Abstract
Objectives. We tested the efficacy of a minimal intervention to create smoke-free homes in low-income households recruited through the United Way of Greater Atlanta 2-1-1, an information and referral system that connects callers to local social services.
Methods. We conducted a randomized controlled trial (n = 498) from June 2012 through June 2013, with follow-up at 3 and 6 months. The intervention consisted of 3 mailings and 1 coaching call.
Results. Participants were mostly smokers (79.7%), women (82.7%), African American (83.3%), and not employed (76.5%), with an annual household income of $10 000 or less (55.6%). At 6-months postbaseline, significantly more intervention participants reported a full ban on smoking in the home than did control participants (40.0% vs 25.4%; P = .002). The intervention worked for smokers and nonsmokers, as well as those with or without children.
Conclusions. Minimal intervention was effective in promoting smoke-free homes in low income households and offers a potentially scalable model for protecting children and adult nonsmokers from secondhand smoke exposure in their homes.
Despite declines in exposure to secondhand smoke (SHS) over the last 2 decades, children and nonsmoking adults who live with a person who smokes still experience significant exposure to SHS.1–3 SHS exposure causes lung cancer, coronary heart disease, and stroke in nonsmoking adults, aexacerbates asthma, and causes impaired lung function, middle ear disease, respiratory illness, and sudden infant death syndrome in children.3–5
Exposure differs markedly between those who live with someone who smokes in the home and those who do not. In 2007 and 2008, 93.4% of nonsmoking adults who lived with someone who smoked inside the home had elevated serum cotinine levels compared with 33.4% of those who did not live with someone who smoked inside the home.6 This pattern was similar, but more striking, for children and youths.6 Certain subgroups of the US population are less likely to have household smoking restrictions and are disproportionately affected by SHS exposure in the home. For instance, African American nonsmokers have an increased prevalence of detectable serum cotinine compared with other major racial/ethnic groups and are less likely to report home smoking bans.6–8 Low income families and those with less education are less likely to have full smoking bans.6,8–11 Other predictors of household smoking bans include the presence of children, the presence of a nonsmoking adult in the home, and fewer friends and family members who smoke.9,10,12–17
Home smoking bans can lead to lower levels of SHS exposure, less smoking, and increased attempts to quit.7,13,18–22 The prevalence of smoke-free homes has increased as states and communities have legislated smoke-free public places.23,24 Intervention studies have typically examined the effects of counseling parents of children with asthma, infants, or medically compromised children on exposure levels.25–29 Effective interventions involve multiple counseling sessions and often combine smoking cessation and smoke-free home messages.30–32 Much of the existing intervention research has taken place or recruited participants through clinical settings.30–33 Minimal interventions to create smoke-free homes in community-based settings have not been adequately studied.31,33,34
Minimal interventions have the potential for greater reach than more intensive interventions, and thus, have the potential for a greater impact at the population level.35–38 Similarly, interventions that target general populations, including households with no young children, can help to achieve population-level reductions in SHS exposure. We tested the efficacy of a minimal intervention with callers to the United Way of Greater Atlanta, Georgia, 2-1-1 number. The 2-1-1 information and referral system consists of more than 200 nonprofit state and local call centers operating in all 50 states and connects more than 16 million callers per year to local health and social services.39 Callers to 2-1-1 are disproportionately low-income, unemployed, uninsured, and have fewer years of education relative to the general population.40 2-1-1 callers have a higher rate of smoking and lower likelihood of a home smoking ban than the general population.41,42 Because 2-1-1 provides extensive reach to vulnerable populations, they are strategic partners for testing, delivering, and ultimately sustaining interventions to reduce risk and improve the lives of low-income persons in the United States.40
We tested the efficacy of a minimal intervention to create smoke-free homes among 2-1-1 callers. Our study builds on formative research on family dynamics related to establishing household smoking bans,43,44 a pilot study to test a brief intervention,45 and a cross-site survey of 2-1-1 callers that showed a relatively low prevalence of smoke-free homes.41 This randomized controlled trial is the first in a series of studies that will move from testing efficacy to effectiveness to dissemination of the intervention through 2-1-1 centers nationally.
METHODS
Atlanta is home to the first and also one of the largest 2-1-1 systems in the United States, with more than 460 000 contacts in the last fiscal year. A random sample of 2-1-1 callers was directed to 1 of 5 trained line agents employed by United Way of Greater Atlanta 2-1-1 and selected for our study based on interest and aptitude. After their reasons for calling were addressed, callers not in crisis (e.g., psychological distress) were asked if there were any smokers in the home. Those who reported having a smoker in the home were given a very brief description of the study that included procedures and compensation, and were asked if they would be interested. We then formally screened all those who were interested for eligibility. We recruited participants from June through October of 2012.
Eligible participants had to be (1) 18 years or older; (2) able to speak and understand English; (3) a smoker living with at least 1 child or other nonsmoker, or a nonsmoker living with a smoker; and (4) allow smoking in the home. The rationale for recruiting both smokers and nonsmokers, as well as households with and without children, was to test an intervention with relatively high generalizability. Eligible callers provided oral consent and were enrolled (n = 498).
Data Collection Procedures
Baseline data were collected by the 2-1-1 line agents following consent. We kept the baseline survey short to minimize disruption to 2-1-1 operations. Following base-line data collection, participants were randomized to receive the brief intervention condition or a measures-only condition using simple randomization (e.g., no blocks). University-based research assistants blinded to study condition collected outcome data at 3 and 6 months postrandomization. Follow-up interviews were conducted over the telephone and lasted approximately 30 minutes. Participants received a $25 Walmart gift card for each interview completed, including the baseline interview. We used a rigorous call protocol of up to 12 call attempts to reach participants, plus sent 2 letters to hard-to-reach participants. We recorded the baseline and follow-up telephone interviews for quality control purposes.
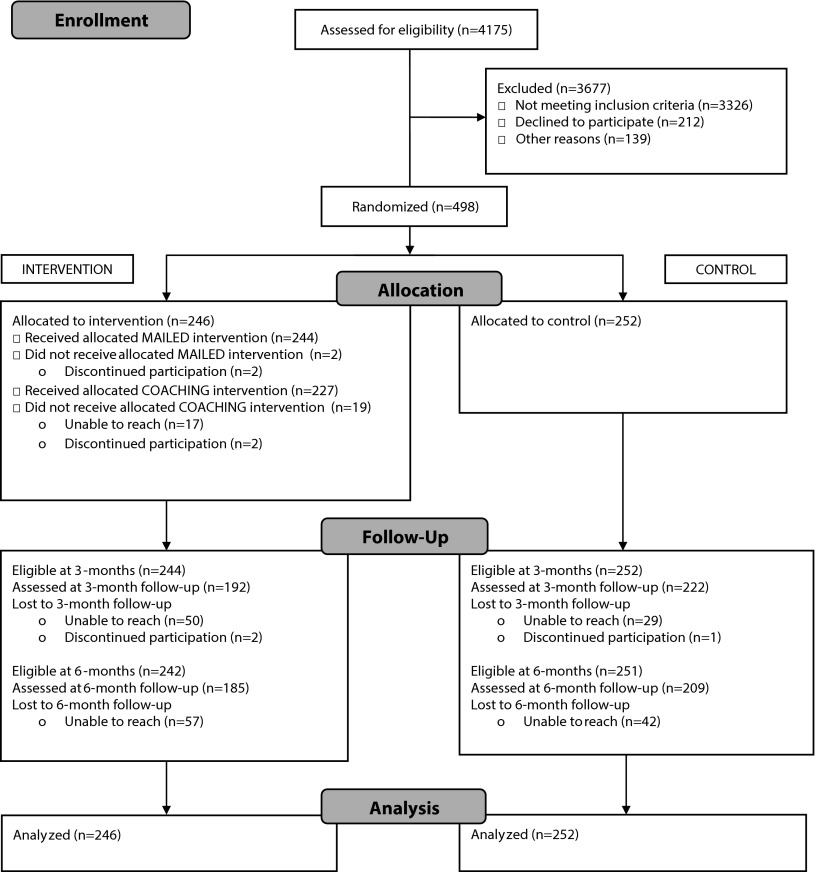
Figure 1 shows the Consolidated Standards of Reporting Trials diagram documenting the participant flow from recruitment through 6 months of data collection. Of the 4175 callers assessed for eligibility, 79.7% were ineligible (71.4% had no smokers in the home, 0.3% had another household member already enrolled, 3.7% had no nonsmokers in the home, 4.3% already had a full ban), 8.4% declined to participate or did not complete eligibility screening or the consent process, and the remaining 498 (11.9%) participants were randomized. Overall, 83.1% (n = 414) completed 3-month data collection, and 79.1% (n = 394) completed 6-month data collection.
FIGURE 1—
Consolidated Standards of Reporting Trials flow diagram for randomized controlled trial to create smoke-free homes: intervention to promote smoke-free homes among 2-1-1 callers, greater Atlanta area, GA, 2012.
Intervention Description
The Smoke-Free Homes intervention, designed for simple and widespread delivery, consisted of 3 mailings and 1 coaching call. The intervention is based on the theme of “Some things are better outside,” with content focused on 5 steps to create a smoke-free home.45 The intervention was delivered over a 6-week period in 2-week intervals as follows: first mailing, coaching call, and then 2 additional mailings.
The first mailed intervention component was delivered immediately following completion of the baseline interview and included a “Five-Step Guide to a Smoke-Free Home,” reasons to have a smoke-free home, a tear-off pledge, tear-off signs, information on SHS, and the state and national Quitline number. The second component was a 15 to 20 minute coaching call based on the 5 steps and methods from motivational interviewing.46 The coaching protocol first assessed importance of and confidence in creating a smoke-free home, then led the participants through a structured, stage-based conversation about making the home smoke-free. The third component was a mailed photo story of a family facing and overcoming challenges to going smoke-free, and a challenges and solutions booklet based on our earlier qualitative research.44,45 The fourth component was a mailed newsletter with success stories, stickers, a window sign, and a thirdhand smoke fact sheet. Thirdhand smoke is the harmful residue that cigarette smoke leaves on surfaces; the fact sheet informed participants that thirdhand smoke remains on surfaces for days, weeks, or even months.47–49
Intervention strategies, based on social cognitive theory and the transtheoretical model’s stages of change, included persuasion, role modeling, goal setting, environmental cues, and written and verbal reinforcement of actions taken to create a smoke-free home.50,51 We developed the intervention to reach both smokers and nonsmokers as home change agents because 2-1-1 callers included both smokers and nonsmokers. The intervention messages focused heavily on creation of smoke-free homes (i.e., smoking outside) rather than on smoking cessation.
Measures
Outcome measures.
The primary outcome measure was the self-reported presence of a full home smoking ban. It was assessed at all 3 time points by asking “Which statement best describes the rules about smoking inside your home: smoking is not allowed anywhere inside your home; smoking is allowed in some places or at some times; smoking is allowed anywhere inside your home; or there are no rules about smoking inside your home?”52 Participants were considered to have no ban if they reported that smoking was allowed anywhere or if there were no rules. They had a partial ban if they allowed smoking in some places or at some times, and had a full ban if smoking was not allowed anywhere inside their home.
Secondary outcomes included self-reported SHS exposure in the home, and among smokers, cessation attempts, number of cigarettes smoked per day, and self-efficacy for quitting.19,20,53 SHS exposure was assessed by asking, “During the past 7 days, how many days have people smoked in your home in your presence?”54 We also asked about smoking restrictions in cars because of the potential for spillover effects from restrictions in 1 type of personal space (homes) to another (vehicles).31 A question adapted from Norman et al. was used to assess smoking restrictions in cars.9
Descriptive measures.
Smoking status and demographic characteristic information on the participant’s race/ethnicity, age, gender, educational level, marital status, household income, household composition, and employment status were also collected. Enforcement of the home smoking ban was assessed by asking, “How often are your smoking rules broken by someone?” Response options were never, rarely, sometimes, and very often.
Process measures.
We included a range of process measures in the 3-month interview, including receipt of mailed materials, proportion of materials read, usefulness and relevance of materials, satisfaction with the coaching call, and intermediate behavioral actions recommended by the intervention (e.g., talking with household members).
Air nicotine to validate self-reported bans.
After the 3-month interview, to validate self-reported home smoking ban status, a passive air nicotine monitor was mailed to all participants who reported a full or no ban and to half of the participants who reported a partial ban (n = 315). We established and successfully executed telephone-based monitor placement and removal protocols in earlier pilot studies.55,56 A total of 272 (86.3%) participants successfully placed the monitor in the home, and 246 (78.1%) monitors were returned intact to the research office. Duplicate and blank monitors were mailed to 10% of assigned households for quality assurance. Participants were compensated with a $25 Walmart gift card for placing and returning the device. The monitors were purchased from and analyzed via gas chromatography by the Johns Hopkins Bloomberg School of Public Health Secondhand Smoke Exposure Assessment Laboratory using previously described methods.57,58
Statistical Analyses
We examined distributions for all relevant variables at each time point. We then assessed the impact of the intervention at each time point using complete case data. We conducted growth analyses with all available data, assuming that data were missing at random after confirming that missingness was not associated with any variables measured at baseline (intent-to-treat analysis) using binary logistic, ordinal logistic, Poisson, and linear multilevel models dependent upon variable type.59 These analyses modeled linear change over time (which was appropriate based upon preliminary investigation of trajectories), as well as a cross-level interaction effect of time and group assignment to model the effectiveness of the intervention. We tested the moderation effects of smoking status, number of smokers in the home, and whether there were any children younger than 18 or younger than 5 years of age in the home using the χ2 and Wilcoxon–Mann–Whitney tests.
In addition, we conducted 2 sensitivity analyses with the same growth curve models used for the intent-to-treat analysis. The first assumed that all participants lost to the intervention would not have made their home smoke-free, and the responses of those who did not have follow-up data were coded as not having a full ban (i.e., a worst-case scenario analysis). The second sensitivity analysis was based on the data from the nicotine monitors. Receiver-operator curve (ROC) analysis found that the area under the curve was 77.9%.60 The optimal threshold was determined 2 ways, closest to ideal and farthest from random, yielding the same threshold of 0.9743 μg/m3 with a sensitivity of 69.5% and a specificity of 81.2% for a home not being smoke-free. Based on the results from the ROC analysis, participants who reported a full ban but had nicotine concentrations above the threshold were recoded as not having a full ban for the second sensitivity analysis. Analyses were conducted using SAS (version 9.3; SAS Institute, Cary, NC), SPSS (version 21; IBM, Armonk, NY), and HLM (version 7; Scientific Software International, Skokie, IL).
RESULTS
Table 1 shows the demographic characteristics of participants and key baseline measures by group assignment. The study population was mostly female (82.7%), African American (83.3%), and not working (76.5%), with a household income of $10 000 or less (55.6%). The majority smoked (79.7%). Most had at least a high school education (75.1%) and were single (55.8%), living with at least 1 child younger than age 18 years in the home (78.9%). The mean age was 40.2 years (SD = 10.87). A large proportion of households had 1 nonsmoking adult in the home (46.4%), but approximately one third of households reported no nonsmoking adults (34.7%). Half of the households had only 1 smoker (50.0%), and the rest had 2 or more. At baseline, 61.4% of participants reported having a partial smoking ban; 38.6% reported having no restriction on smoking in the home. There were no statistically significant differences between those randomized to the intervention or the control group on any baseline variables.
TABLE 1—
Demographic Characteristics of Study Participants at Baseline: Intervention to Promote Smoke-Free Homes Among 2-1-1 Callers, Greater Atlanta Area, GA, 2012
| Characteristic | Total (n = 498), No. (%) or Mean ±SD | Intervention (n = 246), No. (%) or Mean ±SD | Control (n = 252), No. (%) or Mean ±SD |
| Gender | |||
| Male | 86 (17.3) | 47 (19.1) | 39 (15.5) |
| Female | 412 (82.7) | 199 (80.9) | 213 (84.5) |
| Race/ethnicity | |||
| African American | 415 (83.3) | 210 (85.4) | 205 (81.4) |
| White | 57 (11.5) | 22 (8.9) | 35 (13.9) |
| Other | 25 (5.0) | 13 (5.3) | 12 (4.8) |
| Employment | |||
| Employed | 117 (23.5) | 55 (22.4) | 62 (24.6) |
| Unemployed | 229 (46.0) | 118 (48.0) | 111 (44.0) |
| Homemaker/retired/disabled/other | 152 (30.5) | 73 (29.7) | 79 (31.4) |
| Income | |||
| ≤ $10 000 | 277 (55.6) | 134 (54.5) | 143 (56.8) |
| $10 001–$20 000 | 138 (27.7) | 67 (27.2) | 71 (28.2) |
| $20 001–$35 000 | 63 (12.7) | 35 (14.2) | 28 (11.1) |
| $35 001–$50 000 | 13 (2.6) | 5 (2.0) | 8 (3.2) |
| $50 001–$75 000 | 1 (0.2) | 1 (0.4) | 0 (0.0) |
| Education | |||
| Less than/some high school | 124 (24.9) | 60 (24.4) | 64 (25.4) |
| High school graduate/GED | 197 (39.6) | 95 (38.6) | 102 (40.5) |
| Vocational/technical school/ some college | 140 (28.1) | 72 (29.3) | 68 (27.0) |
| College graduate or higher | 37 (7.4) | 19 (7.7) | 18 (7.1) |
| Marital status | |||
| Not married, living with partner | 137 (27.5) | 66 (26.8) | 71 (28.2) |
| Married | 83 (16.7) | 43 (17.5) | 40 (15.9) |
| Single | 278 (55.8) | 137 (55.7) | 141 (56.0) |
| Age, y | 40.2 ±10.87 | 40.0 ±10.99 | 40.4 ±10.77 |
| Smoking status | |||
| Nonsmoker | 101 (20.3) | 55 (22.4) | 46 (18.3) |
| Smoker | 397 (79.7) | 191 (77.6) | 206 (81.8) |
| No. of cigarettes per da | 12.9 ±8.2 | 12.6 ±7.72 | 13.1 ±8.62 |
| No. of smokers in the home | |||
| 1 | 248 (50.0) | 126 (51.6) | 122 (48.4) |
| 2 | 176 (35.5) | 85 (34.8) | 91 (36.1) |
| ≥ 3 | 72 (14.5) | 33 (13.5) | 39 (15.5) |
| No. of nonsmoking adults in the home | |||
| 0 | 173 (34.7) | 83 (33.7) | 90 (35.7) |
| 1 | 231 (46.4) | 116 (47.2) | 115 (45.6) |
| ≥ 2 | 94 (18.9) | 47 (19.1) | 47 (18.7) |
| Children in the home (yes reported) | |||
| Children < 18 y in the home | 393 (78.9) | 199 (80.9) | 194 (77.0) |
| Children < 5 y in the home | 192 (38.6) | 99 (40.4) | 93 (36.9) |
| Children < 1 y in the home | 49 (9.8) | 18 (7.3) | 31 (12.3) |
| Home smoking ban status | |||
| Partial ban | 306 (61.4) | 154 (62.6) | 152 (60.3) |
| No ban | 192 (38.6) | 92 (37.4) | 100 (39.7) |
Note. GED = general equivalency diploma. Percentages might not add up to 100% because of rounding or refusal to answer.
For the 397 participants who smoked.
Process Evaluation
Of the intervention participants who completed the 3-month interviews with complete process evaluation data (n = 180), the majority felt the materials were very (70.0%) or somewhat (20.0%) relevant, and very (82.8%) or somewhat (13.9%) useful.
The coaching call was also well received, with 82.4% reporting they were very satisfied. Of note, a high percentage of participants engaged in actions recommended by the intervention: 92.8% had a family talk and 60.6% put up signs.
Intervention Impact
Household smoking bans.
Significantly more intervention participants reported a full ban on smoking in the home than control participants 3 months postbaseline (30.4% vs 14.9%; P < .001), as well as at 6 months (40.0% vs 25.4%; P = .002; Table 2). The longitudinal intent-to-treat analysis showed that the difference in change was significant over time. When defining success more stringently by including only those reporting a full ban and no enforcement challenges, we found again that more intervention than control participants were successful in having and enforcing their smoke-free home rule at 3 months (11.0% vs 5.6%; P = .03) and at 6 months postbaseline (18.3% vs 8.7%; P = .002).
TABLE 2—
Impact of the Intervention on Primary and Secondary Outcomes at 3 and 6 Months Postbaseline: Intervention to Promote Smoke-Free Homes Among 2-1-1 Callers, Greater Atlanta Area, GA, 2012
| 3 Mo Assessment |
6 Mo Assessment |
ITT Analysis Intervention Group Change |
||||||
| Characteristic | Intervention (n = 192), No. (%) or Mean ±SD | Control (n = 222), No. (%) or Mean ±SD | P | Intervention (n = 185), No. (%) or Mean ±SD | Control (n = 209), No. (%) or Mean ±SD | P | Effect | P |
| Primary outcome | ||||||||
| Home smoking ban | < .001 | .002 | 1.56a | < .001 | ||||
| Full ban | 58 (30.4) | 33 (14.9) | 74 (40.0) | 53 (25.4) | ||||
| No full ban | 133 (69.6) | 189 (85.1) | 111 (60.0) | 156 (74.6) | ||||
| Secondary outcomes—all participants | ||||||||
| No. of d exposed to SHS in past wk | 2.7 ±2.86 | 3.8 ±2.97 | < .001 | 2.1 ±2.66 | 3.2 ±3.05 | < .001 | 0.77b | < .001 |
| Car smoking ban | 0.02 | .189 | N/A | |||||
| Full ban | 50 (26.2) | 40 (18.0) | 59 (31.9) | 54 (25.8) | ||||
| Partial ban | 49 (25.7) | 47 (21.2) | 46 (24.9) | 53 (25.5) | ||||
| No ban | 34 (17.8) | 60 (27.0) | 26 (14.1) | 42 (20.1) | ||||
| No carc | 58 (30.4) | 75 (33.8) | 54 (29.2) | 60 (28.7) | ||||
| Secondary outcomes—smokers only | ||||||||
| Smokers | 139 (72.8) | 168 (75.7) | 130 (70.3) | 153 (73.2) | ||||
| Quit attempts last 3 mo | 2.1 ±2.6 | 1.3 ±1.8 | 0.003 | 1.5 ±1.7 | 1.4 ±1.8 | .9 | 1.12b | .15 |
| No. of cigarettes per d | 9.8 ±6.3 | 13.0 ±8.5 | < .001 | 9.2 ±6.6 | 11.3 ±8.4 | .02 | −0.99d | .004 |
| Confidence to quit smoking | 6.9 ±2.4 | 5.5 ±3.1 | < .001 | 6.7 ±2.5 | 6.2 ±2.8 | .12 | 0.30d | .03 |
Note. ITT = intent to treat; SHS = secondhand smoke.
Odds ratio.
Event rate ratio.
Excluded from analysis.
Unstandardized parameter estimate.
Secondary outcomes.
We saw a larger reduction in self-reported exposure to SHS in the home among intervention participants at both follow-up points, with a significantly larger decrease in the intervention group. In addition, we found a significantly higher percentage of intervention participants (26.2% vs 18.0%) who reported a full smoking ban in cars at 3 months (P = .02), although this difference was not observed 6 months postbaseline.
Smokers in the intervention group reported fewer cigarettes smoked per day at both follow-up points, and the longitudinal analysis indicated that the intervention group had a significantly larger reduction over time. Although we observed no difference in cessation rates between intervention and control groups, smokers in the intervention group had a higher number of quit attempts at the first follow-up point, but not at 6 months postbaseline. We also found that smokers in the intervention group had higher confidence in being able to quit at 3 months, but not at 6 months. The longitudinal intent-to-treat analysis, however, showed a significant difference in self-efficacy to quit.
Moderators of Intervention Effect
We found that a significantly higher percentage of nonsmokers reported a total smoking ban at 3 months than did smokers (Table 3). This was the case in both the intervention and control groups. However, although nonsmokers were more likely to report a smoke-free home than smokers at 6 months, the difference was not statistically significant.
TABLE 3—
Moderators of Intervention Effectiveness: Intervention to Promote Smoke-Free Homes Among 2-1-1 Callers, Greater Atlanta Area, GA, 2012
| Percentage With Full Home Smoking Ban |
||||||||||
| Follow-up Point | Smoker, No. (%) | Nonsmoker, No. (%) | P | Children, No. (%) | No Children, No. (%) | P | 1 Smoker, No. (%) | 2 Smokers, No. (%) | ≥ 3 Smokers, No. (%) | P |
| 3 mo | ||||||||||
| Intervention | 38 (25.9) | 20 (45.5) | .01 | 48 (31.2) | 10 (27.0) | .62 | 33 (33.3) | 17 (25.8) | 8 (32.0) | .58 |
| Control | 21 (11.5) | 12 (30.0) | .003 | 24 (14.3) | 9 (16.7) | .67 | 17 (15.7) | 13 (15.7) | 3 (9.7) | .68 |
| 6 mo | ||||||||||
| Intervention | 54 (37.2) | 20 (50.0) | .14 | 63 (42.6) | 11 (29.7) | .15 | 33 (35.9) | 30 (46.2) | 10 (38.5) | .39 |
| Control | 40 (23.5) | 13 (33.3) | .2 | 43 (27.0) | 10 (20.0) | .33 | 25 (24.0) | 21 (26.9) | 7 (25.9) | .7 |
Note. P values for number of smokers in the home are from the Kruskal–Wallis test.
Having children in the home had no impact on creating a smoke-free home at either follow-up point, although participants with children reported somewhat higher rates of a full smoking ban. In addition, we found no significant difference in the main outcome that was dependent on the number of smokers in the home.
Sensitivity Analyses
The mean nicotine concentration was significantly lower for homes where participants reported a full ban (mean = 0.75 μg/m3; SD = 1.38 μg/m3) than for homes without a full ban (mean = 3.57 μg/m3; SD = 5.66 μg/m3; t = 6.16; P < .001).
The 2 sensitivity analyses confirmed the preceding results. Under the worst-case scenario analysis, intervention participants were significantly more likely to have a smoke-free home at 3 months (P = .003) and at 6 months (P = .02). After re-coding participants with nicotine readings above the ROC threshold or missing nicotine monitor data, there were still significantly more smoke-free homes in the intervention group (n = 35; 14.2%) compared with the control group (n = 21; 8.3%) at 3 months postbaseline (P = .04).
DISCUSSION
The intervention was effective in promoting smoke-free home policies among 2-1-1 callers, with air nicotine monitors validating the self-reported results. Twice as many participants in the intervention group had a full ban at 3 months as in the control group. Significant differences (15% points) were also found at 6 months despite improvement in the control group. Other intervention approaches have achieved declines in SHS exposure, but typically through more intensive counseling approaches and targeting families with young or medically compromised children.26–34,61–63 To our knowledge, this was the first smoke-free homes intervention study that targeted a general population that included households with an adult nonsmoker and no children.
The demographic characteristics of the study population reflected the overall population of callers to 2-1-1 in the greater Atlanta area. Interest in study participation was high, which suggested a brief intervention to create smoke-free homes was acceptable to 2-1-1 callers, albeit combined with a gift card incentive, which is used in most intervention research studies. This was consistent with recent research that documented that minimal contact interventions delivered through 2-1-1 were acceptable to callers, and that they would act upon health referrals when offered, especially when referrals were supplemented with mailed reminders or telephone health coaching.42,64
Our intervention targeted both smokers and nonsmokers as change agents, because both groups are represented among callers to 2-1-1. The majority of those enrolled in the study were smokers. Interestingly, moderator analyses indicated that smokers were just as likely to report full bans at 6 months, which indicated success for this approach in reducing SHS exposure among both children and nonsmokers. The main outcome was not affected by the number of smokers in the home, which was surprising because of the associations between household smoking restrictions and the number of smokers in the home.10
Previous research showed that smoke-free homes were associated with reduced cigarette consumption and increased cessation.18–22,29 In this intervention, which had a strong smoke-free home message and a limited cessation message, we found that smokers reduced consumption of cigarettes and had increased self-efficacy to quit. Examination of potential spillover effects to other personal spaces, particularly cars, was also interesting. Although the differences observed at 3 months were not sustained, expanding the intervention to encourage smoke-free vehicles could be promising. One area in which the intervention could have been strengthened was enforcement. More research is needed to determine how to enhance enforcement practices, perhaps through real-time feedback.65
The sample was largely African American women living in a large southeastern US metropolitan area. The majority reported partial bans at baseline, which might indicate a certain level of readiness to create a smoke-free home. Thus, our study results might not generalize to other groups. Generalizability concerns will be addressed with replication studies under way in North Carolina and Houston, Texas. Our use of self-report raised concerns about socially desirable responses at the follow-up assessments. This risk was offset by findings from nicotine dosimeters, which confirmed reduced exposure in the home. Finally, our final follow-up assessment was conducted at 6 months postbaseline, which might not be the optimal time point for assessing long-term effects. However, we saw increases rather than decreases from our 3- to 6-month assessment, which indicated a minimal diminished effect over time and possible reactivity to the measures.
Overall, this brief intervention, set in a 2-1-1 call center, was effective in creating smoke-free homes. The next 2 trials with North Carolina and Houston 2-1-1 callers will take the intervention 1 more step into “real world” operations and examine the potential for scalability, with the intervention delivered by trained call specialists at a 2-1-1 call center instead of by university research staff. If successful, these studies will set the stage for dissemination of the model through 2-1-1 call centers nationally. This collection of trials offers a model for conducting efficacy to translational research to develop and disseminate new interventions through partnerships poised to reach high-risk populations nationwide.
Acknowledgments
We would like to thank marketzero for their creative contributions to the printed intervention materials. This publication was supported by the National Cancer Institute's State and Community Tobacco Control Research Initiative (grant number U01CA154282). This trial is registered with ClinicalTrials.gov number: NCT01625468.
Human Participant Protection
The Emory University institutional review board approved this protocol.
References
- 1.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the US population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114(6):853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Disparities in secondhand smoke exposure. MMWR Morb Mortal Wkly Rep. 2008;57(27):744–747. [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. Handbooks of Cancer Prevention, Tobacco Control. Lyon, France: World Health Organization; 2009. Evaluating the effectiveness of smoke-free policies; pp. 9–58. [Google Scholar]
- 4.US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: Office of the Surgeon General; 2006. [Google Scholar]
- 5.US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Washington, DC: Office of the Surgeon General; 2014. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Vital signs: nonsmokers’ exposure to secondhand smoke—United States, 1999-2008. MMWR Morb Mortal Wkly Rep. 2010;59(35):1141–1146. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Disparities in secondhand smoke exposure–United States, 1988-1994 and 1999-2004. MMWR Morb Mortal Wkly Rep. 2008;57(27):744–747. [PubMed] [Google Scholar]
- 8.Mills AL, White MM, Pierce JP, Messer K. Home smoking bans among US households with children and smokers: opportunities for intervention. Am J Prev Med. 2011;41(6):559–565. doi: 10.1016/j.amepre.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Norman GJ, Ribisl KM, Howard-Pitney B, Howard KA. Smoking bans in the home and car: do those who really need them have them? Prev Med. 1999;29(6):581–589. doi: 10.1006/pmed.1999.0574. [DOI] [PubMed] [Google Scholar]
- 10.Kegler MC, Malcoe LH. Smoking restrictions in the home and car among rural Native American and white families with young children. Prev Med. 2002;35(4):334–342. doi: 10.1006/pmed.2002.1091. [DOI] [PubMed] [Google Scholar]
- 11.King BA, Dube SR, Homa DM. Smoke-free rules and secondhand smoke exposure in homes and vehicles among US adults, 2009-2010. Prev Chronic Dis. 2013;10:E79. doi: 10.5888/pcd10.120218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster MA, Franke T, Pham CB. Smoking patterns of household members and visitors in homes with children in the United States. Arch Pediatr Adolesc Med. 2002;156(11):1094–1100. doi: 10.1001/archpedi.156.11.1094. [DOI] [PubMed] [Google Scholar]
- 13.Pizacani BA, Martin DP, Stark MJ, Koepsell TD, Thompson B, Diehr P. Household smoking bans: which households have them and do they work? Prev Med. 2003;36(1):99–107. doi: 10.1006/pmed.2002.1123. [DOI] [PubMed] [Google Scholar]
- 14.Okah FA. Effect of children on home smoking restriction by inner-city smokers. Pediatrics. 2002;109(2):244–249. doi: 10.1542/peds.109.2.244. [DOI] [PubMed] [Google Scholar]
- 15.Berg CJDC, Nazir N, Cully A et al. Smoke-free policies in the workplace and in the home among American Indians. J Health Dispar Res Pract. 2012;5(2):81–91. [PMC free article] [PubMed] [Google Scholar]
- 16.King G, Mallett R, Kozlowski L, Bendel RB, Nahata S. Personal space smoking restrictions among African Americans. Am J Prev Med. 2005;28(1):33–40. doi: 10.1016/j.amepre.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins SS, Berkman L. Parental home smoking policies: the protective effect of having a young child in the household. Prev Med. 2011;53(1-2):61–63. doi: 10.1016/j.ypmed.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Hyland A, Higbee C, Travers MJ et al. Smoke-free homes and smoking cessation and relapse in a longitudinal population of adults. Nicotine Tob Res. 2009;11(6):614–618. doi: 10.1093/ntr/ntp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messer K, Mills AL, White MM, Pierce JP. The effect of smoke-free homes on smoking behavior in the US. Am J Prev Med. 2008;35(3):210–216. doi: 10.1016/j.amepre.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Forster JL, Widome R, Bernat DH.Policy interventions and surveillance as strategies to prevent tobacco use in adolescents and young adults Am J Prev Med 2007336, supplS335–S339. [DOI] [PubMed] [Google Scholar]
- 21.Biener L, Nyman AL. Effect of workplace smoking policies on smoking cessation: results of a longitudinal study. J Occup Environ Med. 1999;41(12):1121–1127. doi: 10.1097/00043764-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Pizacani BA, Martin DP, Stark MJ, Koepsell TD, Thompson B, Diehr P. A prospective study of household smoking bans and subsequent cessation related behaviour: the role of stage of change. Tob Control. 2004;13(1):23–28. doi: 10.1136/tc.2003.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mons U, Nagelhout GE, Allwright S et al. Impact of national smoke-free legislation on home smoking bans: findings from the International Tobacco Control Policy Evaluation Project Europe Surveys. Tob Control. 2013;22(e1):e2–e9. doi: 10.1136/tobaccocontrol-2011-050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng KW, Glantz SA, Lightwood JM. Association between smokefree laws and voluntary smokefree-home rules. Am J Prev Med. 2011;41(6):566–572. doi: 10.1016/j.amepre.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stotts AL, Green C, Northrup TF et al. Feasibility and efficacy of an intervention to reduce secondhand smoke exposure among infants discharged from a neonatal intensive care unit. J Perinatol. 2013;33(10):811–816. doi: 10.1038/jp.2013.43. [DOI] [PubMed] [Google Scholar]
- 26.Tyc VL, Huang Q, Nicholson J et al. Intervention to reduce secondhand smoke exposure among children with cancer: a controlled trial. Psychooncology. 2013;22(5):1104–1111. doi: 10.1002/pon.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Effect of counselling mothers on their children’s exposure to environmental tobacco smoke: randomised controlled trial. BMJ. 2000;321(7257):337–342. doi: 10.1136/bmj.321.7257.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hovell MF, Meltzer SB, Wahlgren DR et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: a controlled trial. Pediatrics. 2002;110(5):946–956. doi: 10.1542/peds.110.5.946. [DOI] [PubMed] [Google Scholar]
- 29.Hovell MF, Zakarian JM, Matt GE et al. Counseling to reduce children’s secondhand smoke exposure and help parents quit smoking: a controlled trial. Nicotine Tob Res. 2009;11(12):1383–1394. doi: 10.1093/ntr/ntp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gehrman CA, Hovell MF. Protecting children from environmental tobacco smoke (ETS) exposure: a critical review. Nicotine Tob Res. 2003;5(3):289–301. doi: 10.1080/1462220031000094231. [DOI] [PubMed] [Google Scholar]
- 31.Hovell MF, Hughes SC. The behavioral ecology of secondhand smoke exposure: a pathway to complete tobacco control. Nicotine Tob Res. 2009;11(11):1254–1264. doi: 10.1093/ntr/ntp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen LJ, Noach MB, Winickoff JP, Hovell MF. Parental smoking cessation to protect young children: a systematic review and meta-analysis. Pediatrics. 2012;129(1):141–152. doi: 10.1542/peds.2010-3209. [DOI] [PubMed] [Google Scholar]
- 33.Hopkins DP, Briss PA, Ricard CJ et al. Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. Am J Prev Med. 2001;20(2 suppl):16–66. doi: 10.1016/s0749-3797(00)00297-x. [DOI] [PubMed] [Google Scholar]
- 34.Task Force on Community Preventive Services. The Guide to Community Preventive Services: What Works to Promote Health? New York, NY: Oxford University Press; 2005. [Google Scholar]
- 35.Rose G. Sick individuals and sick populations. 1985. Bull World Health Organ. 2001;79(10):990–996. [PMC free article] [PubMed] [Google Scholar]
- 36.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gierisch JM, DeFrank JT, Bowling JM et al. Finding the minimal intervention needed for sustained mammography adherence. Am J Prev Med. 2010;39(4):334–344. doi: 10.1016/j.amepre.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribisl KM, Leeman J, Glasser A. Pricing health behavior intervention to promote adoption: lessons from the marketing and business literature. Am J Prev Med. 2014;46(6):653–659. doi: 10.1016/j.amepre.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daily LS. Health research and surveillance potential to partner with 2-1-1. Am J Prev Med. 2012;43(6 suppl 5):S422–S424. doi: 10.1016/j.amepre.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreuter MW. Reach, effectiveness, and connections: the case for partnering with 2-1-1 to eliminate health disparities. Am J Prev Med. 2012;43(6 suppl 5):S420–S421. doi: 10.1016/j.amepre.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purnell JQ, Kreuter MW, Eddens KS et al. Cancer control needs of 2-1-1 callers in Missouri, North Carolina, Texas, and Washington. J Health Care Poor Underserved. 2012;23(2):752–767. doi: 10.1353/hpu.2012.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreuter MW, Eddens KS, Alcaraz KI et al. Use of cancer control referrals by 2-1-1 callers: a randomized trial. Am J Prev Med. 2012;43(6 suppl 5):S425–S434. doi: 10.1016/j.amepre.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escoffery C, Kegler MC, Butler S. Formative research on creating smoke-free homes in rural communities. Health Educ Res. 2009;24(1):76–86. doi: 10.1093/her/cym095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kegler MC, Escoffery C, Groff A, Butler S, Foreman A. A qualitative study of how families decide to adopt household smoking restrictions. Fam Community Health. 2007;30(4):328–341. doi: 10.1097/01.FCH.0000290545.56199.c9. [DOI] [PubMed] [Google Scholar]
- 45.Kegler MC, Escoffery C, Bundy L et al. Pilot study results from a brief intervention to create smoke-free homes. J Environ Public Health. 2012;2012:951426. doi: 10.1155/2012/951426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. New York, NY: Guilford Press; 2002. [Google Scholar]
- 47.Winickoff JP, Friebely J, Tanski S et al. Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics. 2009;123(1):e74–e79. doi: 10.1542/peds.2008-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martins-Green M, Adhami N, Frankos M et al. Cigarette smoke toxins deposited on surfaces: implications for human health. PLoS ONE. 2014;9(1):e86391. doi: 10.1371/journal.pone.0086391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Escoffery C, Bundy L, Carvalho M et al. Third-hand smoke as a potential intervention message for promoting smoke-free homes in low-income communities. Health Educ Res. 2013;28(5):923–930. doi: 10.1093/her/cyt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. New York, NY: Prentice-Hall; 1986. [Google Scholar]
- 51.Prochaska JO, DiClemente CC. Transtheoretical therapy: toward a more integrative model of change. Psychotherapy. 1982;19(3):276–288. [Google Scholar]
- 52.Centers for Disease Control and Prevention. 2008 Behavioral Risk Factor Surveillance System Questionnaire. 2007. Available at: http://www.cdc.gov/brfss/annual_data/pdf-ques/2008brfss.pdf. Accessed January 15, 2012.
- 53.Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. Tobacco Free Initiative: Surveillance and Monitoring. Available at: http://www.who.int/tobacco/surveillance/survey/en/index.html. Accessed May 15, 2012.
- 55.Berg CJ, Bundy L, Escoffery C, Haardörfer R, Kegler MC. Telephone-assisted placement of air nicotine monitors to validate self-reported smoke-free home policies. Public Health. 2013;127(4):342–344. doi: 10.1016/j.puhe.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hudman K, Mullen P, Nicol L et al. Telephone-guided placement and removal of nicotine monitors for the assessment of passive exposure to environmental tobacco smoke. Toxicol Ind Health. 1997;13(1):73–80. doi: 10.1177/074823379701300107. [DOI] [PubMed] [Google Scholar]
- 57.Navas-Acien A, Peruga A, Breysse P et al. Secondhand tobacco smoke in public places in Latin American, 2002–2003. JAMA. 2004;291(22):2741–2745. doi: 10.1001/jama.291.22.2741. [DOI] [PubMed] [Google Scholar]
- 58.Wipfli H, Avila-Tang E, Navas-Acien A et al. Exposure to secondhand smoke among women and children worldwide. Am J Public Health. 2008;98(4):672–679. doi: 10.2105/AJPH.2007.126631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford, New York: Oxford University Press; 2003. [Google Scholar]
- 60.Grönen M. Analyzing Receiver Operating Characteristics With SAS. Cary, NC: SAS Institute Inc.; 2007. [Google Scholar]
- 61.Zakarian JM, Hovell MF, Sandweiss RD et al. Behavioral counseling for reducing children’s ETS exposure: implementation in community clinics. Nicotine Tob Res. 2004;6(6):1061–1074. doi: 10.1080/1462220412331324820. [DOI] [PubMed] [Google Scholar]
- 62.Sockrider MM, Hudmon KS, Addy R, Dolan Mullen P. An exploratory study of control of smoking in the home to reduce infant exposure to environmental tobacco smoke. Nicotine Tob Res. 2003;5(6):901–910. doi: 10.1080/14622200310001615240. [DOI] [PubMed] [Google Scholar]
- 63.Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108(1):18–24. doi: 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- 64.Eddens KS, Kreuter MW. Proactive screening for health needs in United Way’s 2-1-1 information and referral service. J Soc Serv Res. 2011;37(2):113–123. doi: 10.1080/01488376.2011.547445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klepeis NE, Hughes SC, Edwards RD et al. Promoting smoke-free homes: a novel behavioral intervention using real-time audio-visual feedback on airborne particle levels. PLoS ONE. 2013;8(8):e73251. doi: 10.1371/journal.pone.0073251. [DOI] [PMC free article] [PubMed] [Google Scholar]