prescription drug monitoring programs. Therefore, state-level action is critical to reversing the prescription drug overdose epidemic.3
In recent years, a number of states have engaged in efforts to address prescription drug overdose. Documentation of state experience in implementing interventions and their impacts is greatly needed. This information can inform other states’ efforts and prevent them from pursuing policies that have minimal impact. Washington State has been an innovative leader in efforts to reduce prescription drug overdose. In this article, we detail Washington’s experience to comprehensively address this serious public health threat.
THE ORIGINS OF THE EPIDEMIC IN WASHINGTON STATE
Use of chronic opioid therapy was historically reserved for patients with cancer or end-of-life pain. The shift toward more liberal use of opioids for chronic, noncancer pain (CNCP) began in the mid- to late 1980s when an early case series suggested that patients with CNCP, if well chosen, could take opioids long term safely and with few severe problems (e.g., abuse or addiction).4 On the basis of this study and similar studies, pain advocacy groups and specialists sought state-based regulatory changes to reverse perceived undertreatment of chronic pain.5 These organizations successfully lobbied state medical boards and legislatures to change statutes and regulations to ensure more permissive use of opioids in the CNCP population, and to reduce the risk of sanction for prescribers. By January 2003, only 5 states and the District of Columbia had not changed their statutes or regulations.6
In Washington State, these laws and regulations were changed in 1999. For example, administrative code (246-919-830, December 1999), with the force of law, stated, “No disciplinary action will be taken against a practitioner based solely on the quantity and/or frequency of opioids prescribed.” This language, in a law since repealed, effectively opened the door to allowing opioid prescribing for CNCP without any restrictions or cautions.
In addition to changes at the state level, national-level policies began to have an impact on opioid prescribing. The development of pain as the “fifth vital sign” by the Joint Commission promoted increased opioid prescribing for acute pain in the hospital setting, especially in emergency departments (EDs) and postoperatively. The numeric pain intensity score was elevated to the sole metric for evaluating “quality” pain care in the hospital.7 Studies have found that these changes have not been associated with improvement in quality of pain management,8 may have increased serious adverse opioid reactions in hospitals,9 and may have contributed to more dangerous levels of postoperative sedation.10 Simultaneously, the term “pseudoaddiction”—referring to patients who look addicted but who, by this definition, were thought to need more opioids—was propagated by drug company surrogates.11
These policy changes, accompanied by aggressive marketing by the pharmaceutical industry to prescribers,12 led to a dramatic increase in prescription opioid sales. Studies have documented a strong linear relationship between mortality and sales of specific prescription opioids, a surrogate measure of volume and dose.13 These deaths are particularly poignant because they affect younger adults, with people aged 35 to 54 years at highest risk.14
In Washington, opioid prescribing increased 500% from 1997 to 2006.15 The Washington workers’ compensation system saw a dramatic increase in schedule II opioid prescribing from 1996 to 2002, and a 50% increase in the average daily morphine-equivalent dose (MED) among injured workers taking these potent medications.16 By 2000, the workers’ compensation program noted a rise in overdose deaths.16 These deaths prompted a manual review of all opioid overdose death certificates by the Washington Department of Health. The initial review showed an increase in the number of overdose deaths involving prescription opioids from 24 in 1995 to 351 in 2004.
By 2006, the Centers for Disease Control and Prevention had identified Washington to be in the highest tertile of mortality (10.8 deaths/100 000) from unintentional drug overdoses in the United States.14 Also in 2006, approximately 10 000 Washington patients in public insurance programs were taking at least 120 milligrams per day MED. These compelling statistics spurred innovative collaboration between state health agencies and pain leaders to begin to address the problem.
MEDICAID’S EFFORTS TO ADDRESS THE PROBLEM
In early 2005, Medicaid staff, using paid claims data on all covered enrollees, identified 320 clients receiving 10 or more opioid prescriptions per month from multiple prescribers. After preliminary discussion with stakeholders, Medicaid implemented the Narcotic Review Program, wherein Medicaid staff initiated direct communication with the clients’ medical and pharmacy providers to ensure the appropriateness of controlled substance prescriptions. Early interventions included providing comprehensive controlled substance prescription histories to all prescribing providers, initiating clinical reviews when high doses and risky behaviors were identified, prior authorization to medically justify prescriptions, coordination with mental health treatment, and referral of selected high-risk clients to a patient review and coordination program (also known as Medicaid Lock-In program).
The patient review and coordination program can restrict clients for 24 months or longer to specific providers and pharmacies. Until 2005, patient review and coordination in Washington was a small program, with about 200 clients. From 2005 to 2012, it grew to more than 3800 clients. The patient review and coordination program resulted in a 33% decrease in ED visits, a 37% decrease in office visits, and a 24% decrease in controlled substance prescriptions after 7 months of patient entry into the program. The program is now run by the Medicaid managed care organizations and has about 3000 clients.
THE FIRST US OPIOID DOSING GUIDELINE
On the basis of the recognition that opioid prescribing practice was a key driver of the epidemic, in 2006 a consortium of all Washington agencies that purchase or regulate health care (the Agency Medical Directors’ Group [AMDG]) collaborated with 15 Washington pain management experts (the Clinical Advisory Group) to develop an opioid prescribing guideline. The guideline was implemented in April 2007 as an educational pilot and focused primarily on dosing guidance for patients at or above 120 milligrams per day MED. High-quality epidemiological studies on dose-related mortality risk were not yet available, so this dosage was based on the experience of the advisory group and unanimously agreed upon. The guideline included 2 parts, each designed to address different aspects of the opioid prescribing problem:
Part I focused on carefully monitoring opioid dosing in opioid-naïve patients on doses of less than 120 milligrams per day MED. The key recommendation was to request specialty pain consultation for patients who have reached a dose of 120 milligrams per day MED without substantial improvements in pain or function. The implication was, in the absence of clinical improvement, opioid tolerance may be developing,17 and dose escalation should not occur without clinical justification.
Part II of the guideline focused on patients already on at least 120 milligrams per day MED, including recommendations for reassessing the patient’s opioid regimen and reducing or withdrawing opioids if significant problems (e.g., dose-limiting side effects, marked tolerance, hyperalgesia) developed.
Initial dissemination efforts included (1) approximately 35 Category I continuing medical education (CME) presentations to primary care groups, (2) two hours of free Category I CME after completing an online test, (3) a Washington State Medical Association resolution allowing a link to the guideline on their Web site as a best practice, and (4) posting on the Agency for Healthcare Research and Quality National Guideline Clearinghouse in June 2008.
In 2009, a brief, Web-based convenience survey was conducted to assess physician acceptance and use of the guideline. Key findings were that only 45% of respondents were familiar with the guideline and had applied it; 54% of physicians who regularly treat chronic pain patients “have frequent concerns about development of psychological dependence, addiction, or diversion”; the vast majority of providers did not use generally accepted best practices for monitoring patients; and 86% believed that the 120 milligrams per day MED “yellow flag” dose was either reasonable or too high.18
An updated Washington AMDG guideline was produced in June 2010 with guidance and publicly available online tools necessary to properly monitor the safe and effective use of opioids for CNCP. These included a 2-question instrument for tracking pain and pain interference with function, screening instruments for substance abuse and depression, and a detailed section on urine drug testing.19
DOSING GUIDANCE AND BEST PRACTICES
In January 2010, on the basis of concerns about the increase in overdose deaths involving prescription opioids, the Washington legislature passed Engrossed Substitute House Bill (ESHB) 2878.20 The bill accomplished 2 key things: it repealed the earlier permissive pain rules, and it mandated that new rules be adopted to address: (1) opioid dosing criteria; (2) guidance on when to seek pain specialty consultation; (3) guidance on tracking clinical progress via assessment tools focusing on pain interference, physical function, and overall risk for poor outcome; and (4) guidance on tracking adherent use of opioids.
Five boards and commissions representing opioid prescribers (physicians, osteopathic physicians, podiatrists, dentists, and advanced registered nurse practitioners) jointly developed rules consistent with the mandates in ESHB 2876. This work resulted in detailed rules similar in scope and content to the Washington AMDG guideline.21 Four of the rules became effective in July 2011, and one became effective in January 2012.
NEW COLLABORATIONS AND INNOVATIONS
In June 2008, the Washington Department of Health staff started an interagency prescription opioid overdose prevention workgroup. In April 2009 an ED physician working subgroup drafted ED opioid prescribing guidelines and encouraged use of an Emergency Department Information Exchange (EDIE) for sharing clinical information between EDs in real-time. In June 2011, the Washington chapter of the American College of Emergency Physicians adopted and disseminated the Washington Emergency Department Opioid Prescribing Guidelines.22,23 Recommendations included limiting opioid prescribing for chronic pain to a single provider and discouraging the administration of intravenous and intramuscular opioids in the ED for the relief of acute exacerbations of chronic pain. The EDIE is a proprietary Internet-based health information exchange that collects and shares clinical information related to ED visits among participating EDs. The EDIE alerts ED providers in real-time when a high-utilization patient presents to the ED.
In parallel to these efforts, Washington Medicaid became increasingly concerned about rising costs related to frequent ED visits for pain-related complaints. A collaboration among Medicaid, the Washington chapter of the American College of Emergency Physicians, the Washington State Hospital Association, and the Washington State Medical Association produced an agreement incorporated into ESHB 2127 requiring 75% of hospitals to start implementing 7 best practices, including the ED opioid guidelines and adoption of EDIE, by June 15, 2012.24 Currently, all EDs attest to implementing the guidelines and all are using EDIE. After passage of ESHB 2127, Medicaid experienced a 24% decline in controlled substance prescribing after an ED visit between July 2012 and July 2013.
PRESCRIPTION DRUG MONITORING PROGRAM
Washington is one of 49 states with an operational prescription drug monitoring program (PDMP). In 2010, Washington received 2 federal grants for PDMP implementation. Data collection began in October 2011 and health care provider access started in January 2012. In 2013, the program was permanently funded from the Medicaid Fraud Penalty account because of the success providing data to public payers.
The Washington Department of Labor and Industries (DLI) and Medicaid both receive PDMP data through a bulk data transfer. At DLI, PDMP data were used to (1) track the longer-term effectiveness of opioid detoxification and tapering programs, and (2) identify 2% of new claimants taking opioids chronically before their injury. Moreover, Medicaid discovered PRC clients who were paying cash for opioid prescriptions.
Like other state PDMPs without mandated registration or use, registration is less than 30% of providers with a Drug Enforcement Administration registration. Emergency department provider enrollment with the PDMP is 1 of Medicaid’s 7 ED best practices. By December 31, 2012, 90% of ED providers were expected to be registered with the PDMP; however, use is not mandated. The DLI’s new opioid guideline25 recommends that providers check the PDMP before writing the first opioid prescription and requires checking if they prescribe opioids beyond 6 weeks postinjury.
NEW WORKERS’ COMPENSATION OPIOID GUIDELINE
In July 2013, the DLI implemented new guidelines and rules meant to address opioid prescribing issues not previously addressed in the Washington Interagency Guideline. Specifically, the guidelines focus on (1) use of opioids for acute and subacute pain, (2) use of opioids in the perioperative period for opioid-tolerant patients undergoing elective surgery, (3) guidance on when to taper opioids, and (4) a definition of clinically meaningful improvement in pain and function.25,26
The AMDG has recalled the advisory group in 2014 to update the guideline, and is reassessing the “yellow-flag” dosing threshold in consideration of more recent high-quality epidemiological studies.27–29 Moreover, an important new dimension could include more direct guidance regarding use of opioids during acute and subacute pain periods, before embarking on chronic opioid therapy.30
INCREASING ACCESS TO PAIN EXPERTS
To help address a significant shortage of qualified pain specialists, the University of Washington implemented telemedicine technology; this program was modeled after the University of New Mexico successful “Project ECHO.”31 In March 2011, “UW TelePain/ECHO” was launched providing free educational consultations by a multidisciplinary group of pain experts.32
The program is offered twice weekly, one focused on management of chronic pain, and another on addiction and dependence issues. By the end of 2013, more than 660 unique providers had participated, 230 consultations were completed, and more than 1600 hours of didactic lectures had been provided.
TAKE-BACK PROGRAM AND SAMARITAN LAW
Local initiatives, beginning in 2003, established Washington as an early adopter and leader in prescription drug take-back programs. In 2013, there were more than 100 ongoing medicine drop-off sites provided by local and state law enforcement. Despite this, not every community has a site and current sites are not widely promoted because of limited resources.
In 2010, Washington became the second state to enact a naloxone-related Samaritan law.33 The law (1) provided legal immunity from drug possession prosecutions for people who have a controlled substance overdose or who seek medical aid for someone having an overdose, and (2) allowed for the opioid antidote, naloxone, to be prescribed to anyone at risk for having or witnessing an opioid overdose and allowed them to possess naloxone and administer it to a person having an apparent opioid overdose. Because the law passed with no funding for implementation or evaluation, the primary vehicle for educating the public has been the Web site, http://www.stopoverdose.org, hosted by University of Washington’s Alcohol and Drug Abuse Institute. The Web site provides general information for the public, law enforcement, pharmacists, and prescribers, and a locator feature to determine where naloxone can be obtained locally.
An evaluation of the law’s implementation showed that awareness of the law among opioid users, police, and paramedics was very low.34 In the 4 years since the law passed, implementation still lags well behind the information and service needs of the general public, and key government and for-profit sector stakeholders.
THE HEALTH IMPACT OF WASHINGTON’S EFFORTS
We have begun to see changes in opioid prescribing and health outcomes, although it is impossible to attribute declines to a single intervention. Prescription opioid overdose death rates in Washington declined by 27% from 2008 to 2012, and overdose hospitalization rates declined for the first time in 2012 (Figure 1). Among workers’ compensation patients, the percentage with new opioid use who became chronic opioid users declined significantly from 26% in 2004 to 11% in 2010,35 the average daily MED declined by 27%,36 those reaching 120 milligrams per day MED declined from 6.3% to 4.7%,36 and the rate of opioid poisonings and opioid adverse effects did not increase between 2004 and 2010, despite an increase nationally.37 Within Washington Medicaid, average opioid doses have declined since 2007. Prescription opioid–involved deaths reached a high of 300 Medicaid clients in 2007, and declined to 198 in 2012.
FIGURE 1—
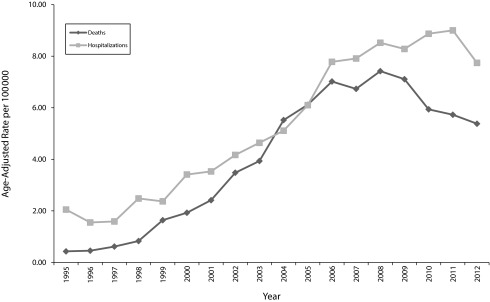
Prescription opioid–involved overdoses: Washington State, 1995–2013.
Source. Washington State Department of Health, Death Certificate and Hospital Discharge Data.
Among Washington 10th graders, the percentage using prescription pain relievers to “get high” declined significantly from 10% in 2006 to 6% in 2012. According to the National Survey on Drug Use and Health, the percentage of Washington residents who have used prescription pain medication nonmedically in the past year declined from 6.2% in 2009 to 2010 to 5.1% in 2011 to 2012.38
LESSONS LEARNED TO REDUCE MORBIDITY AND MORTALITY
The box on page 467 includes the essential elements of Washington’s approach to reduce prescription opioid–related morbidity and mortality. Washington’s experiences serve to inform action in other states, and are described more fully in the next paragraphs.
Essential Elements From Washington State to Reduce Prescription Opioid Morbidity and Mortality
| Facilitate collaboration among state agencies. |
| Create strong pain management laws. |
| Establish dosing and best-practice guidelines and rules related to opioid use for acute, subacute, and chronic noncancer pain. |
| Implement effective prescription drug monitoring programs and leverage health information technology to inform real-time decision-making. |
| Institute robust surveillance to track prescribing and use practices and health outcomes. |
| Incentivize use of best practices. |
| Initiate overdose education programs to mitigate risk to those already using opioids. |
| Increase access to medication-assisted treatment. |
| Evaluate impact of interventions. |
Collaboration among state agencies at the highest levels of state government has been critical to our success. Like many other states, public payers of health care in Washington claim a larger portion of the opioid-related morbidity and mortality. More than half of the deaths in Washington are among Medicaid enrollees,39 and although deaths in workers’ compensation are much lower, the emergence of increasing dependence and disability related to opioid use among injured workers30 is a serious threat to the public health and financial stability of the system. Given these realities, policy change is best served by collaboration and strong leadership among state departments of health, employee benefits, Medicaid, corrections, and workers’ compensation.
The Washington legislature’s creation of strong pain management laws was a significant step in the state.20 The law also repealed existing pain management policies, which previously made it virtually impossible to sanction problematic opioid prescribers and clinics. Until new policies—based on current science and expert consensus—are adopted, state efforts to curb inappropriate opioid prescribing will likely be minimized.
The creation of dosing and best practice guidelines in collaboration among public agencies and clinical and academic pain leaders in the state has led to wide acceptance of these changes in the practicing community. The most critical element of the Washington AMDG guideline19 was the inclusion of a 120-milligrams-per-day MED dosing threshold. This served as a new “set-point,” with a focus on whether patients reaching that level of dosing were meaningfully better, in both pain and function. Strong epidemiological studies now support a dosing threshold or range around 80 to 100 milligrams per day MED,27–29 and should be a critical component of future guidelines.
The institution of robust surveillance to track prescribing practices and overdose events has been another example of extraordinary collaboration among state agencies. Washington public agencies collaborated to clearly define and track prescription opioid- and heroin-involved deaths, overdose hospitalizations, patterns of high-dose prescribing and use, and adolescent use of prescription pain relievers to “get high.” A more accurate accounting of opioid-related deaths requires application of formal case criteria to identify deaths from prescription opioids and heroin on death certificates,16,39 and involvement of medical examiners and coroners to follow national recommendations on investigation and certification of deaths involving opioids.40 States have the responsibility to track overdose deaths and overdose hospitalizations, and to collaborate in feeding back this information to the public agencies with the greatest proportion of morbidity and mortality, such as Medicaid.
Identifying high-dose patients and prescribers through computerized bill payment systems is an effective way for public and commercial payers to offer assistance or intervene to prevent harm. These data system inquiries must be timely and should apply a standard set of metrics.
The recent implementation and funding of an effective PDMP has been important to Washington prescribers in more effectively and safely monitoring patients on opioids. The PDMPs offer great promise as a tool to assist providers in preventing and managing risk when prescribing opioids and other controlled substances. The PDMPs can become a standard part of opioid prescribing best practices, but identified barriers include inadequate funding, underutilization, and lack of interoperability across state lines or health care systems (e.g., with the Veterans Affairs Health System).41
Financial and nonfinancial incentives provided by payers could increase the use of opioid best practices, and of alternatives to use of opioids. For example, DLI pays substantially more for receipt of complete best practice information documented in the medical record.26 Free CME is a good nonfinancial incentive offered for the Washington pain rules21 and 2013 workers’ compensation opioid guideline.25
Initiation of overdose education programs to mitigate risk to those already using opioids is an important tertiary prevention tool. The majority of opioid overdoses continue to involve prescription opioids, and many more people are regular users of prescription opioids than of heroin. However, heroin users may be at higher risk for overdose than prescription opioid users.42,43 In 2013, the Substance Abuse and Mental Health Services Administration released an opioid overdose prevention toolkit, which encourages prescribers to provide overdose education and prescribe naloxone to those at risk for overdose.44
Nationally and in Washington, there are documented increases in use of heroin and heroin-related overdose deaths, most of which represent transition from prescription opioids to heroin among some users.45–47 Factors influencing this transition include tolerance, availability, and costs.45 Changes in prescribing of opioids will likely prevent a future generation of opioid-addicted individuals, but efforts are necessary now to assist those already addicted. These include overdose education efforts, including expanding access to naloxone, and increased access to medication-assisted treatment. Evaluation of state policies and programs to determine impact is an essential part of any state effort to prevent opioid overdose, dependence, and addiction; funding opportunities in this area have recently improved substantially. Most of the results reported previously in this article were supported by a Centers for Disease Control and Prevention grant focused on assessing the impact of the Washington AMDG opioid dosing guideline. The Centers for Disease Control and Prevention has also presented recent collaborative program funding opportunities to state departments of health, and the budget for these contracts is likely to increase in the next fiscal year.48
CONCLUSIONS
It is critically important that the opioid epidemic is understood as much greater than an epidemic of mortality—it is also an epidemic of dependence, addiction, disability, and other severe adverse events affecting millions of people in the United States. These layers of the epidemic are primarily associated with the overprescribing of opioids for many common chronic pain conditions for which evidence does not support their use.49 State opioid policies, clinical practices, patients, health system and payer policies, and agencies responsible for oversight of health care practice all play a crucial role in implementing solutions effective in reversing this public health crisis.
In Washington, collaboration at the highest levels of government, and among state agencies, researchers, medical providers, and community stakeholders, led to a statewide effort to implement policies sufficient to begin to see health impacts. These efforts required a strong coalition of stakeholders driven by effective analyses of prescription drug utilization and related morbidity and mortality data. Furthermore, the coalition focused on interventions that targeted the key drivers of the epidemic. Primary among them was mandatory use of evidence-based best prescribing practices, implementation of a robust PDMP, leveraging technology, and innovative Medicaid and workers' compensation policies. Important, though less robustly implemented, were public health and treatment approaches to mitigate risk among those already regularly using opioids. Washington’s experience navigating various stakeholder priorities, sustaining a diverse coalition, and demonstrating changes in clinical practice and health outcomes underscores the power of a collaborative state-based approach.
Human Participant Protection
Institutional board review was not necessary as a result of the nonintervention nature of this article.
References
- 1.Calcaterra S, Glanz J, Binswanger IA. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug Alcohol Depend. 2013;131(3):263–270. doi: 10.1016/j.drugalcdep.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy M. Containing the opioid overdose epidemic. BMJ. 2012;345:e8340. doi: 10.1136/bmj.e8340. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Policy impact: prescription painkiller overdoses. 2011. Available at: http://www.cdc.gov/homeandrecreationalsafety/rxbrief. Accessed August 29, 2014.
- 4.Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25(2):171–186. doi: 10.1016/0304-3959(86)90091-6. [DOI] [PubMed] [Google Scholar]
- 5.Hill CS., Jr Government regulatory influences on opioid prescribing and their impact on the treatment of pain of nonmalignant origin. J Pain Symptom Manage. 1996;11(5):287–298. doi: 10.1016/0885-3924(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 6.Pain and Policies Study Group. Database on statutes, regulations, and other policies for pain management. Available at: http://www.painpolicy.wisc.edu/database-statutes-regulations-other-policies-pain-management. Accessed August 29, 2014.
- 7.Tormoehlen LM, Mowry JB, Bodle JD, Rusyniak DE. Increased adolescent opioid use and complications reported to a poison control center following the 2000 JCAHO pain initiative. Clin Toxicol (Phila) 2011;49(6):492–498. doi: 10.3109/15563650.2011.587819. [DOI] [PubMed] [Google Scholar]
- 8.Mularski RA, White-Chu F, Overbay D, Miller L, Asch SM, Ganzini L. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med. 2006;21(6):607–612. doi: 10.1111/j.1525-1497.2006.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vila H, Jr, Smith RA, Augustyniak MJ et al. The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: is patient safety compromised by treatment based solely on numerical pain ratings? Anesth Analg. 2005;101(2):474–480. doi: 10.1213/01.ANE.0000155970.45321.A8. [DOI] [PubMed] [Google Scholar]
- 10.Taylor S, Voytovich AE, Kozol RA. Has the pendulum swung too far in postoperative pain control? Am J Surg. 2003;186(5):472–475. doi: 10.1016/j.amjsurg.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Weissman DE, Haddox JD. Opioid pseudoaddiction—an iatrogenic syndrome. Pain. 1989;36(3):363–366. doi: 10.1016/0304-3959(89)90097-3. [DOI] [PubMed] [Google Scholar]
- 12.Van Zee A. The promotion and marketing of Oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221–227. doi: 10.2105/AJPH.2007.131714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618–627. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 14.Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999–2006. NCHS Data Brief. 2009;(22):1–8. [PubMed] [Google Scholar]
- 15.Washington State Department of Health. Drug poisoning and overdose. 2013. Available at: http://www.doh.wa.gov/portals/1/Documents/2900/DOH530090Poison.pdf. Accessed on January 10, 2015.
- 16.Franklin GM, Mai J, Wickizer T, Turner JA, Fulton-Kehoe D, Grant L. Opioid dosing trends and mortality in Washington State workers’ compensation, 1996–2002. Am J Ind Med. 2005;48(2):91–99. doi: 10.1002/ajim.20191. [DOI] [PubMed] [Google Scholar]
- 17.Ballantyne JC. Opioid analgesia: perspectives on right use and utility. Pain Physician. 2007;10(3):479–491. [PubMed] [Google Scholar]
- 18.Morse JS, Stockbridge H, Egan KB, Mai J, Wickizer T, Franklin GM. Primary care survey of the value and effectiveness of the Washington State Opioid Dosing Guideline. J Opioid Manag. 2011;7(6):427–433. doi: 10.5055/jom.2011.0083. [DOI] [PubMed] [Google Scholar]
- 19.WA Agency Medical Director’s Group. Opioid dosing guideline for chronic, non-cancer pain. 2010. Available at: http://www.agencymeddirectors.wa.gov/opioiddosing.asp. Accessed August 29, 2014.
- 20.Engrossed Substitute House Bill 2876-An act relating to pain management. 2010. Available at: http://apps.leg.wa.gov/documents/billdocs/2009-10/Pdf/Bills/Session Laws/House/2876-S.SL.pdf. Accessed August 29, 2014.
- 21.Washington State Department of Health. Pain management rules. Available at: http://www.doh.wa.gov/ForPublicHealthandHealthcareProviders/HealthcareProfessionsandFacilities/PainManagement/AdoptedRules.aspx. Accessed August 29, 2014.
- 22.Washington Emergency Department Opioid Prescribing Guidelines. Available at: http://washingtonacep.org/Postings/edopioidabuseguidelinesfinal.pdf. Accessed August 29, 2014.
- 23.Neven DE, Sabel JC, Howell DN, Carlisle RJ. The development of the Washington State emergency department opioid prescribing guidelines. J Med Toxicol. 2012;8(4):353–359. doi: 10.1007/s13181-012-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Third Engrossed Substitute House Bill 2127, § 43 (a-g) (2012) Available at: http://apps.leg.wa.gov/documents/billdocs/2011-12/Pdf/Bills/House Passed Legislature/2127-S.PL.pdf. Accessed August 29, 2014.
- 25.Guideline for prescribing opioids to treat pain in injured workers, effective July 1, 2013. Available at: http://lni.wa.gov/ClaimsIns/Files/OMD/MedTreat/FINALOpioidGuideline010713.pdf. Accessed August 29, 2014. [DOI] [PubMed]
- 26.Washington State Department of Labor and Industries. Prescribing opioids to treat pain in injured workers; rules, and other resources. Available at: http://www.lni.wa.gov/ClaimsIns/Providers/TreatingPatients/ByCondition/Opioids/default.asp?utm_source=shortmarketingurl&utm_medium=url&utm_campaign=Opioids. Accessed August 29, 2014.
- 27.Dunn KM, Saunders KW, Rutter CM et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686–691. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- 29.Bohnert AS, Valenstine M, Bair MJ et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 30.Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer T. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine. 2008;33(2):199–204. doi: 10.1097/BRS.0b013e318160455c. [DOI] [PubMed] [Google Scholar]
- 31.Arora S, Kalishman S, Dion D et al. Partnering urban academic medical centers and rural primary care clinicians to provide complex chronic disease care. Health Aff Millwood. 2011;30(6):1176–1184. doi: 10.1377/hlthaff.2011.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.University of Washington Pain Medicine. Telepain. Available at: http://depts.washington.edu/anesth/care/pain/telepain/index.shtml. Accessed August 29, 2014.
- 33.Washington State Statutes: RCW 69.50.315: medical assistance—drug-related overdose—naloxone—prosecution for possession. 2010. Available at: http://app.leg.wa.gov/rcw/default.aspx?cite=69.50.315. Accessed October 29, 2014.
- 34.Banta-Green CJ, Beletsky L, Schoeppe JA, Coffin PO, Kuszler PC. Police officers’ and paramedics’ experiences with overdose and their knowledge and opinions of Washington State’s drug overdose-naloxone-Good Samaritan law. J Urban Health. 2013;90(6):1102–1111. doi: 10.1007/s11524-013-9814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garg RK, Fulton-Kehoe D, Turner JA et al. Changes in opioid prescribing for Washington workers’ compensation claimants after implementation of an opioid dosing guideline for chronic noncancer pain: 2004 to 2010. J Pain. 2013;14(12):1620–1628. doi: 10.1016/j.jpain.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Franklin GM, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D. Bending the prescription opioid dosing and mortality curves: impact of the Washington State opioid dosing guideline. Am J Ind Med. 2012;55(4):325–331. doi: 10.1002/ajim.21998. [DOI] [PubMed] [Google Scholar]
- 37.Fulton-Kehoe D, Garg RK, Turner JA et al. Opioid poisonings and opioid adverse effects in workers in Washington State. Am J Ind Med. 2013;56(12):1452–1462. doi: 10.1002/ajim.22266. [DOI] [PubMed] [Google Scholar]
- 38. Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health. State estimates of substance abuse and mental disorders. Available at: https://nsduhweb.rti.org/respweb/homepage.cfm. Accessed October 29, 2014.
- 39.Centers for Disease Control and Prevention. Overdose deaths involving prescription opioids among Medicaid enrollees—Washington, 2004–2007. MMWR Morb Mortal Wkly Rep. 2009;58(42):1171–1175. [PubMed] [Google Scholar]
- 40.Davis GG National Association of Medical Examiners and American College of Medical Toxicology Expert Panel on Evaluating and Reporting Opioid Deaths. Complete republication: National Association of Medical Examiners position paper: recommendations for the investigation, diagnosis, and certification of deaths related to opioid drugs. J Med Toxicol. 2014;10(1):100–106. doi: 10.1007/s13181-013-0323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deyo RA, Irvine JM, Millet LM et al. Measures such as interstate cooperation would improve the efficacy of programs to track controlled drug prescriptions. Health Aff (Millwood) 2013;32(3):603–613. doi: 10.1377/hlthaff.2012.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darke S, Mattick RP, Degenhardt L. The ratio of non-fatal to fatal heroin overdose. Addiction. 2003;98(8):1169–1171. doi: 10.1046/j.1360-0443.2003.00474.x. [DOI] [PubMed] [Google Scholar]
- 43.Substance Abuse and Mental Health Services Administration. Opioid overdose prevention toolkit. 2013. Available at: http://store.samhsa.gov/product/Opioid-Overdose-Prevention-Toolkit/SMA13-4742. Accessed August 29, 2014.
- 44.Jenkins LM, Banta-Green CJ, Maynard C et al. Risk factors for nonfatal overdose at Seattle-area syringe exchanges. J Urban Health. 2011;88(1):118–128. doi: 10.1007/s11524-010-9525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers—United States, 2002–2004 and 2008–2010. Drug Alcohol Depend. 2013;132(1-2):95–100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Peavy KM, Banta-Green CJ, Kingston S, Hanrahan M, Merrill JO, Coffin PO. “Hooked on” prescription-type opiates prior to using heroin: results from a survey of syringe exchange clients. J Psychoactive Drugs. 2012;44(3):259–265. doi: 10.1080/02791072.2012.704591. [DOI] [PubMed] [Google Scholar]
- 47.Banta-Green C. Heroin trends across Washington State. Info brief prepared for the UW Alcohol & Drug Abuse Institute. 2013. Available at: http://adai.uw.edu/pubs/InfoBriefs/ADAI-IB-2013-02.pdf. Accessed August 29, 2014.
- 48.Centers for Disease Control and Prevention. Prescription drug overdose: boost for state prevention. Available at: http://www.grants.gov/web/grants/view-opportunity.html?oppId=253411. Accessed August 29, 2014.
- 49.Franklin GM. Opioids for chronic noncancer pain. A position paper of the American Academy of Neurology. Neurology. 2014;83(14):1277–1284. doi: 10.1212/WNL.0000000000000839. [DOI] [PubMed] [Google Scholar]


