Abstract
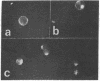
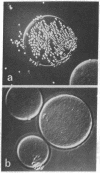
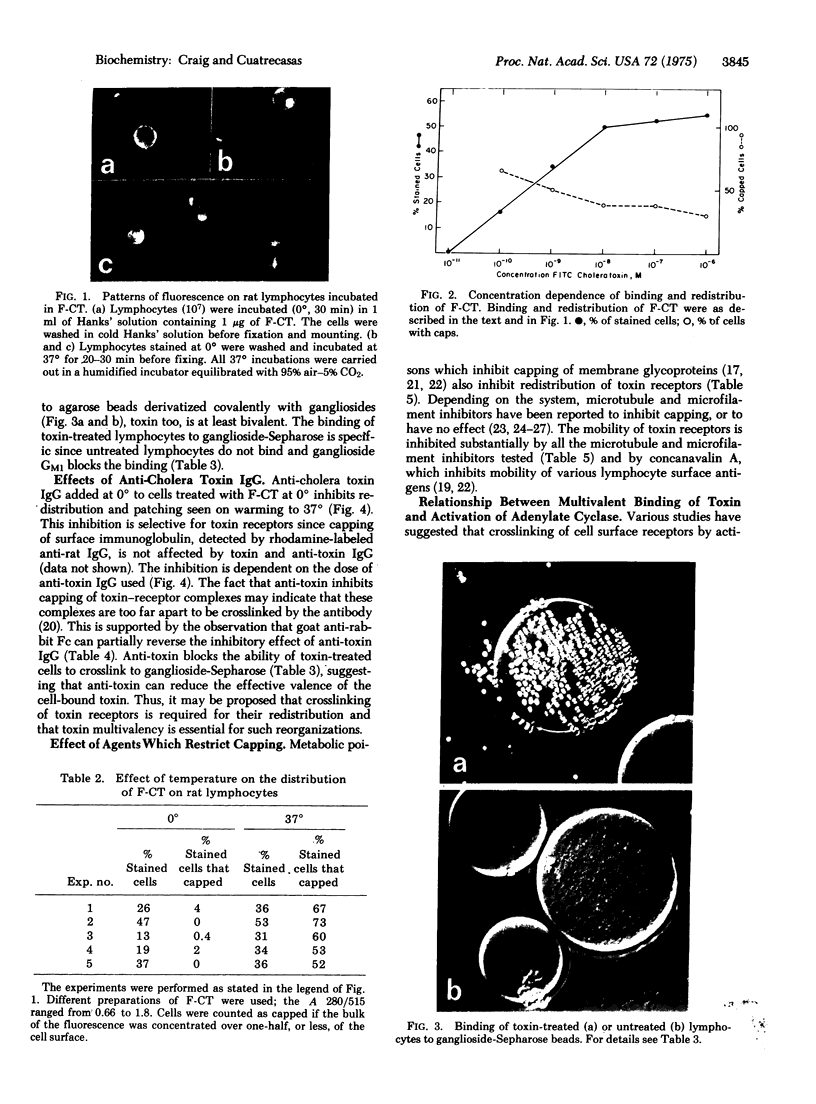
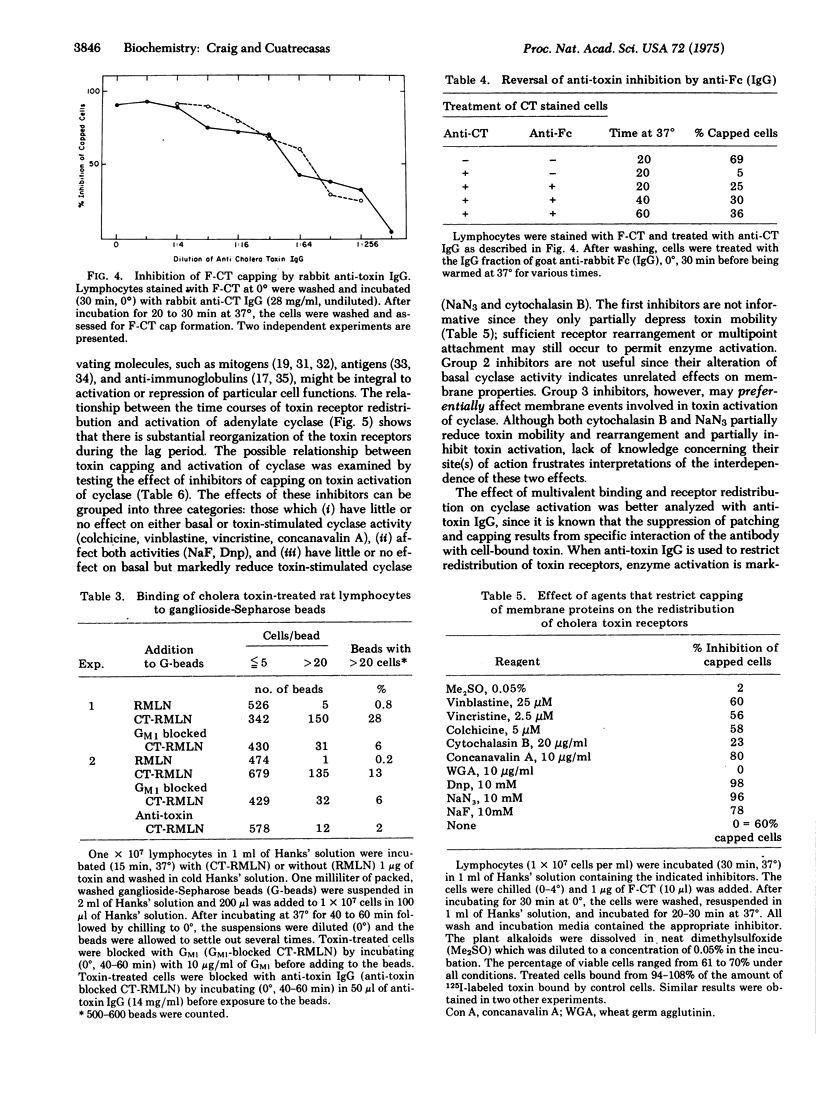
Fluorescein-labeled cholera toxin binds detectably to 40-60% of rat mesenteric lymph node cells and induces a temperature-dependent redistribution (patch and cap formation) of cell surface toxin receptors. The redistribution is inhibited by several "metabolic," "microtubule," and "microfilament" inhibitors, by concanavalin A, and by anticholera toxin IgG. Various studies indicate that cholera toxin is at least bivalent, and that this property may be related to both the induction of receptor redistribution and to the activation of adenylate cyclase. Membrane components which are probably identical to the sialo-glycolipid, GM1 ganglioside, appear to be mobile in the plane of the membrane. The possible role of toxin multivalency and receptor mobility in the mechanism of toxin action is considered.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Cuatrecasas P. Mechanism of activation of adenylate cyclase by Vibrio cholerae enterotoxin. J Membr Biol. 1975 Jun 3;22(1):29–52. doi: 10.1007/BF01868162. [DOI] [PubMed] [Google Scholar]
- Bennett V., O'Keefe E., Cuatrecasaş P. Mechanism of action of cholera toxin and the mobile receptor theory of hormone receptor-adenylate cyclase interactions. Proc Natl Acad Sci U S A. 1975 Jan;72(1):33–37. doi: 10.1073/pnas.72.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. C., Greenough W. B., 3rd Response of the canine duodenum to intraluminal challenge with cholera exotoxin. J Clin Invest. 1968 Dec;47(12):2600–2607. doi: 10.1172/JCI105942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Goldstein G. Chromatographic purification of tetramethylrhodamine-immune globulin conjugates and their use in the cellular localization of rabbit gamma-globulin polypeptide chains. J Immunol. 1965 Aug;95(2):230–245. [PubMed] [Google Scholar]
- Cohen H. J. Human lymphocyte surface immunoglobulin capping. Normal characteristics and anomalous behavior of chronic lymphocytic leukemic lymphocytes. J Clin Invest. 1975 Jan;55(1):84–93. doi: 10.1172/JCI107921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. Some observations on the neutralization of cholera vascular permeability factor in vivo. J Infect Dis. 1970 May;121(Suppl):100+–100+. doi: 10.1093/infdis/121.supplement.s100. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I., Hollenberg M. D. Affinity chromatography and structural analysis of Vibrio cholerae enterotoxin-ganglioside agarose and the biological effects of ganglioside-containing soluble polymers. Biochemistry. 1973 Oct 9;12(21):4253–4264. doi: 10.1021/bi00745a033. [DOI] [PubMed] [Google Scholar]
- De Petris S. Inhibition and reversal of capping by cytochalasin B, vinblastine and colchicine. Nature. 1974 Jul 5;250(461):54–56. doi: 10.1038/250054a0. [DOI] [PubMed] [Google Scholar]
- De Petris S., Raff M. C. Ultrastructural distribution and redistribution of alloantigens and concanavalin A receptors on the surface of mouse lymphocytes. Eur J Immunol. 1974 Feb;4(2):130–137. doi: 10.1002/eji.1830040213. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Weiss A. Antigen cap formation in cultured fibroblasts: a reflection of membrane fluidity and of cell motility. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2456–2459. doi: 10.1073/pnas.69.9.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger M. W., Hart D. A., Wells J. V., Nisonoff A. Requirement for cross-linkage in the stimulation of transformation of rabbit peripheral lymphocytes by antiglobulin reagents. J Immunol. 1970 Dec;105(6):1484–1492. [PubMed] [Google Scholar]
- Feldmann M., Easten A. The relationship between antigenic structure and the requirement for thymus-derived cells in the immune response. J Exp Med. 1971 Jul 1;134(1):103–119. doi: 10.1084/jem.134.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S. Activation of T and B lymphocytes by insoluble phytomitogens. Nat New Biol. 1972 Jan 19;235(55):67–70. doi: 10.1038/newbio235067a0. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Chen L. C., Sharp G. W. Intestinal adenyl-cyclase activity in canine cholera: correlation with fluid accumulation. J Infect Dis. 1972 Apr;125(4):377–381. doi: 10.1093/infdis/125.4.377. [DOI] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Fishman P. H., Bennett V., Cuatrecasas P. Cholera toxin and cell growth: role of membrane gangliosides. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4224–4228. doi: 10.1073/pnas.71.10.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Fixation and inactivation of cholera toxin by GM1 ganglioside. Scand J Infect Dis. 1973;5(1):77–78. doi: 10.3109/inf.1973.5.issue-1.15. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T. H. Cross-linking of glycolipids in erythrocyte ghost membrane. J Biol Chem. 1974 Dec 25;249(24):7841–7847. [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lotan R., Lis H., Rosenwasser A., Novogrodsky A., Sharon N. Enhancement of the biological activities of soybean agglutinin by cross-linking with glutaraldehyde. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1347–1355. doi: 10.1016/s0006-291x(73)80042-7. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. Control of the immune response by events at the lymphocyte surface. In Vitro. 1971 Sep-Oct;7(2):88–94. doi: 10.1007/BF02628267. [DOI] [PubMed] [Google Scholar]
- O'Keefe E., Cuatrecasas P. Cholera toxin mimics melanocyte stimulating hormone in inducing differentiation in melanoma cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2500–2504. doi: 10.1073/pnas.71.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F. Differential inhibitory effects of cholera toxoids and ganglioside on the enterotoxins of Vibrio cholerae and Escherichia coli. J Exp Med. 1973 Apr 1;137(4):1009–1023. doi: 10.1084/jem.137.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran J. A new simple method for separation of adenosine 3',5'-cyclic monophosphate from other nucleotides and its use in the assay of adenyl cyclase. Anal Biochem. 1971 Sep;43(1):227–239. doi: 10.1016/0003-2697(71)90128-x. [DOI] [PubMed] [Google Scholar]
- Springer G. F., Adye J. C., Bezkorovainy A., Jirgensons B. Properties and activity of the lipopolysaccharide-receptor from human erythrocytes. Biochemistry. 1974 Mar 26;13(7):1379–1389. doi: 10.1021/bi00704a011. [DOI] [PubMed] [Google Scholar]
- Stackpole C. W., Jacobson J. B., Lardis M. P. Two distinct types of capping of surface receptors on mouse lymphoid cells. Nature. 1974 Mar 15;248(445):232–234. doi: 10.1038/248232a0. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Modified procedure for the synthesis of 32P-labelled ribonucleoside 5'-monophosphates of high specific activity. Biochim Biophys Acta. 1968 Feb 26;155(2):609–610. doi: 10.1016/0005-2787(68)90205-0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Karnovsky M. J., Engers H. D. Ligand-induced movement of lymphocyte membrane macromolecules. 3. Relationship between the formation and fate of anti-Ig-surface Ig complexes and cell metabolism. J Exp Med. 1973 Mar 1;137(3):675–689. doi: 10.1084/jem.137.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Karnovsky M. J. Redistribution and fate of Ig complexes on surface of B lymphocytes: functional implications and mechanisms. Transplant Rev. 1973;14:184–210. doi: 10.1111/j.1600-065x.1973.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Wolff J., Temple R., Cook G. H. Stimulation of steroid secretion in adrenal tumor cells by choleragen. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2741–2744. doi: 10.1073/pnas.70.10.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]