Abstract
In complex multicellular organisms, epithelia lining body cavities regulate absorption and secretion of ions, organic molecules, and water. Proper function of epithelia depends on apically and basolaterally situated ion channels as well as tight junctions which seal the apical intercellular space. Without tight junctions, transepithelial concentration gradients of ions and nutrients would be dissipated through the paracellular space. Elevated tight junction permeability is a feature of many diseases of multiple organs, including the gastrointestinal tract [1,2,3*,4*], kidney [5,6], and lungs [7,8]. In the intestines, epithelial barrier dysfunction is a major contributor to diarrhea and malnutrition and is associated with significant morbidity and mortality worldwide.
Introduction to tight junction proteins
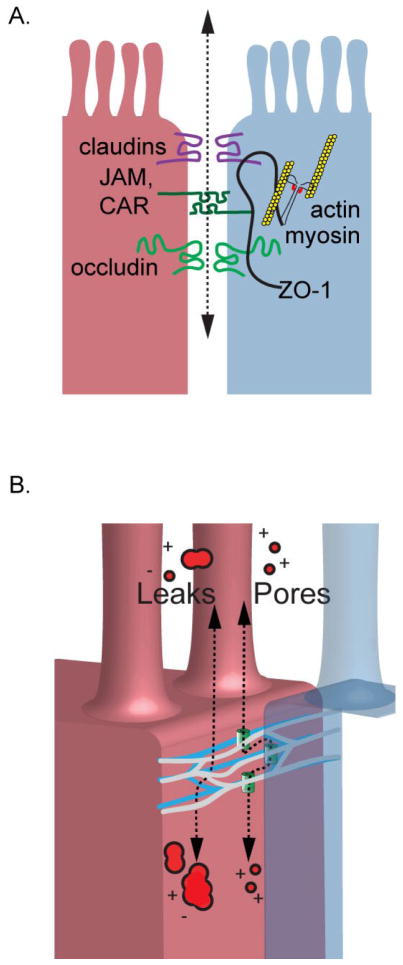
In 1986, the first tight junction protein, zonula occludens-1 (ZO-1), was discovered [9], and since that time, many additional proteins and interactions have been discovered. These include a large number of transmembrane proteins such as claudins, junctional adhesion molecules (JAMs), coxsackie adenovirus receptor (CAR), and members of the tight junction associated marvel protein (TAMP) family, including marvelD3, occludin, and tricellulin. These proteins are situated at the apical intercellular space with the extracellular domains interacting between adjacent cells to form the paracellular barrier. In addition to the these transmembrane tight junction proteins, cytoplasmic scaffolding proteins including members of the ZO family, cingulin, and related proteins, which provide coupling to the cytoskeleton (Figure 1a) and a means of interactions with multiple cellular signaling pathways which regulate paracellular flux.
Figure 1.
Tight junctions establish the rate limiting step for paracellular flux between epithelial cells. (a) The barrier is established by multiple interactions between transmembrane proteins situated on adjacent cells. Transmembrane proteins are linked to the actin cytoskeleton providing one means of barrier regulation. (b) Ultrastructurally, the tight junction appears and a meshwork of anastomosing strands that encircle the epithelial cells. Current models of barrier function suggest at least two distinct pathways of trans-tight junction flux. A high capacity pore pathway regulates paracellular flux of small ions and molecules but does not pass macromolecules. A low capacity leak pathway passes ions and macromolecules in a charge and relatively size non-selective manner.
Extracellular stimuli modulate tight junction barrier function
Multiple signaling pathways have the capacity to regulate tight junction barrier function. Physiologic mechanisms of tight junction barrier regulation include cross talk between plasma membrane ion channels and transporters and the tight junction. For example, in the intestine, ingested glucose and amino acids modulate paracellular permeability by interacting with the plasma membrane sodium glucose cotransporter (SGLT1), which activates myosin light chain kinase (MLCK) through activating another apical membrane transporter, Na+/H+ antiporter (NHE3), to promote contraction of the apical actin cytoskeleton [10]. This form of barrier regulation is thought to promote increased passive paracellular flux of ions and water soon after a meal. Other plasma membrane ion channels and transporters, including the Na+/K+ ATPase, and the chloride channel ClC-2, have also been reported to regulate the tight junction barrier. The Na+/K+ ATPase regulates tight junction permeability by inhibiting protein phosphatase 2A activity to induce occludin phosphorylation [11]. ClC-2 channels influence tight junction permeability via caveolar trafficking of occluding to the tight junction [12]. Thus physiologic tight junction regulation depends not only on tight junction protein expression and localization, but also on the expression of plasma membrane channels and transporters.
In addition to these physiological regulators of tight junction barrier function, pathological stimuli such as enteric pathogens [13], or basolateral inflammatory cytokines [7,14–16] mediate changes in tight junction conductance. Many signaling pathways including protein kinase C, mitogen activated protein kinases, and Rho GTPases have been shown to regulate tight junctions in cytoskeleton dependent and independent mechanisms [17]. Recent studies have highlighted novel roles of non-coding micro RNAs (miRNAs) in tight junction maintenance and regulation [18]. MiR-21 is upregulated in patients with ulcerative colitis, induces barrier dysfunction, and decreased occludin protein in vitro. These effects are correlated with degradation of RhoB mRNA [19]. Another miRNA, MiR-122a, directly controls occludin expression by degrading occludin mRNA [20]. Thus, numerous physiological and pathophysiological signaling pathways converge at the tight junction for fine-tuned regulation of paracellular flux. We now understand that the full capacity of barrier regulation is not achieved by simple paracellular tightening and loosening, but via regulation of multiple dynamic paracellular charge and size selective permeability pathways.
A Dynamic Model of Tight Junction Function
Historically, the tight junction was often assumed to be a simple static paracellular seal, but evidence supports that tight junctions are far more complex with more than one distinct permeability pathway and the capacity to dynamically regulate paracellular flux in a size and charge selective manner. Early evidence favoring a more complex mechanism of barrier regulation comes from freeze fracture scanning electron microscopy studies. Using this approach, the lipid bilayer at the apical intercellular space is fractured along hydrophobic planes providing a lateral view of the tight junction. These studies showed that the tight junction is composed of multiple parallel branching stands encircling the cell at its apical surface (Figure 1b). Leaky epithelia, like the kidney proximal convoluted tubule and gallbladder, generally have 1–2 strands, and tight epithelia like the bladder contain 5 or more strands [21]. Remarkably, it was shown that the number of tight junction strands does not correlate linearly with transepithelial resistance, as would be expected of resistors arranged in series. Rather, transepithelial resistance follows a logarithmic function of strand number [22]. Such non-linear behavior could be explained by a model in which each strand is populated by conductive pathways which can open and close [22]. In such a model, conductance depends not only on the number of strands, but also the number of open pores in each strand.
To date it has been impossible to directly test this model through direct measurements due to limitations in our ability to study tight junction function at the molecular level. Instead, we have relied on temporally and spatially averaged measurements of barrier function across small areas of epithelium containing thousands of epithelial cells and intercellular spaces.
Additional insight into the dynamics of tight junction structure can be obtained by studying the diffusion of fluorescent tagged tight junction proteins. Using an approach known as fluorescence recovery after photobleaching (FRAP), a small (e.g., several microns) area of fluorescently tagged tight junction protein is transiently bleached. The rate and extent of fluorescence recovery in this area is followed over time. This provides both a measurement of the rate of recovery (i.e. t1/2) as well as percent recovery (i.e. mobile fraction). Using this approach and a similar approach, fluorescence loss in photobleaching (FLIP), it was discovered that the interactions between tight junction proteins and the actin cytoskeleton are labile and undergo continuous remodeling at steady state [23]. ZO-1, occludin, and claudin-1 each have distinct recovery kinetics. Specifically, claudin-1 and occludin both diffuse within the plasma membrane but differ dramatically with respect to their mobile fraction. In contrast, the scaffolding protein ZO-1 does not diffuse within the plasma membrane, but rather exchanges with intracellular pools. Its exchange depends on interactions with the actin cytoskeleton and MLCK activity. Remarkably, the mobile fraction of ZO-1 correlates with MLCK activity and tight junction barrier function [24]. Thus, these data support a dynamic model of tight junction barrier in which tight junction mobility is reflective of tight junction electrical conductance.
Pore and Leak Pathways of tight junction conductance
Measurements of tight junction barrier function usually rely on either electrical measurements of tight junction conductance and/or flux assays of molecular tracers. These two approaches have historically been used as interchangeable methods to study the tight junction barrier. However, examples where electrical conductance and flux are not correlated suggest a more complicated model in which the barrier to macromolecules is distinct from the barrier to small ions [25,26]. To better understand this phenomenon, additional assays of barrier function to measure tight junction size selectivity and charge selectivity are required [27]. Tight junction size selectivity can be assessed by measuring the permeability of a graded series of uncharged polyethylene glycol oligomers as a function of their radius [14]. A second approach is to substitute different sized monovalent cations for Na+ and to measure relative permeabilities electrically from shifts in equilibrium potential [28]. From such measurements, it is clear that the relationship between tracer size and tight junction flux is biphasic. There is a high permeability for molecules smaller than ~4 Å radius and relatively low permeability for larger molecules [27,29]. Tight junction charge selectivity can be assessed from the relative permeabilities of Na+ and Cl− determined from the equilibrium potential measured after reducing the concentration of NaCl on one side of the epithelium.
From such measurements, it was determined that expression of claudins are the principal determinants of tight junction charge selectivity and define tight junction permeability to small molecules [30]. Stimuli that affect ZO-1, occludin, or tricellulin were shown to modulate flux of larger molecules irrespective of charge [31]. Based on these data, the prevailing hypothesis is that there are at least two distinct pathways of trans-tight junction flux: a low capacity ZO-1- and occludin-dependent “leak” pathway that defines the permeability of macromolecules and a high capacity claudin-dependent “pore” pathway that regulates permeability of the tight junction to ions and small molecules.
Further data support that these two pathways are differentially regulated by pathophysiological stimuli. For example, activation of SGLT1, which regulates tight junction permeability via communications through the actin cytoskeleton [10], results in increased tight junction conductance to small ions (i.e. reduced transepithelial resistance). However, when one assesses macromolecular flux using tracer molecules, it becomes apparent that permeability is not always increased by activation of SGLT1. There is a size cutoff such inulin (radius = 11.5 Å) cannot cross the tight junction, while the smaller molecule mannitol (radius = 3.6 Å), can pass. These data are consistent with activation of small tight junction pores which would be expected to augment paracellular ion and water absorption without allowing significant paracellular loss of larger proteins and nutrients.
Cytokines, which are produced by lamina propria inflammatory cells, also have the capacity to differentially regulate pore and leak pathways. Interleukin-13 (IL-13) [15,25,32,33] and tumor necrosis factor (TNF)-α [33–35], in particular, have been extensively studied because of their relevance to inflammatory bowel disease. Interestingly, these cytokines differentially regulate pore and leak pathways. IL-13 acts to specifically increase tight junction claudin-2 expression and specifically increases tight junction permeability to Na+ and small cations, but does not significantly alter transepithelial permeability of negatively charged ions or macromolecules [25]. TNF-α causes increased permeability of small ions irrespective of their charge and increased flux of uncharged macromolecules and is associated with internalization of occludin [25,36*].
Thus, there are at least two distinct pathways of trans-tight junction conductance: pore and leak. We still do not fully understand the relative contributions of these pathways or significance of alterations to diseases. Bridging this gap in knowledge of molecular mechanisms of these pathways is expected to help with the development of more specific approaches to treat barrier dysfunction in the future.
Molecular mechanisms of pore pathway flux
There are 24 members of the claudin family of tight junction proteins. Claudin expression varies between types of epithelium, depending on physiological requirements [37–40], changes over the course of development [41], and is altered in a large number of different disease states [1–3,15,33,42,43]. The pattern of claudins expressed is the major determinant of pore pathway permeability. Some claudins form relatively tight paracellular barriers to ions [44], while others, such as claudin-2 and claudin-15 make tight junctions selectively permeable to small cations, but impermeable to anions and macromolecules [45]. It is notable that knockout of claudins 2 and 15 in mice was recently shown to cause dramatic defects in paracellular Na+ flux and this was coupled with defects in cellular sodium coupled nutrient transport [46**]. Other claudins (e.g. claudin-4, 10a, 17), induce anion selectivity [47*–49].
The crystal structure of a claudin-15 monomer was recently discovered, and agrees with mathematical predictions that claudins consist of four transmembrane alpha helices and two extracellular loops [50**]. The extracellular loops interact to form a beta sheet fold. Claudin monomers are believed to assemble into polymeric structures. The structure of these polymers is defined by conserved residues within transmembrane segment 3 of claudins, and this also contributes to strand ultrastructure [51*]. It appears that a disulfide bond between conserved extracellular cysteines is important to intermolecular interactions and pore formation [52*]. Although the structure of the claudin pore remains undefined, mutagenesis studies have pinpointed several amino acids in the first extracellular loop, which are important to defining pore ion selectivity properties [28,53]. Bulky covalent modifications in this region of the pore have the capacity to reduce paracellular conductance [54].
Molecules which interact with residues lining claudin pores are potential modulators of pore pathway function. Multivalent cations Ca2+ and La3+ are competitive inhibitors of the pore pathway function which compete with monovalent cations for binding to negatively charged residues within the pore [54]. However, since these ions interact with other cell membrane transporters, they are not specific modulators of paracellular flux. A possible pharmacologic approach to treat barrier dysfunction may be to design peptides that act as claudin binding partners. For example, a peptide derived from the claudin-1 extracellular loop has been shown to specifically disrupt barrier function [55]. However, this effect is associated with a drop in barrier function and as such, appears to be due to defects in tight junction assembly rather than pore blockade. Another claudin binder derived from Clostridium perfringens enterotoxin increases tight junction permeability to macromolecules [56,57]. Improved understanding of claudin pore structure is expected to aid in the development of agents to specifically block pore function.
Since tight junctions are dynamic structures that undergo continuous remodeling, stimuli which modulate the ability of claudins to form stable pore structures is another approach which can be used to reduce pore permeability. One such stimulus is casein kinase 2 (CK2). CK2-dependent phosphorylation of the occludin C-terminal tail favors the formation of a stable complex between claudin-1, claudin-2 and ZO-1, preventing formation of claudin-2 pores after IL-13-induced increases in permeability [58]. In addition to phosphorylation of occludin, other stimuli which directly phosphorylate of claudin-2 at its cytoplasmic tail can regulate trafficking and retention of claudin-2 to the tight junction [59*].
Mechanisms of leak pathway flux
Much less is known about the mechanisms of the tight junction leak pathway. The leak pathway is often associated with reduced expression or internalization of tight junction proteins such as occludin, ZO-1, or tricellulin [25,36,60,61].
There are several models to account for increased leak pathway, and they are not mutually exclusive. One possibility is that increased large molecule flux is due to transient strand separations allowing large molecules to cross the tight junction in a stepwise manner. Another possible explanation is that leak may be due to the physical geometry of tricellular contacts. Overexpression of tricellulin, a member of the TAMP family, which localizes to tricellular tight junctions, appears to diminish this type of leak [61]. Finally, loss of occludin is associated with a ~62.5 Å barrier defect suggests a model of leak associated with large paracellular channels [58]. The structural basis of these channels remains unclear.
Tight junction dynamics are also an important part of leak pathway regulation. TNF-α treatment increases the diffusion rate of occludin within the plasma membrane and promotes occludin endocytosis. These effects can be blocked through specific inhibition of MLCK [16,25] or by blocking occludin/ZO-1 interactions at the occludin OCEL domain [36].
Summary
Tight junctions form the essential paracellular barrier in all epithelia. While these structures were once thought to be static and largely impermeant, we now understand that tight junctions are dynamic structures. Further, paracellular flux is regulated via at least two distinct mechanisms: pore and leak. The pore pathway selectively regulates transepithelial permeability to small ions, whereas the leak pathway is a charge non-selective pathway which allows much larger macromolecules to pass. Improved understanding of the molecular mechanisms and differential roles of these pathways in disease will lead to novel therapeutic approaches to treat barrier dysfunction.
Highlights.
Paracellular flux is regulated by dynamic tight junction protein interactions.
Claudin interactions define small molecule flux across tight junction pores.
Macromolecular flux occurs via the non-selective tight junction leak pathway.
Pore and leak pathways are differentially regulated in health and disease.
Acknowledgments
The authors are supported by the National Institutes of Health K08DK088953 and The University of Chicago, Digestive Diseases and Research Core Center Pilot and Feasibility Study, P30 DK42086.
Footnotes
Nothing declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schumann M, Kamel S, Pahlitzsch ML, Lebenheim L, May C, Krauss M, Hummel M, Daum S, Fromm M, Schulzke JD. Defective tight junctions in refractory celiac disease. Ann N Y Acad Sci. 2012;1258:43–51. doi: 10.1111/j.1749-6632.2012.06565.x. [DOI] [PubMed] [Google Scholar]
- *3.Lameris AL, Huybers S, Kaukinen K, Makela TH, Bindels RJ, Hoenderop JG, Nevalainen PI. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol. 2013;48:58–69. doi: 10.3109/00365521.2012.741616. The authors analyzed claudin mRNA levels of claudins in gastrointestinal biopsies of healthy individuals and patients with active and inactive inflammatory bowel diseases (IBD). Claudin 18 was only expressed in the stomach, claudin-2 and -15 were expressed in the proximal gastrointestinal tract, claudin-3, -4, -7 and -8, were expressed in the distal parts, and claudin-12 was expressed throughout the gastrointestinal tract. Claudin expression changes correlated with inflammatory activity in IBD. [DOI] [PubMed] [Google Scholar]
- *4.Bergmann KR, Liu SX, Tian R, Kushnir A, Turner JR, Li HL, Chou PM, Weber CR, De Plaen IG. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol. 2013;182:1595–1606. doi: 10.1016/j.ajpath.2013.01.013. In a neonatal mouse model of necrotizing enterocolitis (NEC), the authors demonstrate that increased intestinal permeability and tight junction alterations preceded NEC and changes were prevented by administration of the probiotic Bifidobacterium infantis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon O, Nelson WJ, Sibley R, Huie P, Scandling JD, Dafoe D, Alfrey E, Myers BD. Backleak, tight junctions, and cell-cell adhesion in postischemic injury to the renal allograft. J Clin Invest. 1998;101:2054–2064. doi: 10.1172/JCI772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 7.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfrey RW, Severs NJ, Jeffery PK. Structural alterations of airway epithelial tight junctions in cystic fibrosis: comparison of transplant and postmortem tissue. Am J Respir Cell Mol Biol. 1993;9:148–156. doi: 10.1165/ajrcmb/9.2.148. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 11.Rajasekaran SA, Barwe SP, Gopal J, Ryazantsev S, Schneeberger EE, Rajasekaran AK. Na-K-ATPase regulates tight junction permeability through occludin phosphorylation in pancreatic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G124–133. doi: 10.1152/ajpgi.00297.2006. [DOI] [PubMed] [Google Scholar]
- 12.Nighot PK, Blikslager AT. Chloride channel ClC-2 modulates tight junction barrier function via intracellular trafficking of occludin. Am J Physiol Cell Physiol. 2012;302:C178–187. doi: 10.1152/ajpcell.00072.2011. [DOI] [PubMed] [Google Scholar]
- 13.Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson CJ, Hoare CJ, Garrod DR, Carlson GL, Warhurst G. Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J Cell Sci. 2005;118:5221–5230. doi: 10.1242/jcs.02630. [DOI] [PubMed] [Google Scholar]
- 15.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 17.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Rao JN, Wang JY. Posttranscriptional Regulation of Intestinal Epithelial Tight Junction Barrier by RNA-binding Proteins and microRNAs. Tissue Barriers. 2014;2:e28320. doi: 10.4161/tisb.28320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Ma Y, Shi C, Chen H, Zhang H, Chen N, Zhang P, Wang F, Yang J, Zhu Q, et al. Overexpression of miR-21 in patients with ulcerative colitis impairs intestinal epithelial barrier function through targeting the Rho GTPase RhoB. Biochem Biophys Res Commun. 2013;434:746–752. doi: 10.1016/j.bbrc.2013.03.122. [DOI] [PubMed] [Google Scholar]
- 20.Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323–1333. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claude P, Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claude P. Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J Membr Biol. 1978;39:219–232. doi: 10.1007/BF01870332. [DOI] [PubMed] [Google Scholar]
- 23.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci U S A. 2010;107:8237–8241. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008 doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 31.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Laboratory Investigation. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald TT, Hutchings P, Choy MY, Murch S, Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990;81:301–305. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Buschmann MM, Shen L, Rajapakse H, Raleigh DR, Wang Y, Wang Y, Lingaraju A, Zha J, Abbott E, McAuley EM, et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell. 2013;24:3056–3068. doi: 10.1091/mbc.E12-09-0688. In cultured epithelial monolayers, the authors showed that TNF-induced tight junction leak allows passage of molecules with radii of approximately 62.5 angstroms or less. This is the first such demonstration of a size cuttoff for the leak pathway flux, thus demonstrating that leak is not explained by complete absence of a barrier. The authors further demonstrated that TNF-induced barrier dysfunction is associated with accelerated EGFP-occludin fluorescence recovery after photobleaching, highlighting the importance of tight junction dynamic interactions to barrier regulation. Finally, they demonstrated the importance of cystoplasmic interactions at the occludin OCEL K433 region for this process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reyes JL, Lamas M, Martin D, del Carmen Namorado M, Islas S, Luna J, Tauc M, Gonzalez-Mariscal L. The renal segmental distribution of claudins changes with development. Kidney Int. 2002;62:476–487. doi: 10.1046/j.1523-1755.2002.00479.x. [DOI] [PubMed] [Google Scholar]
- 38.Peppi M, Ghabriel MN. Tissue-specific expression of the tight junction proteins claudins and occludin in the rat salivary glands. J Anat. 2004;205:257–266. doi: 10.1111/j.0021-8782.2004.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 40.Kaarteenaho R, Merikallio H, Lehtonen S, Harju T, Soini Y. Divergent expression of claudin-1, -3, -4, -5 and -7 in developing human lung. Respir Res. 2010;11:59. doi: 10.1186/1465-9921-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006;6:581–588. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 43.Bergmann KR, Liu SX, Tian R, Kushnir A, Turner JR, Li HL, Chou PM, Weber CR, De Plaen IG. Bifidobacteria Stabilize Claudins at Tight Junctions and Prevent Intestinal Barrier Dysfunction in Mouse Necrotizing Enterocolitis. Am J Pathol. 2013 doi: 10.1016/j.ajpath.2013.01.013. In a neonatal mouse model of necrotizing enterocolitis (NEC), the authors demonstrate that increased intestinal permeability and tight junction alterations preceded NEC and changes were prevented by administration of the probiotic Bifidobacterium infantis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- **46.Wada M, Tamura A, Takahashi N, Tsukita S. Loss of Claudins 2 and 15 From Mice Causes Defects in Paracellular Na(+) Flow and Nutrient Transport in Gut and Leads to Death from Malnutrition. Gastroenterology. 2013;144:369–380. doi: 10.1053/j.gastro.2012.10.035. The concentration of Na+ in the lumen was greatly reduced in Cldn2(−/−)Cldn15(−/−), double-knockout mice. This was associated with decreased absorption of glucose, amino acids, and fats and ultimately mouse death from malnutrition at day 25. This is an excellent illustration of the essential role for claudins in regulation of transepithelial ion gradients and demonstrates the importance of ion selective sodium pores established by claudin-2 and -15. [DOI] [PubMed] [Google Scholar]
- *47.Krug SM, Gunzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, Schulzke JD, Fromm M. Claudin-17 forms tight junction channels with distinct anion selectivity. Cell Mol Life Sci. 2012;69:2765–2778. doi: 10.1007/s00018-012-0949-x. Claudin-17 forms anion selective pores, which is in contrast to most claudins which form cation selective pores. This is likely associated with a somewhat unique physiological function on the proximal nephron where claudin-17 is principally expressed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol. 2006;291:F1288–1299. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 49.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A. 2010;107:18010–18015. doi: 10.1073/pnas.1009399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **50.Suzuki H, Nishizawa T, Tani K, Yamazaki Y, Tamura A, Ishitani R, Dohmae N, Tsukita S, Nureki O, Fujiyoshi Y. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science. 2014;344:304–307. doi: 10.1126/science.1248571. This is the first demonstration of the crystal structure of any claudin molecule. The structure consists of a transmembrane four-helix bundle and two extracellular loops which forming a beta-sheet fold. [DOI] [PubMed] [Google Scholar]
- *51.Rossa J, Ploeger C, Vorreiter F, Saleh T, Protze J, Gunzel D, Wolburg H, Krause G, Piontek J. Claudin-3 and claudin-5 protein folding and assembly into the tight junction are controlled by nonconserved residues in the transmembrane 3 (TM3) and extracellular loop 2 (ECL2) segments. J Biol Chem. 2014;289:7641–7653. doi: 10.1074/jbc.M113.531012. In this study, the authors begin to define the molecular determinants of claudin strand assembly. They showed that residues in transmembrane segment 3 and the transition of transmembrane segment 3 to extracellular loop 2 were involved in claudin interactions and defined tight junction strand ultrastructure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *52.Li J, Angelow S, Linge A, Zhuo M, Yu AS. Claudin-2 pore function requires an intramolecular disulfide bond between two conserved extracellular cysteines. Am J Physiol Cell Physiol. 2013;305:C190–196. doi: 10.1152/ajpcell.00074.2013. In MDCK epithelial cell lines, disulfide bonds between two conserved cysteine residues, C54 and C64, in the extracellular loop (ECL) 1 of claudin-2 is necessary for claudin-2 pore formation, probably by stabilizing the ECL1 fold. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Itallie CM, Anderson JM. The role of claudins in determining paracellular charge selectivity. Proc Am Thorac Soc. 2004;1:38–41. doi: 10.1513/pats.2306013. [DOI] [PubMed] [Google Scholar]
- 54.Angelow S, Yu AS. Structure-function studies of claudin extracellular domains by cysteine-scanning mutagenesis. J Biol Chem. 2009;284:29205–29217. doi: 10.1074/jbc.M109.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mrsny RJ, Brown GT, Gerner-Smidt K, Buret AG, Meddings JB, Quan C, Koval M, Nusrat A. A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am J Pathol. 2008;172:905–915. doi: 10.2353/ajpath.2008.070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi A, Saito Y, Kondoh M, Matsushita K, Krug SM, Suzuki H, Tsujino H, Li X, Aoyama H, Matsuhisa K, et al. Creation and biochemical analysis of a broad-specific claudin binder. Biomaterials. 2012;33:3464–3474. doi: 10.1016/j.biomaterials.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 57.Veshnyakova A, Piontek J, Protze J, Waziri N, Heise I, Krause G. Mechanism of Clostridium perfringens Enterotoxin Interaction with Claudin-3/-4 Protein Suggests Structural Modifications of the Toxin to Target Specific Claudins. J Biol Chem. 2012;287:1698–1708. doi: 10.1074/jbc.M111.312165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193:565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *59.Van Itallie CM, Tietgens AJ, Logrande K, Aponte A, Gucek M, Anderson JM. Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes. J Cell Sci. 2012 doi: 10.1242/jcs.111237. Phosphorylation of a serine residue in the claudin-2 tail (S208) is important to retention of claudin-2 at the plasma membrane. These data suggest a means by which claudin-2 expression is regulated, and a potential means by which tight junction sodium permeability could be increased in a number of inflammatory disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krug SM, Amasheh S, Richter JF, Milatz S, Gunzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–3724. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]